Proteomics Analysis of Colostrum Samples from Sows Housed under Different Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Treatments, Management, and Feeding
2.2. Colostrum Samples
2.3. Isolation of Colostrum Proteins
2.4. Protein In-Solution Digestion and Tagging
2.5. Classification by High-Performance Liquid Chromatography (HPLC) and Analysis with Liquid Chromatography (LC) Mass Spectrometry (MS)
2.6. MS Data Analysis
2.7. Screening of Differentially Expressed Proteins
2.8. Bioinformatics Analysis
2.9. Statistical Analysis
3. Results
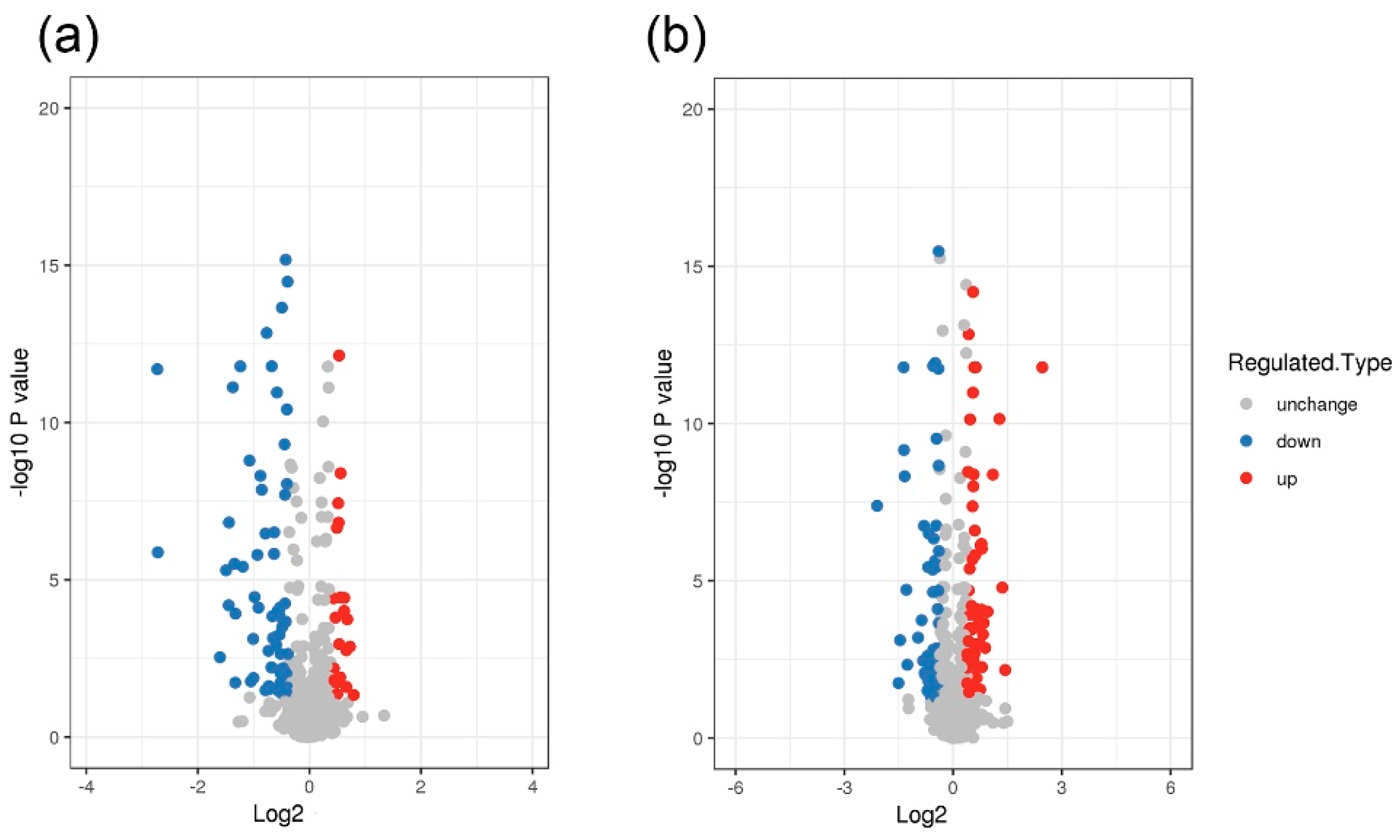
3.1. Differentially Expressed Proteins
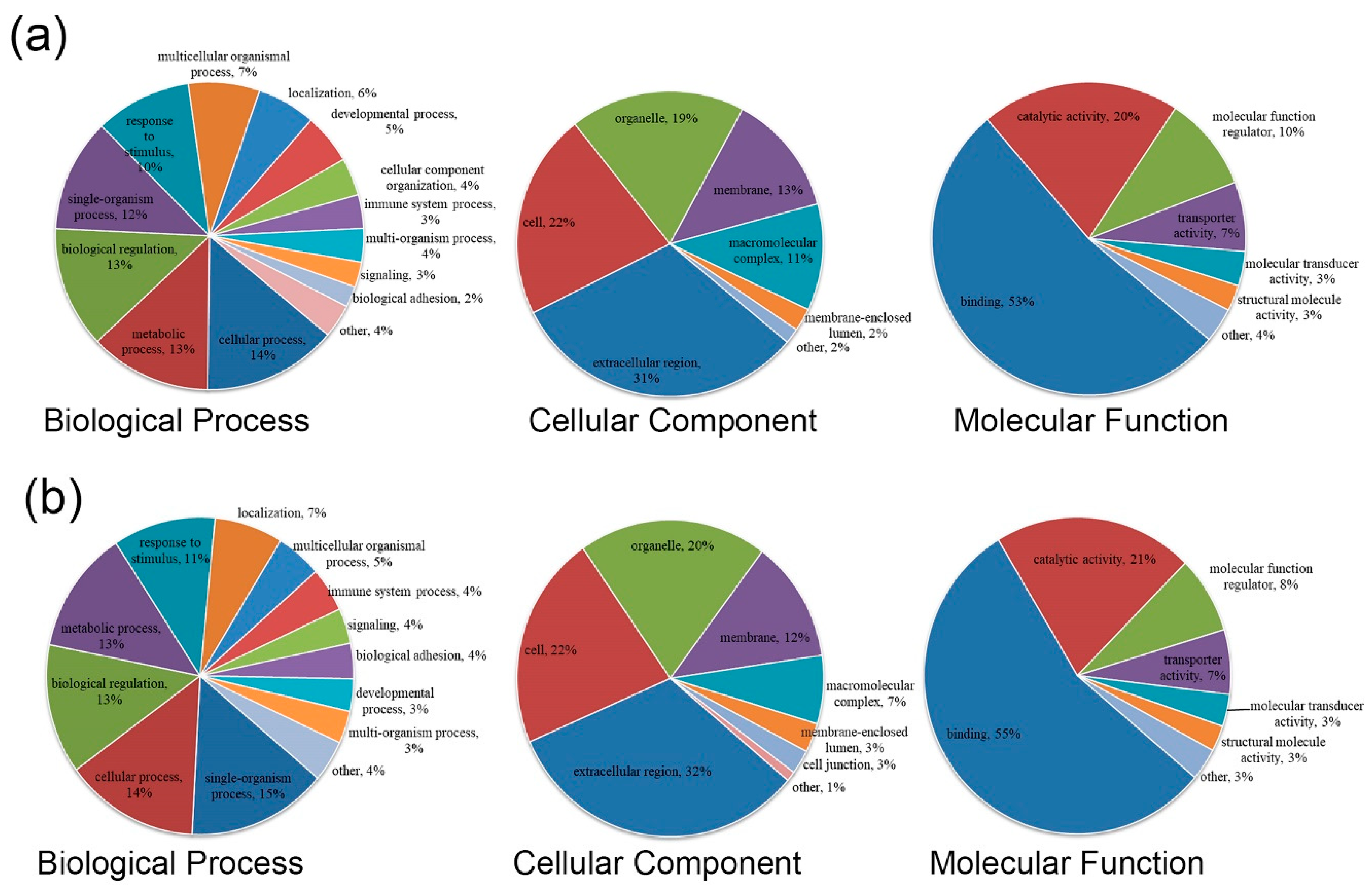
3.2. GO Functional Annotation
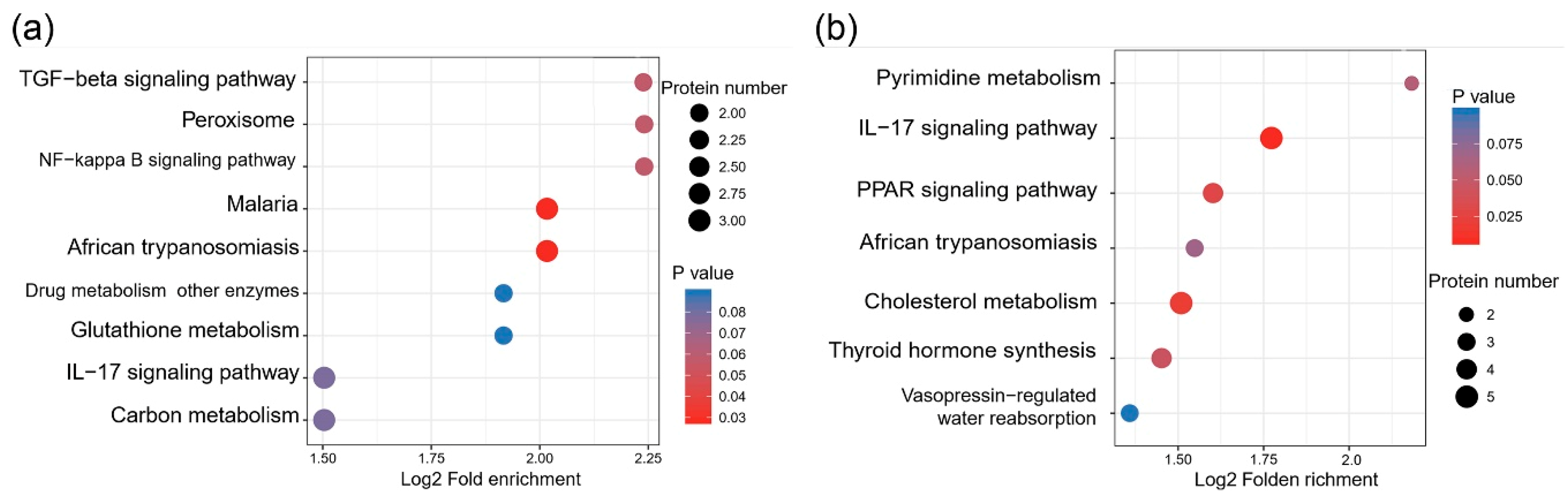
3.3. KEGG Pathway Enrichment of Differentially Expressed Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robert, C.F.S. Hormonal, behavioural and performance characteristics of Meishan sows during pregnancy and lactation. Can. J. Anim. Sci. 2003, 83, 1–12. [Google Scholar] [CrossRef]
- Hernandezcastellano, L.E.; Almeida, A.M.; Castro, N.; Arguello, A. The Colostrum Proteome, Ruminant Nutrition and Immunity: A Review. Curr. Protein Pept. Sci. 2014, 15, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Quesnel, H. Nutritional, hormonal, and environmental effects on colostrum in sows. J. Anim. Sci. 2009, 87, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Tsukahara, T.; Nishibayashi, R.; Nakatani, M.; Okutani, M.; Nakanishi, N.; Ushida, K.; Inoue, R. Shotgun proteomic analysis of porcine colostrum and mature milk. Anim. Sci. J. 2014, 85, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, X.; Wu, G.; Kim, S.; Chen, F.; Wang, J. Differential composition of proteomes in sow colostrum and milk from anterior and posterior mammary glands. J. Anim. Sci. 2010, 88, 2657–2664. [Google Scholar] [CrossRef]
- Yun, J.; Swan, K.; Vienola, K.; Kim, Y.Y.; Oliviero, C.; Peltoniemi, O.A.T.; Valros, A. Farrowing environment has an impact on sow metabolic status and piglet colostrum intake in early lactation. Livest. Sci. 2014, 163, 120–125. [Google Scholar] [CrossRef]
- Yun, J.; Swan, K.; Vienola, K.; Farmer, C.; Oliviero, C.; Peltoniemi, O.; Valros, A. Nest-building in sows: Effects of farrowing housing on hormonal modulation of maternal characteristics. Appl. Anim. Behav. Sci. 2013, 148, 77–84. [Google Scholar] [CrossRef]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 2016, 184, 46–57. [Google Scholar] [CrossRef]
- Quesnel, H.; Farmer, C.; Devillers, N. Colostrum intake: Influence on piglet performance and factors of variation. Livest. Sci. 2012, 146, 105–114. [Google Scholar] [CrossRef]
- Dividich, J.L.; Charneca, R.; Thomas, F. Relationship between birth order, birth weight, colostrum intake, acquisition of passive immunity and pre-weaning mortality of piglets. Span. J. Agric. Res. 2017, 15, 0603. [Google Scholar] [CrossRef]
- Theil, P.K.; Lauridsen, C.; Quesnel, H. Neonatal piglet survival: Impact of sow nutrition around parturition on fetal glycogen deposition and production and composition of colostrum and transient milk. Animal 2014, 8, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-C.; Lim, C.-Y.; Chong, K.-Y.; Tsai, T.-C.; Shen, C.-J.; Lim, M.-F.; Su, C.-Y.; Chen, H.-L.; Chen, C.-M. Lactoferrin as a natural regimen for selective decontamination of the digestive tract: Recombinant porcine lactoferrin expressed in the milk of transgenic mice protects neonates from pathogenic challenge in the gastrointestinal tract. J. Infect. Dis. 2009, 199, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, M.; Pedersen, L.J.; Bendixen, E. An in vivo characterization of colostrum protein uptake in porcine gut during early lactation. J. Proteom. 2011, 74, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Roncada, P.; Piras, C.; Soggiu, A.; Turk, R.; Urbani, A.; Bonizzi, L. Farm animal milk proteomics. J. Proteom. 2012, 75, 4259–4274. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Wang, L.; Zhao, X.; Yu, L.; Chen, B. Effects of pregnancy and lactation environments on maternal performance of primiparous sows during lactation. Int. J. Agric. Biol. 2020, 23, 409–416. [Google Scholar]
- Hemsworth, P.H. Key determinants of pig welfare: Implications of animal management and housing design on livestock welfare. Anim. Prod. Sci. 2018, 58, 1375–1386. [Google Scholar] [CrossRef]
- Miller, G.E.; Cohen, S.; Ritchey, A.K. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychol. 2002, 21, 531–541. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Webster, E.L.; Torpy, D.J.; Chrousos, G.P. Stress, Corticotropin-Releasing Hormone, Glucocorticoids, and the Immune/Inflammatory Response: Acute and Chronic Effectsa. Ann. N. Y. Acad. Sci. 1999, 876, 1–13. [Google Scholar] [CrossRef]
- Vitlic, A.; Lord, J.M.; Phillips, A.C. Stress, ageing and their influence on functional, cellular and molecular aspects of the immune system. Age 2014, 36, 9631. [Google Scholar] [CrossRef]
- Chang, S.H.; Dong, C. IL-17F: Regulation, signaling and function in inflammation. Cytokine 2009, 46, 7–11. [Google Scholar] [CrossRef]
- Ogura, H.; Murakami, M.; Okuyama, Y.; Tsuruoka, M.; Kitabayashi, C.; Kanamoto, M.; Nishihara, M.; Iwakura, Y.; Hirano, T. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 2008, 29, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Gaffen, S.L. Structure–function relationships in the IL-17 receptor: Implications for signal transduction and therapy. Cytokine 2008, 41, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.O.; Martin, L.; Østergaard, M.V.; Rudloff, S.; Roggenbuck, M.; Nguyen, D.N.; Sangild, P.T.; Bering, S.B. Human milk oligosaccharide effects on intestinal function and inflammation after preterm birth in pigs. J. Nutr. Biochem. 2017, 40, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Yuan, L.; Azevedo, M.S.; Jeong, K.-I.; González, A.-M.; Saif, L.J. Transfer of maternal cytokines to suckling piglets: In vivo and in vitro models with implications for immunomodulation of neonatal immunity. Vet. Immunol. Immunopathol. 2007, 117, 236–248. [Google Scholar] [CrossRef]
- Foisnet, A.; Farmer, C.; David, C.; Quesnel, H. Relationships between colostrum production by primiparous sows and sow physiology around parturition. J. Anim. Sci. 2010, 88, 1672–1683. [Google Scholar] [CrossRef]
- Loisel, F.; Farmer, C.; Ramaekers, P.; Quesnel, H. Colostrum yield and piglet growth during lactation are related to gilt metabolic and hepatic status prepartum. J. Anim. Sci. 2014, 92, 2931–2941. [Google Scholar] [CrossRef]
- Hayes, J.D.; McLELLAN, L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic. Res. 1999, 31, 273–300. [Google Scholar] [CrossRef]
- Reddy, K.E.; Jeong, J.; Lee, S.D.; Baek, Y.-C.; Oh, Y.; Kim, M.; So, K.M.; Kim, D.W.; Kim, J.H.; Park, S. Effect of different early weaning regimens for calves on adipogenic gene expression in Hanwoo loin at the fattening stage. Livest. Sci. 2017, 195, 87–98. [Google Scholar] [CrossRef]
- Cipolletta, D.; Feuerer, M.; Li, A.; Kamei, N.; Lee, J.; Shoelson, S.E.; Benoist, C.; Mathis, D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue T reg cells. Nature 2012, 486, 549–553. [Google Scholar] [CrossRef]
- Islinger, M.; Grille, S.; Fahimi, H.D.; Schrader, M. The peroxisome: An update on mysteries. Histochem. Cell Biol. 2012, 137, 547–574. [Google Scholar] [CrossRef] [PubMed]
| Nutrient Composition | Pregnancy 0 to 90 Days | Pregnancy 90 to 107 Days | Pregnancy 107 Days to Weaning |
|---|---|---|---|
| DE (Mcal/kg) | 3.0 | 3.2 | 3.39 |
| CP (%) | 14.66 | 15.47 | 18.31 |
| SID Lys (%) | 0.6 | 0.65 | 1.00 |
| KEGG Pathway | Protein Accession | Protein Description | Regulated Type |
|---|---|---|---|
| TGF-beta signaling pathway | F1S6B5 | Fibromodulin | Up |
| TGF-beta signaling pathway | P15203 | Transforming growth factor beta-3 | Down |
| IL-17 signaling pathway | C3S7K5 | Protein S100 | Down |
| IL-17 signaling pathway | C3S7K6 | Calcium-binding protein | Down |
| IL-17 signaling pathway | A0A287A9T4 | Heat shock protein | Down |
| African trypanosomiasis | P01965 | Hemoglobin subunit alpha | Down |
| African trypanosomiasis | A0A286ZJL9 | Uncharacterized protein | Up |
| African trypanosomiasis | F1RII7 | Hemoglobin subunit beta | Down |
| NF-kappa B signaling pathway | A0A286ZJL9 | Uncharacterized protein | Up |
| NF-kappa B signaling pathway | A0A287B5Y6 | Lipopolysaccharide-binding protein precursor | Down |
| Glutathione metabolism | F1RIF8 | 6-phosphogluconate dehydrogenase | Down |
| Glutathione metabolism | P80031 | Glutathione S-transferase P | Up |
| Malaria | P01965 | Hemoglobin subunit alpha | Down |
| Malaria | P15203 | Transforming growth factor beta-3 | Down |
| Malaria | F1RII7 | Hemoglobin subunit beta | Down |
| Peroxisome | F1S3Y7 | Xanthine dehydrogenase/oxidase | Down |
| Peroxisome | A0A287APD5 | Long-chain-fatty-acid-CoA ligase 3 | Down |
| Carbon metabolism | F1RIF8 | 6-phosphogluconate dehydrogenase | Down |
| Carbon metabolism | A0A287BBI5 | Glyceraldehyde-3-phosphate dehydrogenase, decarboxylating | Down |
| Carbon metabolism | I3LK59 | Enolase 1 | Down |
| Drug metabolism other enzymes | F1S3Y7 | Xanthine dehydrogenase | Down |
| Drug metabolism other enzymes | P80031 | Glutathione S-transferase P | Up |
| KEGG Pathway | Protein Accession | Protein Description | Regulated Type |
|---|---|---|---|
| Vasopressin-regulated water reabsorption | A0A287BNU5 | RAB5B | Up |
| Vasopressin-regulated water reabsorption | F2Z536 | Dynein light chain | Down |
| Vasopressin-regulated water reabsorption | A0A287BN36 | Ras-related protein Rab-5C | Up |
| IL-17 signaling pathway | F1RRX1 | Neutrophil gelatinase-associated lipocalin precursor | Down |
| IL-17 signaling pathway | C3S7K5 | Protein S100 | Down |
| IL-17 signaling pathway | C3S7K6 | Calcium-binding protein A9 | Down |
| IL-17 signaling pathway | A0A287A9T4 | Heat shock protein | Up |
| IL-17 signaling pathway | Q29092 | Endoplasmin | Up |
| African trypanosomiasis | P01965 | Hemoglobin subunit alpha | Down |
| African trypanosomiasis | K7GM40 | Apolipoprotein A-I | Down |
| African trypanosomiasis | F1RII7 | Hemoglobin subunit beta | Down |
| Pyrimidine metabolism | Q2EN76 | Nucleoside diphosphate kinase B | Up |
| Pyrimidine metabolism | I3LH72 | Ectonucleoside triphosphate Diphosphohydrolase 6 | Down |
| Cholesterol metabolism | P27917 | Apolipoprotein C-III | Down |
| Cholesterol metabolism | A0A287AZ36 | Low-density lipoprotein receptor-related protein 2 | Up |
| Cholesterol metabolism | P49923 | Lipoprotein lipase | Up |
| Cholesterol metabolism | K7GM40 | Apolipoprotein A-I | Down |
| Cholesterol metabolism | Q2TNK5 | Angiopoietin-related protein 4 | Up |
| Thyroid hormone synthesis | P50390 | Transthyretin | Down |
| Thyroid hormone synthesis | F1RXM6 | Thyroxine-binding globulin | Down |
| Thyroid hormone synthesis | A0A287AZ36 | Low-density lipoprotein receptor-related protein 2 | Up |
| Thyroid hormone synthesis | Q29092 | Endoplasmin | Up |
| PPAR signaling pathway | P27917 | Apolipoprotein C-III | Down |
| PPAR signaling pathway | P49923 | Lipoprotein lipase | Up |
| PPAR signaling pathway | K7GM40 | Apolipoprotein A-I | Down |
| PPAR signaling pathway | Q2TNK5 | Angiopoietin-related protein 4 | Up |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, G.; Wang, L.; Zhao, X.; Yu, L.; Huang, D. Proteomics Analysis of Colostrum Samples from Sows Housed under Different Conditions. Animals 2020, 10, 355. https://doi.org/10.3390/ani10020355
Yin G, Wang L, Zhao X, Yu L, Huang D. Proteomics Analysis of Colostrum Samples from Sows Housed under Different Conditions. Animals. 2020; 10(2):355. https://doi.org/10.3390/ani10020355
Chicago/Turabian StyleYin, Guoan, Lei Wang, Xiaoyu Zhao, Langchao Yu, and Dapeng Huang. 2020. "Proteomics Analysis of Colostrum Samples from Sows Housed under Different Conditions" Animals 10, no. 2: 355. https://doi.org/10.3390/ani10020355
APA StyleYin, G., Wang, L., Zhao, X., Yu, L., & Huang, D. (2020). Proteomics Analysis of Colostrum Samples from Sows Housed under Different Conditions. Animals, 10(2), 355. https://doi.org/10.3390/ani10020355





