Metformin Improves Quality of Post-Thaw Canine Semen
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Semen Collection
2.2. Cryopreservation and Thawing Method
2.3. Computer-Assisted Semen Analysis
2.4. Criteria of Semen Quality Analysed by Flow Cytometry
2.5. Sperm Membrane Integrity
2.6. Mitochondrial Activity
2.7. Oxidative Stress Analysis
2.8. Energetic Metabolites Analysis
2.9. Statistical Analysis
3. Results
3.1. Viability and Motility
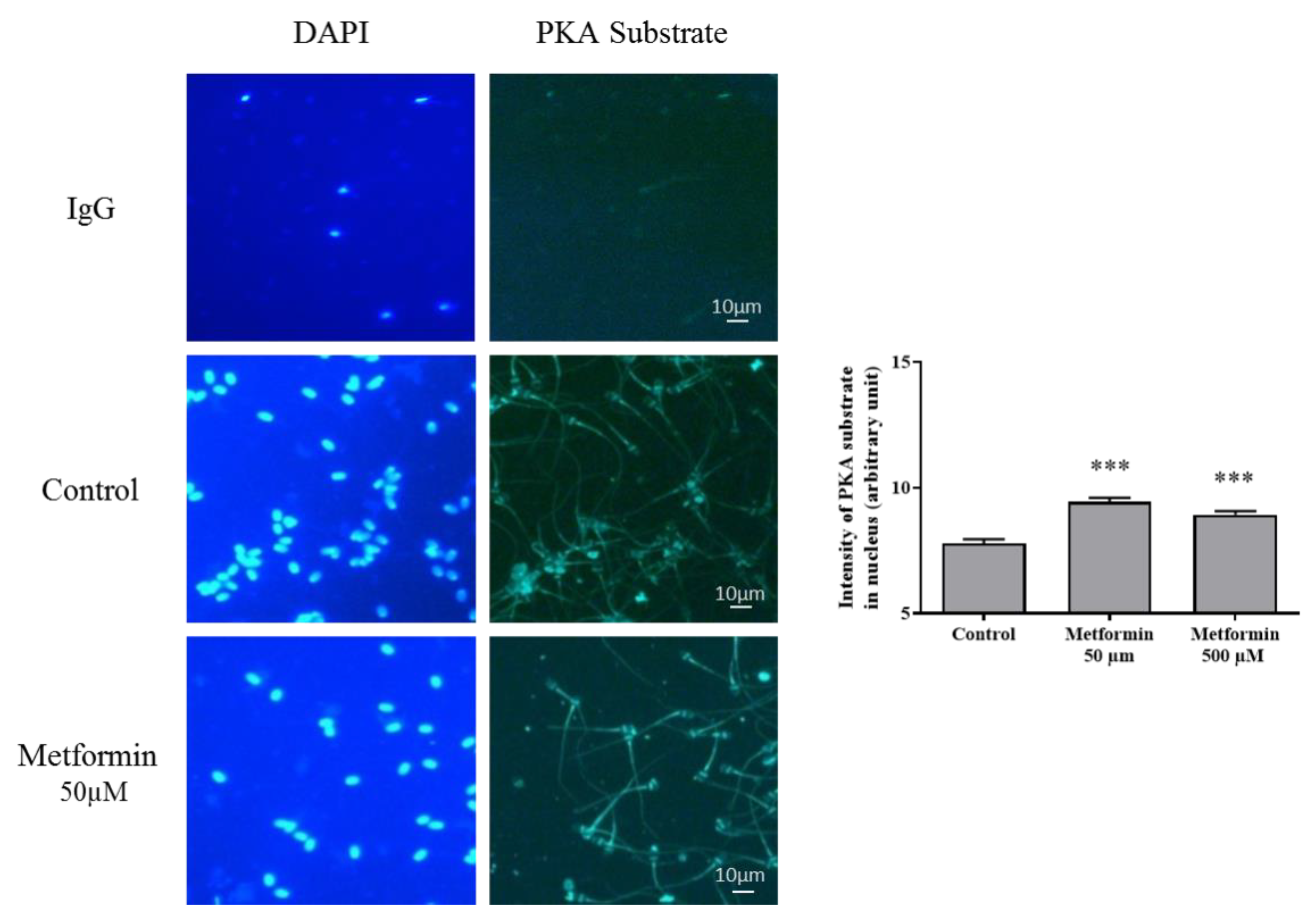
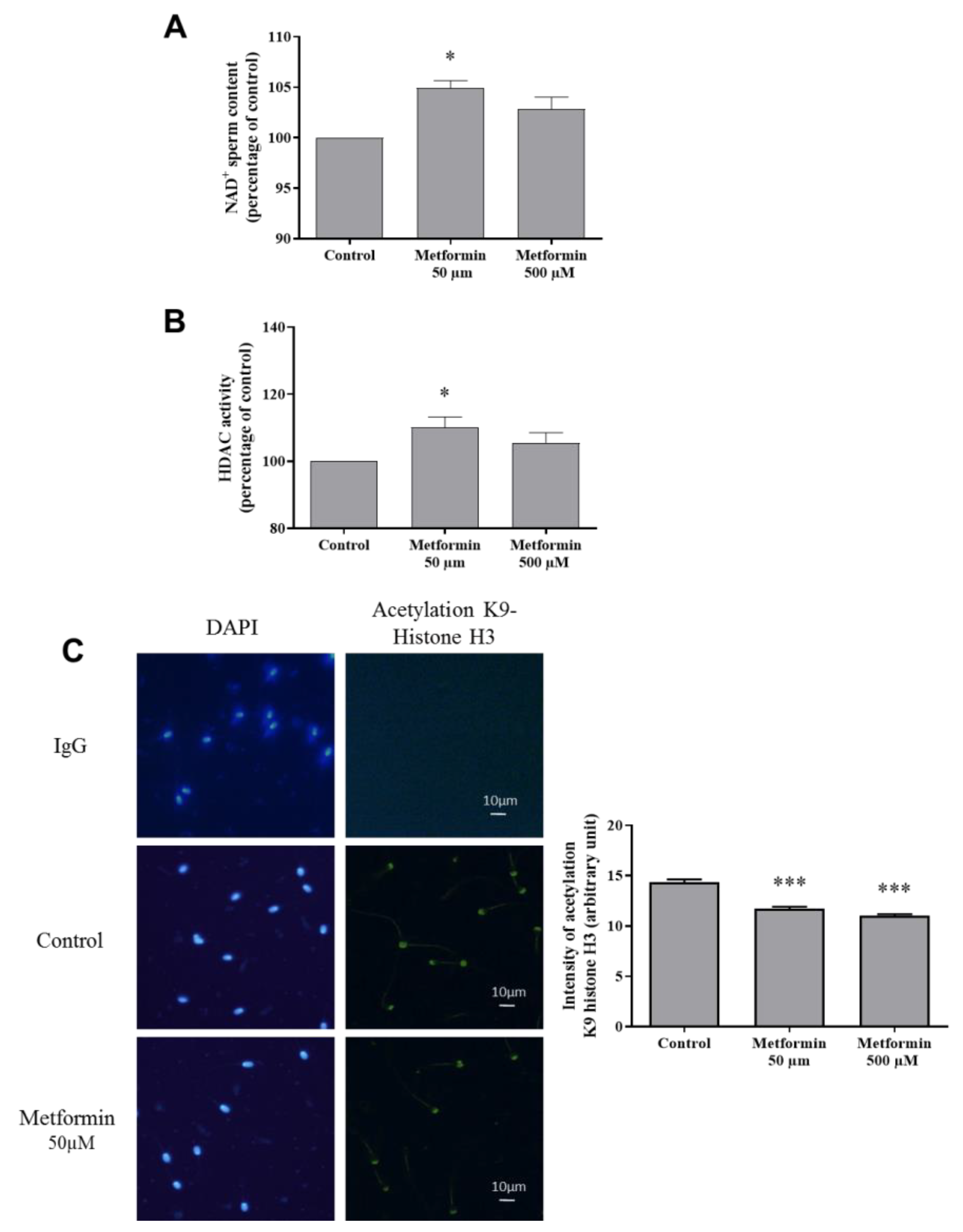
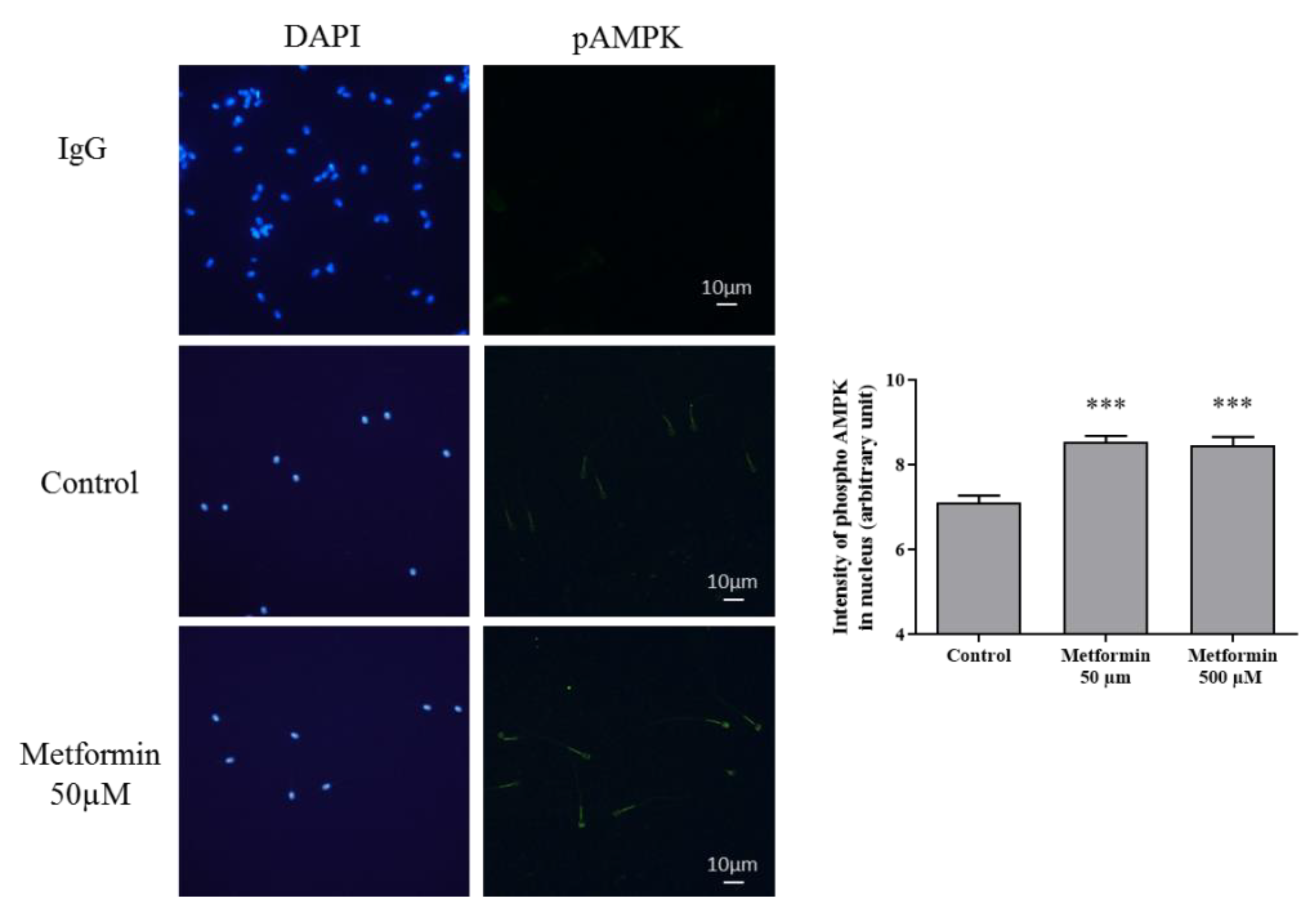
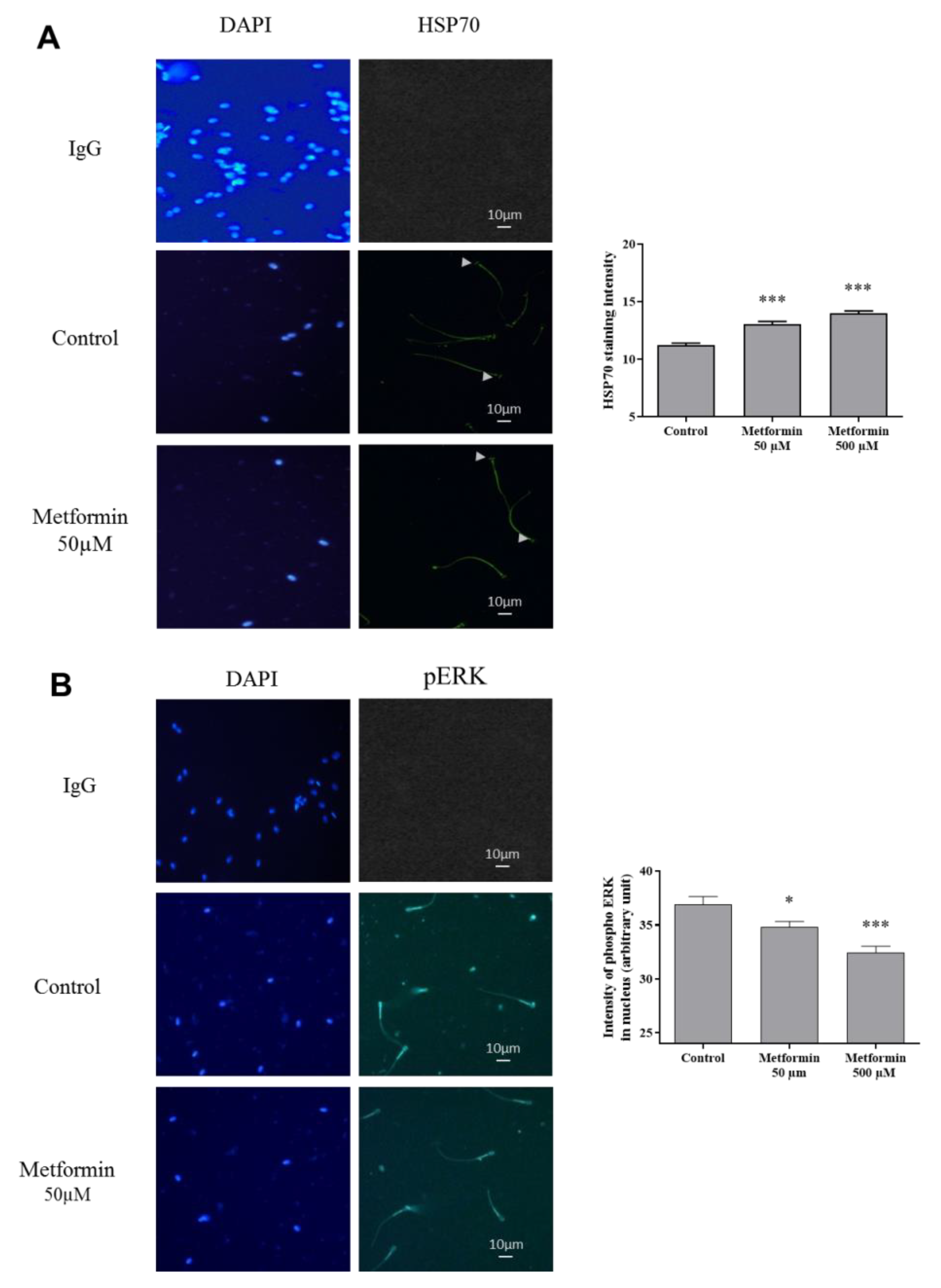
3.2. Molecular Markers Associated to Semen Quality
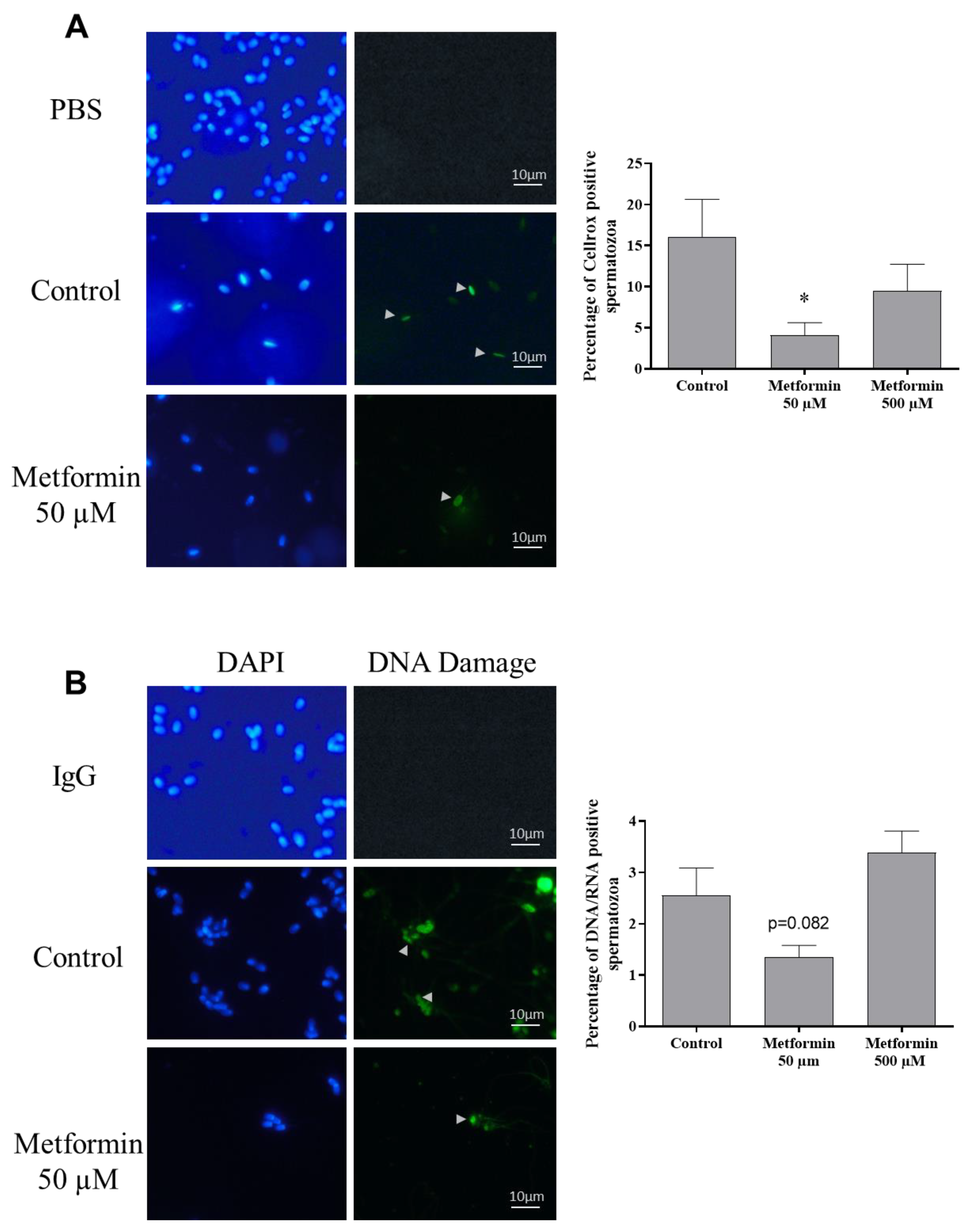
3.3. Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hori, T.; Yoshikuni, R.; Kobayashi, M.; Kawakami, E. Effects of Storage Temperature and Semen Extender on Stored Canine Semen. J. Vet. Med. Sci. 2014, 76, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.R.F.; Paccamonti, D.L.; Eilts, B.E. Fertility in bitches artificially inseminated with extended, chilled semen. Theriogenology 1999, 52, 609–616. [Google Scholar] [CrossRef]
- Ponglowhapan, S.; Essén-Gustavsson, B.; Linde Forsberg, C. Influence of glucose and fructose in the extender during long-term storage of chilled canine semen. Theriogenology 2004, 62, 1498–1517. [Google Scholar] [CrossRef] [PubMed]
- Bucci, D.; Rodriguez-Gil, J.E.; Vallorani, C.; Spinaci, M.; Galeati, G.; Tamanini, C. GLUTs and mammalian sperm metabolism. J. Androl. 2011, 32, 348–355. [Google Scholar] [CrossRef]
- Drobnis, E.Z.; Crowe, L.M.; Berger, T.; Anchordoguy, T.J.; Overstreet, J.W.; Crowe, J.H. Cold shock damage is due to lipid phase transitions in cell membranes: A demonstration using sperm as a model. J. Exp. Zool. 1993, 265, 432–437. [Google Scholar] [CrossRef]
- Bencharif, D.; Amirat, L.; Anton, M.; Schmitt, E.; Desherces, S.; Delhomme, G.; Langlois, M.-L.; Barrière, P.; Larrat, M.; Tainturier, D. The advantages of LDL (low density lipoproteins) in the cryopreservation of canine semen. Theriogenology 2008, 70, 1478–1488. [Google Scholar] [CrossRef]
- Hollinshead, F.K.; Hanlon, D.W. Factors affecting the reproductive performance of bitches: A prospective cohort study involving 1203 inseminations with fresh and frozen semen. Theriogenology 2017, 101, 62–72. [Google Scholar] [CrossRef]
- Linde-Forsberg, C. Achieving canine pregnancy by using frozen or chilled extended semen. Vet. Clin. N. Am. Small Anim. Pract. 1991, 21, 467–485. [Google Scholar] [CrossRef]
- Niżański, W.; Klimowicz, M.; Partyka, A.; Savić, M.; Dubiel, A. Effects of the Inclusion of Equex STM into Tris-Based Extender on the Motility of Dog Spermatozoa Incubated at 5 °C. Reprod. Domest. Anim. 2009, 44, 363–365. [Google Scholar] [CrossRef]
- Okano, T.; Murase, T.; Asano, M.; Tsubota, T. Effects of final dilution rate, sperm concentration and times for cooling and glycerol equilibration on post-thaw characteristics of canine spermatozoa. J. Vet. Med. Sci. 2004, 66, 1359–1364. [Google Scholar] [CrossRef]
- Santos, S.E.C.; Vannucchi, C.I.; Satzinger, S.I.; Assumpcao, M.E.; Visintin, J.A. Comparison of five extenders for canine semen freezing. Braz. J. Vet. Res. Anim. Sci. 1999, 36. [Google Scholar] [CrossRef]
- Axner, E.; Lagerson, E. Cryopreservation of Dog Semen in a Tris Extender with 1% or 2% Soya Bean Lecithin as a Replacement of Egg Yolk. Reprod. Domest. Anim. 2016, 51, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.J.; Núñez-Martínez, I.; Morán, J.M. Semen technologies in dog breeding: An update. Reprod. Domest. Anim. 2006, 41 (Suppl. 2), 21–29. [Google Scholar] [CrossRef]
- Iguer-ouada, M.; Verstegen, J.P. Long-term preservation of chilled canine semen: Effect of commercial and laboratory prepared extenders. Theriogenology 2001, 55, 671–684. [Google Scholar] [CrossRef]
- Aitken, R.J.; Buckingham, D.W.; Carreras, A.; Irvine, D.S. Superoxide dismutase in human sperm suspensions: Relationship with cellular composition, oxidative stress, and sperm function. Free Radic. Biol. Med. 1996, 21, 495–504. [Google Scholar] [CrossRef]
- Kim, S.-H.; Yu, D.-H.; Kim, Y.-J. Effects of cryopreservation on phosphatidylserine translocation, intracellular hydrogen peroxide, and DNA integrity in canine sperm. Theriogenology 2010, 73, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Yu, D.-H.; Kim, Y.-J. Apoptosis-like change, ROS, and DNA status in cryopreserved canine sperm recovered by glass wool filtration and Percoll gradient centrifugation techniques. Anim. Reprod. Sci. 2010, 119, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci. 2000, 60–61, 481–492. [Google Scholar] [CrossRef]
- Lucio, C.F.; Regazzi, F.M.; Silva, L.C.G.; Angrimani, D.S.R.; Nichi, M.; Vannucchi, C.I. Oxidative stress at different stages of two-step semen cryopreservation procedures in dogs. Theriogenology 2016, 85, 1568–1575. [Google Scholar] [CrossRef]
- Sabeti, P.; Pourmasumi, S.; Rahiminia, T.; Akyash, F.; Talebi, A.R. Etiologies of sperm oxidative stress. Int. J. Reprod. Biomed. 2016, 14, 231–240. [Google Scholar] [CrossRef]
- Taylor, K.; Roberts, P.; Sanders, K.; Burton, P. Effect of antioxidant supplementation of cryopreservation medium on post-thaw integrity of human spermatozoa. Reprod. Biomed. Online 2009, 18, 184–189. [Google Scholar] [CrossRef]
- Kalthur, G.; Raj, S.; Thiyagarajan, A.; Kumar, S.; Kumar, P.; Adiga, S.K. Vitamin E supplementation in semen-freezing medium improves the motility and protects sperm from freeze-thaw-induced DNA damage. Fertil. Steril. 2011, 95, 1149–1151. [Google Scholar] [CrossRef] [PubMed]
- Khodayari Naeini, Z.; Hassani Bafrani, H.; Nikzad, H. Evaluation of ebselen supplementation on cryopreservation medium in human semen. Iran. J. Reprod. Med. 2014, 12, 249–256. [Google Scholar] [PubMed]
- Bertoldo, M.J.; Guibert, E.; Tartarin, P.; Guillory, V.; Froment, P. Effect of metformin on the fertilizing ability of mouse spermatozoa. Cryobiology 2014, 68, 262–268. [Google Scholar] [CrossRef]
- Nguyen, T.M.D.; Alves, S.; Grasseau, I.; Métayer-Coustard, S.; Praud, C.; Froment, P.; Blesbois, E. Central role of 5′-AMP-activated protein kinase in chicken sperm functions. Biol. Reprod. 2014, 91, 121. [Google Scholar] [CrossRef]
- Córdova, A.; Strobel, P.; Vallejo, A.; Valenzuela, P.; Ulloa, O.; Burgos, R.A.; Menarim, B.; Rodríguez-Gil, J.E.; Ratto, M.; Ramírez-Reveco, A. Use of hypometabolic TRIS extenders and high cooling rate refrigeration for cryopreservation of stallion sperm: Presence and sensitivity of 5′ AMP-activated protein kinase (AMPK). Cryobiology 2014, 69, 473–481. [Google Scholar] [CrossRef]
- Hurtado de Llera, A.; Martin-Hidalgo, D.; Gil, M.C.; Garcia-Marin, L.J.; Bragado, M.J. AMPK up-activation reduces motility and regulates other functions of boar spermatozoa. Mol. Hum. Reprod. 2015, 21, 31–45. [Google Scholar] [CrossRef]
- Tsuda, T.; Watanabe, M.; Ohshima, K.; Norinobu, S.; Choi, S.W.; Kawakishi, S.; Osawa, T. Antioxidative Activity of the Anthocyanin Pigments Cyanidin 3-O-Beta-d-Glucoside and Cyanidin. J. Agric. Food Chem. 1994, 42, 2407–2410. [Google Scholar] [CrossRef]
- Tsuda, T.; Shiga, K.; Ohshima, K.; Kawakishi, S.; Osawa, T. Inhibition of lipid peroxidation and the active oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris L. Biochem. Pharmacol. 1996, 52, 1033–1039. [Google Scholar] [CrossRef]
- Esteghamati, A.; Eskandari, D.; Mirmiranpour, H.; Noshad, S.; Mousavizadeh, M.; Hedayati, M.; Nakhjavani, M. Effects of metformin on markers of oxidative stress and antioxidant reserve in patients with newly diagnosed type 2 diabetes: A randomized clinical trial. Clin. Nutr. 2013, 32, 179–185. [Google Scholar] [CrossRef]
- Onken, B.; Driscoll, M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS ONE 2010, 5, e8758. [Google Scholar] [CrossRef] [PubMed]
- Ashabi, G.; Khalaj, L.; Khodagholi, F.; Goudarzvand, M.; Sarkaki, A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab. Brain Dis. 2015, 30, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, Y.; Zhang, W.; Peng, X.; Zhou, J.; Li, F.; Han, B.; Liu, X.; Ou, Y.; Yu, X. Delphinidin induced protective autophagy via mTOR pathway suppression and AMPK pathway activation in HER-2 positive breast cancer cells. BMC Cancer 2018, 18, 342. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Horman, S.; Hussain, N.; Dilworth, S.M.; Storey, K.B.; Rider, M.H. Evaluation of the role of AMP-activated protein kinase and its downstream targets in mammalian hibernation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 142, 374–382. [Google Scholar] [CrossRef]
- Nizanski, W.; Dubiel, A.; Bielas, W.; Dejneka, G.J. Effects of three cryopreservation methods and two semen extenders on the quality of dog semen after thawing. J. Reprod. Fertil. Suppl. 2001, 57, 365–369. [Google Scholar]
- Nizanski, W. Intravaginal insemination of bitches with fresh and frozen-thawed semen with addition of prostatic fluid: Use of an infusion pipette and the Osiris catheter. Theriogenology 2006, 66, 470–483. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef]
- Bertoldo, M.J.; Guibert, E.; Faure, M.; Guillou, F.; Ramé, C.; Nadal-Desbarats, L.; Foretz, M.; Viollet, B.; Dupont, J.; Froment, P. Specific deletion of AMP-activated protein kinase (α1AMPK) in mouse Sertoli cells modifies germ cell quality. Mol. Cell. Endocrinol. 2016, 423, 96–112. [Google Scholar] [CrossRef]
- Faure, M.; Bertoldo, M.J.; Khoueiry, R.; Bongrani, A.; Brion, F.; Giulivi, C.; Dupont, J.; Froment, P. Metformin in Reproductive Biology. Front. Endocrinol. 2018, 9, 675. [Google Scholar] [CrossRef]
- Partyka, A.; Niżański, W.; Łukaszewicz, E. Evaluation of fresh and frozen-thawed fowl semen by flow cytometry. Theriogenology 2010, 74, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Partyka, A.; Nizanski, W.; Bajzert, J.; Lukaszewicz, E.; Ochota, M. The effect of cysteine and superoxide dismutase on the quality of post-thawed chicken sperm. Cryobiology 2013, 67, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Partyka, A.; Łukaszewicz, E.; Niżański, W.; Twardoń, J. Detection of lipid peroxidation in frozen-thawed avian spermatozoa using C11-BODIPY581/591. Theriogenology 2011, 75, 1623–1629. [Google Scholar] [CrossRef]
- Ouslimani, N.; Peynet, J.; Bonnefont-Rousselot, D.; Thérond, P.; Legrand, A.; Beaudeux, J.-L. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metab. Clin. Exp. 2005, 54, 829–834. [Google Scholar] [CrossRef]
- Otsuka, M.; Matsumoto, T.; Morimoto, R.; Arioka, S.; Omote, H.; Moriyama, Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc. Natl. Acad. Sci. USA 2005, 102, 17923–17928. [Google Scholar] [CrossRef] [PubMed]
- Hurtado de Llera, A.; Martin-Hidago, D.; Gil, M.; Garcia-Marin, L.; Bragado, M. The AMPK activator metformin inhibits one of the main functions of boar spermatozoa, motility. FEBS J. 2012, 279, 52–576. [Google Scholar]
- Nguyen, T.M.D.; Seigneurin, F.; Froment, P.; Combarnous, Y.; Blesbois, E. The 5′-AMP-Activated Protein Kinase (AMPK) Is Involved in the Augmentation of Antioxidant Defenses in Cryopreserved Chicken Sperm. PLoS ONE 2015, 10, e0134420. [Google Scholar] [CrossRef]
- Gallardo Bolaños, J.M.; Balao da Silva, C.M.; Martín Muñoz, P.; Morillo Rodríguez, A.; Plaza Dávila, M.; Rodríguez-Martínez, H.; Aparicio, I.M.; Tapia, J.A.; Ortega Ferrusola, C.; Peña, F.J. Phosphorylated AKT preserves stallion sperm viability and motility by inhibiting caspases 3 and 7. Reproduction 2014, 148, 221–235. [Google Scholar] [CrossRef]
- McGann, L.E.; Yang, H.Y.; Walterson, M. Manifestations of cell damage after freezing and thawing. Cryobiology 1988, 25, 178–185. [Google Scholar] [CrossRef]
- Aitken, R.J.; Koopman, P.; Lewis, S.E. Seeds of concern. Nature 2004, 432, 48–52. [Google Scholar] [CrossRef]
- Olaciregui, M.; Luño, V.; Gonzalez, N.; De Blas, I.; Gil, L. Freeze-dried dog sperm: Dynamics of DNA integrity. Cryobiology 2015, 71, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Ballot, C.; Jouy, N.; Thomas, P.; Marchetti, C. Influence of mitochondrial membrane potential of spermatozoa on in vitro fertilisation outcome. Andrologia 2012, 44, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Menzies, K.J.; Auwerx, J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S. Emerging therapeutic roles for NAD+ metabolism in mitochondrial and age-related disorders. Clin. Trans. Med. 2016, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Szrejder, M.; Rachubik, P.; Rogacka, D.; Audzeyenka, I.; Rychłowski, M.; Kreft, E.; Angielski, S.; Piwkowska, A. Metformin reduces TRPC6 expression through AMPK activation and modulates cytoskeleton dynamics in podocytes under diabetic conditions. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165610. [Google Scholar] [CrossRef]
- Almog, T.; Lazar, S.; Reiss, N.; Etkovitz, N.; Milch, E.; Rahamim, N.; Dobkin-Bekman, M.; Rotem, R.; Kalina, M.; Ramon, J.; et al. Identification of Extracellular Signal-regulated Kinase 1/2 and p38 MAPK as Regulators of Human Sperm Motility and Acrosome Reaction and as Predictors of Poor Spermatozoan Quality. J. Biol. Chem. 2008, 283, 14479–14489. [Google Scholar] [CrossRef]
- Bai, C.; Kang, N.; Zhao, J.; Dai, J.; Gao, H.; Chen, Y.; Dong, H.; Huang, C.; Dong, Q. Cryopreservation disrupts lipid rafts and heat shock proteins in yellow catfish sperm. Cryobiology 2019, 87, 32–39. [Google Scholar] [CrossRef]
- Cocchia, N.; Pasolini, M.P.; Mancini, R.; Petrazzuolo, O.; Cristofaro, I.; Rosapane, I.; Sica, A.; Tortora, G.; Lorizio, R.; Paraggio, G.; et al. Effect of sod (superoxide dismutase) protein supplementation in semen extenders on motility, viability, acrosome status and ERK (extracellular signal-regulated kinase) protein phosphorylation of chilled stallion spermatozoa. Theriogenology 2011, 75, 1201–1210. [Google Scholar] [CrossRef]
- Huang, S.Y.; Chen, M.Y.; Lin, E.C.; Tsou, H.L.; Kuo, Y.H.; Ju, C.C.; Lee, W.C. Effects of single nucleotide polymorphisms in the 5′-flanking region of heat shock protein 70.2 gene on semen quality in boars. Anim. Reprod. Sci. 2002, 70, 99–109. [Google Scholar] [CrossRef]
- Huang, S.Y.; Kuo, Y.H.; Lee, Y.P.; Tsou, H.L.; Lin, E.C.; Ju, C.C.; Lee, W.C. Association of heat shock protein 70 with semen quality in boars. Anim. Reprod. Sci. 2000, 63, 231–240. [Google Scholar] [CrossRef]
- Matwee, C.; Kamaruddin, M.; Betts, D.H.; Basrur, P.K.; King, W.A. The effects of antibodies to heat shock protein 70 in fertilization and embryo development. Mol. Hum. Reprod. 2001, 7, 829–837. [Google Scholar] [CrossRef]
- Nikbin, S.; Panandam, J.M.; Yaakub, H.; Murugaiyah, M.; Sazili, A.Q. Novel SNPs in heat shock protein 70 gene and their association with sperm quality traits of Boer goats and Boer crosses. Anim. Reprod. Sci. 2014, 146, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Spinaci, M.; Volpe, S.; Bernardini, C.; Ambrogi, M.D.; Tamanini, C.; Seren, E.; Galeati, G. Immunolocalization of heat shock protein 70 (Hsp 70) in boar spermatozoa and its role during fertilization. Mol. Reprod. Dev. 2005, 72, 534–541. [Google Scholar] [CrossRef]
- Liu, S.; Xu, J.; Fang, C.; Shi, C.; Zhang, X.; Yu, B.; Yin, Y. Over-expression of heat shock protein 70 protects mice against lung ischemia/reperfusion injury through SIRT1/AMPK/eNOS pathway. Am. J. Transl. Res. 2016, 8, 4394–4404. [Google Scholar] [PubMed]
- Reddy, V.S.; Yadav, B.; Yadav, C.L.; Anand, M.; Swain, D.K.; Kumar, D.; Kritania, D.; Madan, A.K.; Kumar, J.; Yadav, S. Effect of sericin supplementation on heat shock protein 70 (HSP70) expression, redox status and post thaw semen quality in goat. Cryobiology 2018, 84, 33–39. [Google Scholar] [CrossRef]
- Dix, D.J.; Allen, J.W.; Collins, B.W.; Mori, C.; Nakamura, N.; Poorman-Allen, P.; Goulding, E.H.; Eddy, E.M. Targeted gene disruption of Hsp70-2 results in failed meiosis, germ cell apoptosis, and male infertility. Proc. Natl. Acad. Sci. USA 1996, 93, 3264–3268. [Google Scholar] [CrossRef]
- Elliott, R.M.A.; Lloyd, R.E.; Fazeli, A.; Sostaric, E.; Georgiou, A.S.; Satake, N.; Watson, P.F.; Holt, W.V. Effects of HSPA8, an evolutionarily conserved oviductal protein, on boar and bull spermatozoa. Reproduction 2009, 137, 191–203. [Google Scholar] [CrossRef]
- Xia, Z.; Huang, L.; Yin, P.; Liu, F.; Liu, Y.; Zhang, Z.; Lin, J.; Zou, W.; Li, C. L-Arginine alleviates heat stress-induced intestinal epithelial barrier damage by promoting expression of tight junction proteins via the AMPK pathway. Mol. Biol. Rep. 2019, 46, 6435–6451. [Google Scholar] [CrossRef]
- Ashizawa, K.; Hashimoto, K.; Higashio, M.; Tsuzuki, Y. The addition of mitogen-activated protein kinase and p34cdc2 kinase substrate peptides inhibits the flagellar motility of demembranated fowl spermatozoa. Biochem. Biophys. Res. Commun. 1997, 240, 116–121. [Google Scholar] [CrossRef]
- Weidinger, S.; Mayerhofer, A.; Kunz, L.; Albrecht, M.; Sbornik, M.; Wunn, E.; Hollweck, R.; Ring, J.; Kohn, F.M. Tryptase inhibits motility of human spermatozoa mainly by activation of the mitogen-activated protein kinase pathway. Hum. Reprod. 2005, 20, 456–461. [Google Scholar] [CrossRef] [PubMed]
| Group | Fresh Semen | Control | Metformin 50 µM | Metformin 500 µM |
|---|---|---|---|---|
| Viable | 80.83 ± 1.47 | 49.78 ± 5.4 | 48.63 ± 4.8 | 46.67 ± 8.0 |
| Live intact acrosomes | 79.69 ± 2.8 | 50.19 ± 3.7 | 50.45 ± 3.6 | 50.71 ± 4.3 |
| Group | Fresh Semen | Control | Metformin 50 µM | Metformin 500 µM |
|---|---|---|---|---|
| MOT (%) | 94.00 ± 2.1 | 40.63 ± 4.5 a | 57.03 ± 5.3 b | 56.44 ± 7.0 a,b |
| PMOT (%) | 69.00 ± 1.5 | 19.08 ± 3.2 a | 30.42 ± 2.9 b | 29.82 ± 3.7 b |
| RAPID (%) | 75.33 ± 1.8 | 21.40 ± 3.9 a | 34.39 ± 3.3 b | 33.89 ± 4.1 a,b |
| VAP (µm/s) | 151.83 ± 5.1 | 117.45 ± 7.4 | 130.24 ± 4.0 | 128.83 ± 2.6 |
| VSL (µm/s) | 139.47 ± 5.4 | 104.57 ± 6.3 | 114.43 ± 4.4 | 112.90 ± 3.4 |
| VCL (µm/s) | 191.90 ± 4.3 | 168.31 ± 13.5 | 187.55 ± 6.6 | 188.52 ± 6.0 |
| STR (%) | 90.67 ± 0.3 | 88.20 ± 1.2 a | 86.44 ± 0.9 a,b | 86.33 ± 1.1 b |
| LIN (%) | 73.33 ± 0.9 | 64.64 ± 2.7 | 62.36 ± 2.7 | 61.38 ± 2.7 |
| Group | Fresh Semen | Control | Metformin 50 µM | Metformin 500 µM |
|---|---|---|---|---|
| High mitochondrial membrane potential (HMMP) | 80.27 ± 6.2 | 48.87 ± 6.4 a | 61.92 ± 8.6 b | 60.29 ± 10.4 a,b |
| Group | Fresh Semen | Control | Metformin 50 µM | Metformin 500 µM |
|---|---|---|---|---|
| Live with LPO | 5.3 ± 1.4 | 1.84 ± 0.3 | 1.32 ± 0.2 | 1.61 ± 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grandhaye, J.; Partyka, A.; Ligocka, Z.; Dudek, A.; Niżański, W.; Jeanpierre, E.; Estienne, A.; Froment, P. Metformin Improves Quality of Post-Thaw Canine Semen. Animals 2020, 10, 287. https://doi.org/10.3390/ani10020287
Grandhaye J, Partyka A, Ligocka Z, Dudek A, Niżański W, Jeanpierre E, Estienne A, Froment P. Metformin Improves Quality of Post-Thaw Canine Semen. Animals. 2020; 10(2):287. https://doi.org/10.3390/ani10020287
Chicago/Turabian StyleGrandhaye, Jérémy, Agnieszka Partyka, Zuzanna Ligocka, Agata Dudek, Wojciech Niżański, Eric Jeanpierre, Anthony Estienne, and Pascal Froment. 2020. "Metformin Improves Quality of Post-Thaw Canine Semen" Animals 10, no. 2: 287. https://doi.org/10.3390/ani10020287
APA StyleGrandhaye, J., Partyka, A., Ligocka, Z., Dudek, A., Niżański, W., Jeanpierre, E., Estienne, A., & Froment, P. (2020). Metformin Improves Quality of Post-Thaw Canine Semen. Animals, 10(2), 287. https://doi.org/10.3390/ani10020287





