Analytical and Clinical Validation of a New Immunoenzymatic Method for the Measurement of Canine Parathyroid Hormone
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. PTH Measurement
2.3. Creatinine and Phosphorus Measurement
2.4. Analytical Validation
2.4.1. Precision and Accuracy
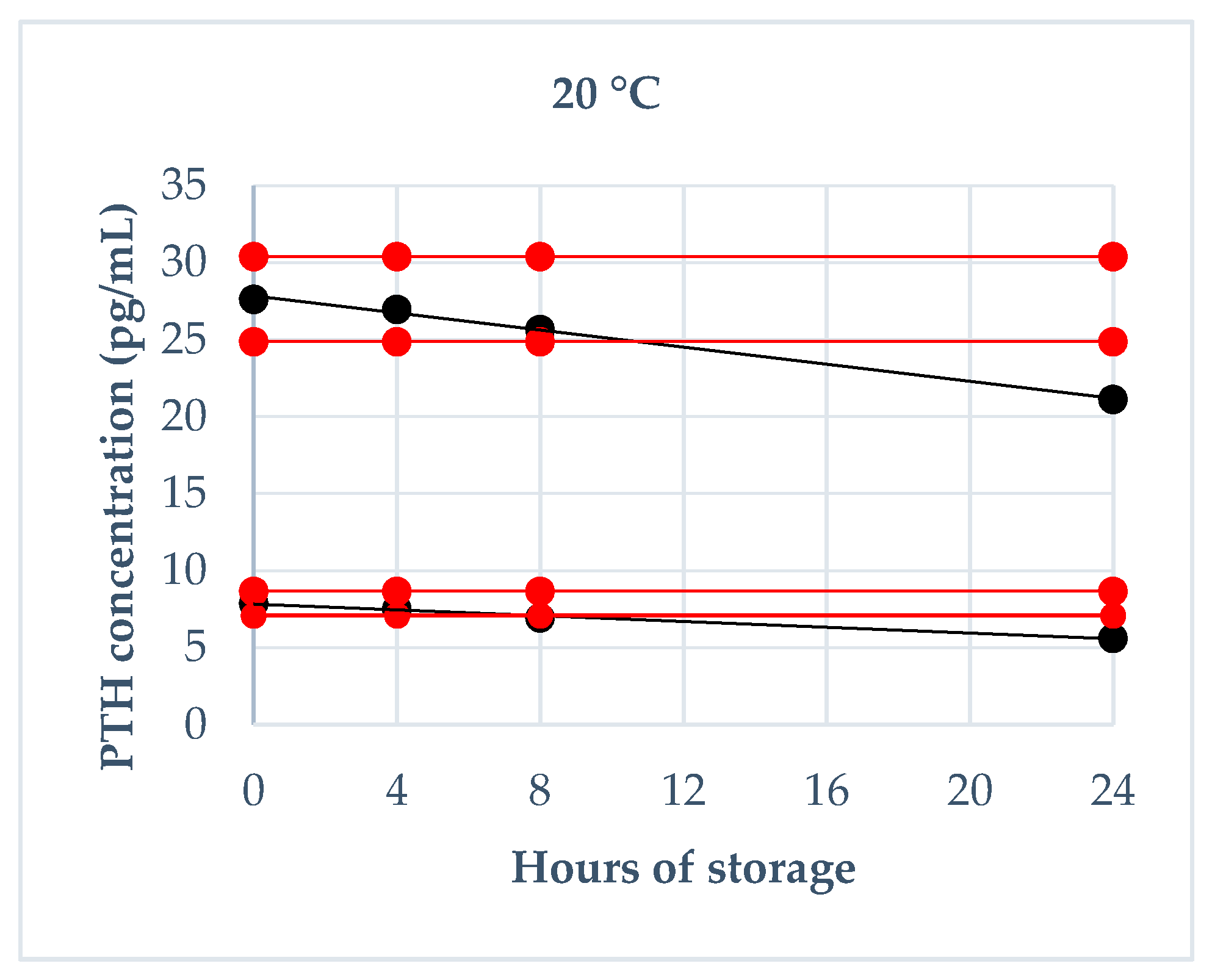
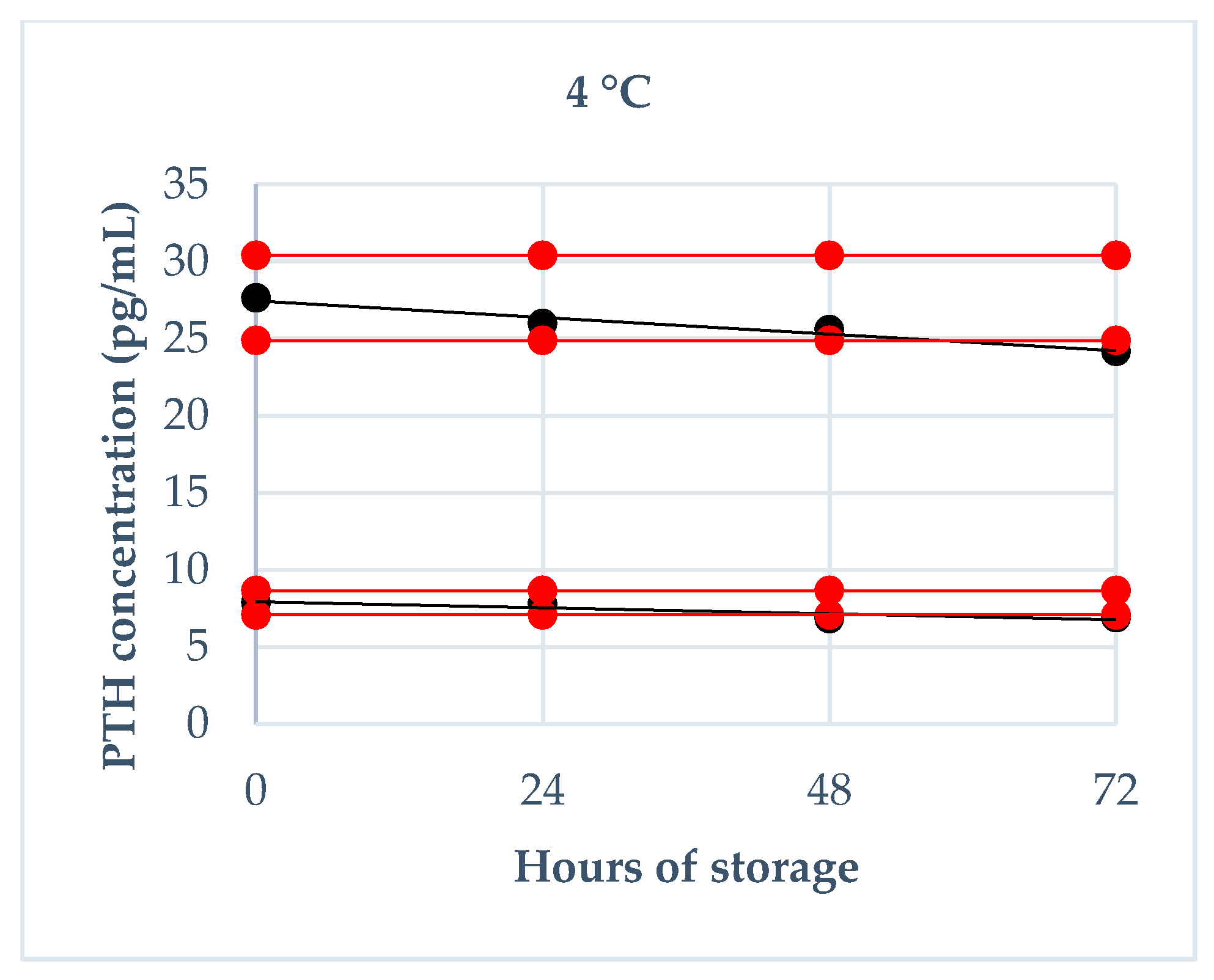
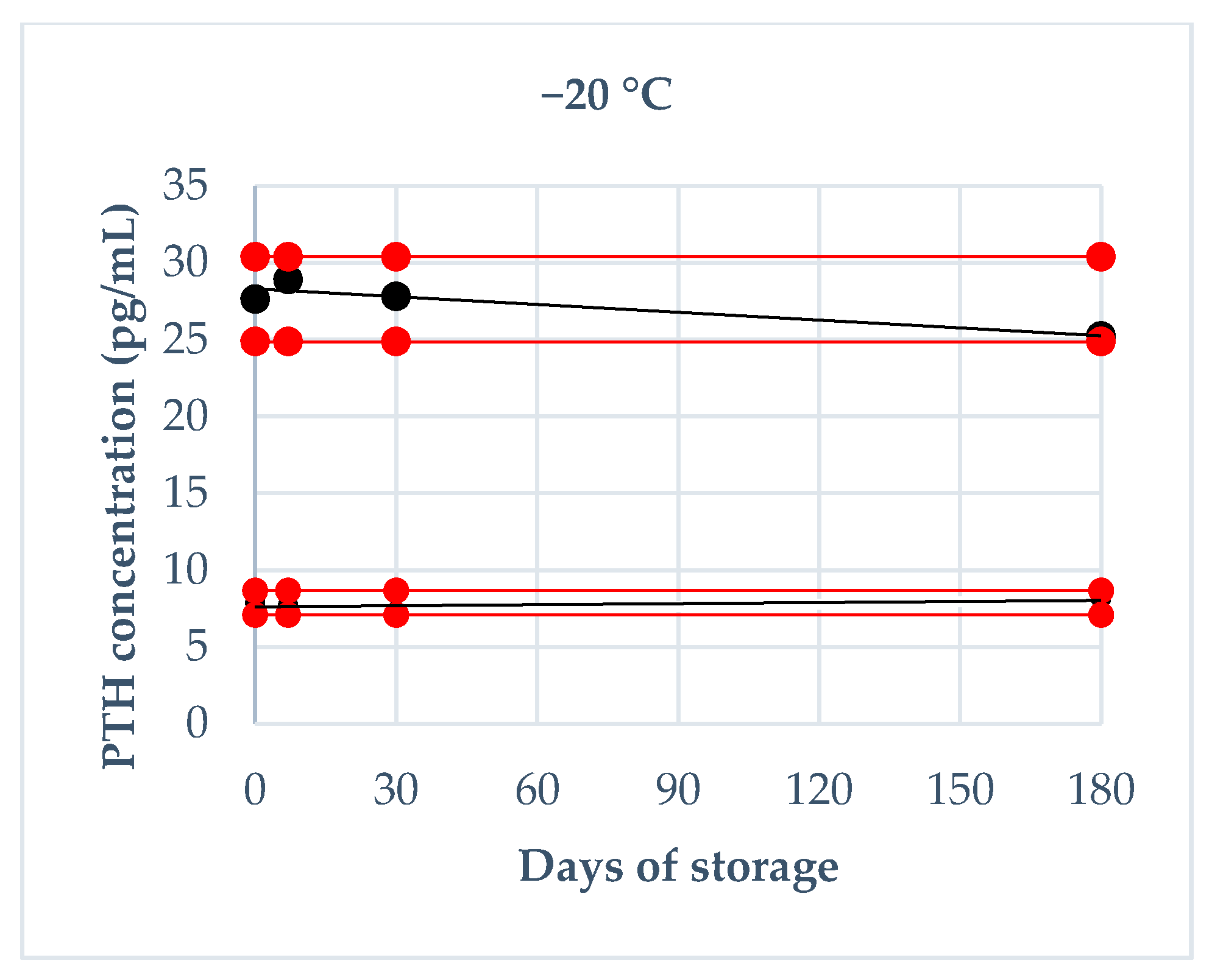
2.4.2. Storage Stability
2.5. Clinical Validation
3. Results
3.1. Animals
3.2. Precision and Accuracy
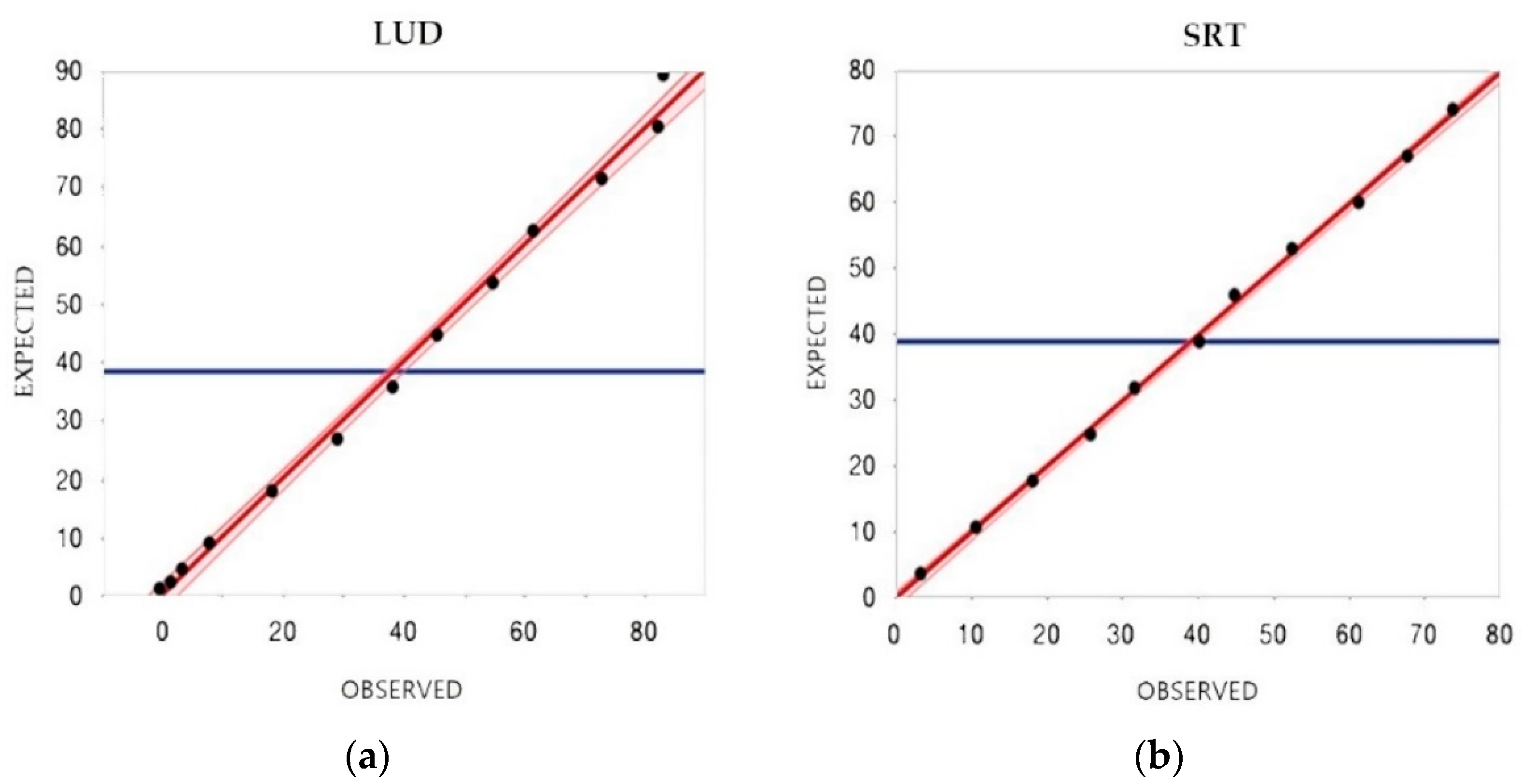
3.3. Storage Stability
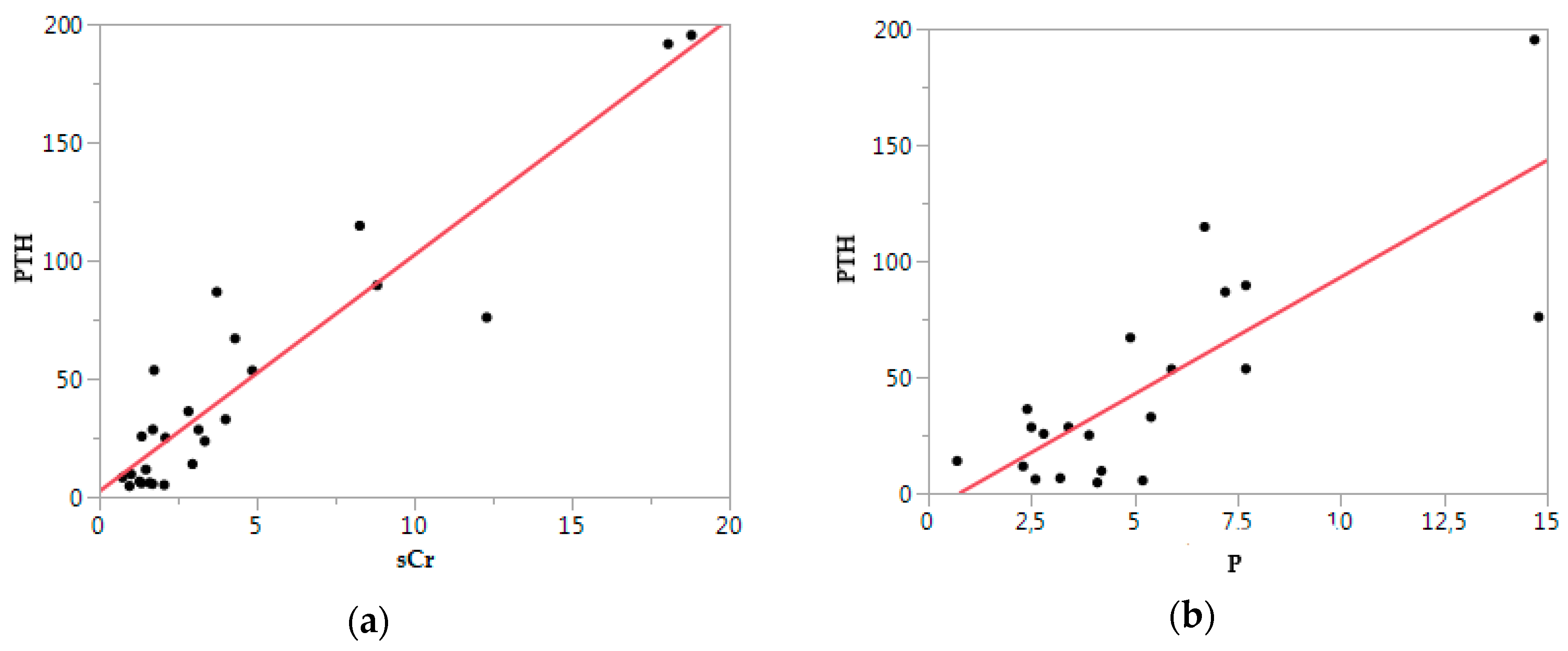
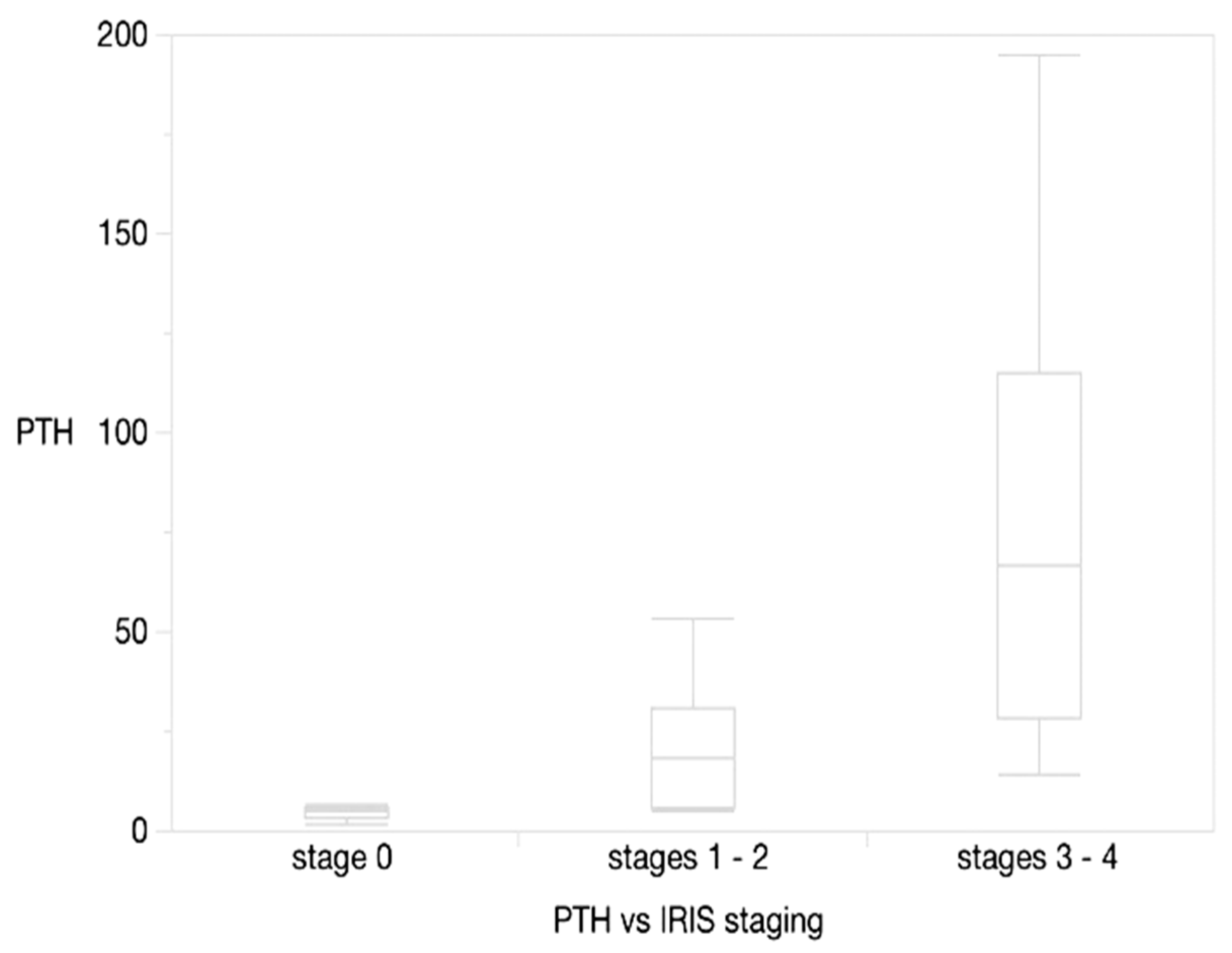
3.4. Clinical Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosol, T.J.; Steinmeyer, C.L.; McCauley, L.K.; Gröne, A.; DeWille, J.W.; Capen, C.C. Sequences of the cDNA encoding canine parathyroid hormone-related protein and parathyroid hormone. Gene 1995, 160, 241–243. [Google Scholar] [CrossRef]
- Foster, D.J. Update on mineral and bone disorders in chronic kidney disease. Vet. Clin. Small Anim. 2016, 46, 1131–1149. [Google Scholar] [CrossRef]
- Cortadellas, O.; Fernández del Palacio, M.J.; Talavera, J.; Bayón, A. Calcium and phosphorus homeostasis in dogs with spontaneous chronic kidney disease at different stages of severity. J. Vet. Intern. Med. 2010, 24, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Parker, V.J.; Harjes, L.M.; Dembek, K.; Young, G.S.; Chew, D.J.; Toribio, R.E. Association of vitamin D metabolites with parathyroid hormone, fibroblast growth factor-23, calcium, and phosphorus in dogs with various stages of chronic kidney disease. J. Vet. Intern. Med. 2017, 31, 791–798. [Google Scholar] [CrossRef] [PubMed]
- de Brito Galvao, J.F.; Nagode, L.A.; Schenck, P.A.; Chew, D.J. Calcitriol, calcidiol, parathyroid hormone, and fibroblast growth factor-23 interactions in chronic kidney disease. J. Vet. Emerg. Crit. Care 2013, 23, 134–162. [Google Scholar] [CrossRef] [PubMed]
- Estepa, J.C.; Lopez, I.; Felsenfeld, A.J.; Gao, P.; Cantor, T.; Rodríguez, M.; Aguilera-Tejero, E. Dynamics of secretion and metabolism of PTH during hypo- and hypercalcemia in the dog as determined by the ‘intact’ and ‘whole’ PTH assays. Nephrol. Dial. Transplant. 2003, 18, 1101–1107. [Google Scholar] [CrossRef][Green Version]
- Harjes, L.M.; Parker, V.J.; Dembek, K.; Young, G.S.; Giovannini, L.H.; Kogika, M.M.; Chew, D.J.; Toribio, R.E. Fibroblast growth factor-23 concentration in dogs with chronic kidney disease. J. Vet. Intern. Med. 2017, 31, 784–790. [Google Scholar] [CrossRef]
- Dittmer, K.E.; Perera, K.C.; Elder, P.A. Serum fibroblast growth factor 23 concentrations in dogs with chronic kidney disease. Res. Vet. Sci. 2017, 114, 348–350. [Google Scholar] [CrossRef]
- Smit, M.A.; van Kinschot, M.J.; van der Linden, J.; van Noord, C.; Kos, S. Clinical guidelines and PTH measurement: Does assay generation matter? Endocr. Rev. 2019, 40, 1468–1480. [Google Scholar] [CrossRef]
- Torrance, A.G.; Nachreiner, R. Human-parathormone assay for use in dogs: Validation, sample handling studies, and parathyroid function testing. Am. J. Vet. Res. 1989, 50, 1123–1127. [Google Scholar]
- Torrance, A.G.; Nachreiner, R. Intact parathyroid hormone assay and total calcium concentration in the diagnosis of disorders of calcium metabolism in dogs. J. Vet. Intern. Med. 1989, 3, 86–89. [Google Scholar] [CrossRef] [PubMed]
- López, I.; Aguilera-Tejero, E.; Estepa, J.C.; Bas, S.; Mayer-Valor, R.; Jiménez, A.; Rodríguez, M. Diurnal variations in the plasma concentration of parathyroid hormone in dogs. Vet. Rec. 2005, 157, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Martiarena, B.; Kogovsek, N.; Salerni, H.; Castillo, V.; Brandi, G.; Regonat, M.; Visintini, A.; Quintana, E.; Otero, P. Reference values of parathyroid hormone and vitamin D hormone by chemiluminescent automated assay. Rev. MVZ Córdoba 2015, 20, 4581–4590. [Google Scholar] [CrossRef]
- Gerber, B.; Hässig, M.; Reusch, C.E. Serum concentrations of 1,25-dihydroxycholecalciferol and 25-hydroxycholecalciferol in clinically normal dogs and dogs with acute and chronic renal failure. Am. J. Vet. Res. 2003, 64, 1161–1166. [Google Scholar] [CrossRef]
- Selting, K.A.; Sharp, C.R.; Ringold, R.; Thamm, D.H.; Backus, R. Serum 25-hydroxyvitamin D concentrations in dogs – correlation with health and cancer risk. Vet. Comp. Oncol. 2016, 14, 295–305. [Google Scholar] [CrossRef]
- Mooney, C.T.; Shiel, R.E.; Fawcett, K.; Matthews, E.; Gunn, E. A comparison of canine whole and intact parathyroid hormone concentrations as measured by different assays. J. Small Anim. Pract. 2019, 60, 507–513. [Google Scholar] [CrossRef]
- Rudinsky, A.J.; Harjes, L.M.; Byron, J.; Chew, D.J.; Toribio, R.E.; Langston, C.; Parker, V.J. Factor associated with survival in dogs with chronic kidney disease. J. Vet. Intern. Med. 2018, 32, 1977–1982. [Google Scholar] [CrossRef]
- O’Brien, M.A.; McMichael, M.A.; Le Boedec, K. 25-Hydroxyvitamin D concentrations in dogs with naturally acquired blastomycosis. J. Vet. Intern. Med. 2018, 32, 1684–1691. [Google Scholar] [CrossRef]
- Ham, K.; Greenfield, C.L.; Barger, A.; Schaeffer, D.; Ehrhart, E.J.; Pinkerton, M.; Valli, V.E.O. Validation of a rapid parathyroid hormone assay and intraoperative measurement of parathyroid hormone in dogs with benign naturally occurring primary hyperparathyroidism. Vet. Surg. 2009, 38, 122–132. [Google Scholar] [CrossRef]
- Graham, K.J.; Wilkinson, M.; Culvenor, J.; Dhand, N.K.; Churcher, R.K. Intraoperative parathyroid hormone concentration to confirm removal of hypersecretory parathyroid tissue and time to postoperative normocalcemia in ine dogs with primary hyperparathyroidism. Aust. Vet. J. 2012, 90, 203–209. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. (i)), S49–S52. [Google Scholar] [PubMed]
- Hillström, A.; Hagman, R.; Tvedten, H.; Kielgaard-Hansen, M. Validation of a commercially available automated canine-specific immunoturbidimetric method for measuring canine C-reactive protein. Vet. Clin. Pathol. 2014, 43, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Lazaretti, P.; Kogika, M.M.; Hagiwara, M.K.; Lustoza, M.D.; Mirandola, R.M.S. Serum concentration of intact parathormone in dogs with chronic renal failure. Arq. Bras. Med. Vet. Zootec 2006, 58, 489–494. [Google Scholar] [CrossRef]
- Ankrah-Tetteh, T.; Wijeratne, S.; Swaminathan, R. Intraindividual variation in serum thyroid hormones, parathyroid hormone and insulin-like growth factor-1. Ann. Clin. Biochem. 2008, 45, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.; Singh, D.K.; Twomey, P.J.; Farrington, K. Analytical quality goals for parathyroid hormone based on biological variation. Clin. Chem. Lab. Med. 2008, 46, 1438–1442. [Google Scholar] [CrossRef] [PubMed]
- Guideline on Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 19 September 2020).
- Hanon, E.A.; Sturgeon, C.M.; Lamb, E.J. Sampling and storage conditions influencing the measurement of parathyroid hormone in blood samples: A systematic review. Clin. Chem. Lab. Med. 2013, 51, 1925–1941. [Google Scholar] [CrossRef] [PubMed]
- Davies, C. Principles of competitive and immunometric assays (including ELISA). In The Immunoassay Handbook, 4th ed.; Wild, D., Ed.; Elsevier: Oxford, UK, 2013; pp. 11–26. [Google Scholar]
- Kritmetapak, K.; Pongchaiyakul, C. Parathyroid hormone measurement in chronic kidney disease: From basics to clinical implications. Int. J. Nephrol. 2019, 2019, 5496710. [Google Scholar] [CrossRef]
| Parameter | Low | Medium | High |
|---|---|---|---|
| INTRA-ASSAY | |||
| Mean | 3.52 | 29.13 | 95.15 |
| SD | 0.23 | 0.77 | 5.68 |
| CV | 6.59 | 2.66 | 5.97 |
| INTER-ASSAY | |||
| Mean | 3.67 | 28.41 | 96.5 |
| SD | 0.13 | 0.71 | 1.76 |
| CV | 3.42 | 2.51 | 1.83 |
| Recovery Percentage | Expected | Obtained | BIAS | % |
|---|---|---|---|---|
| 100% | 3.5 | 3.5 | 0 | 0 |
| 90% | 10.54 | 10.8 | 0.26 | 2.5 |
| 80% | 17.58 | 18.3 | 0.72 | 4.1 |
| 70% | 24.62 | 26 | 1.38 | 5.6 |
| 60% | 31.66 | 31.8 | 0.14 | 0.4 |
| 50% | 38.7 | 40.4 | 1.7 | 4.4 |
| 40% | 45.74 | 45 | −0.74 | −1.6 |
| 30% | 52.78 | 52.6 | −0.18 | −0.3 |
| 20% | 59.82 | 61.4 | 1.58 | 2.6 |
| 10% | 66.86 | 67.9 | 1.04 | 1.6 |
| 0% | 73.9 | 73.9 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambarbieri, J.; Tagliasacchi, F.; Moretti, P.; Giordano, A.; Scarpa, P. Analytical and Clinical Validation of a New Immunoenzymatic Method for the Measurement of Canine Parathyroid Hormone. Animals 2020, 10, 2411. https://doi.org/10.3390/ani10122411
Zambarbieri J, Tagliasacchi F, Moretti P, Giordano A, Scarpa P. Analytical and Clinical Validation of a New Immunoenzymatic Method for the Measurement of Canine Parathyroid Hormone. Animals. 2020; 10(12):2411. https://doi.org/10.3390/ani10122411
Chicago/Turabian StyleZambarbieri, Jari, Filippo Tagliasacchi, Pierangelo Moretti, Alessia Giordano, and Paola Scarpa. 2020. "Analytical and Clinical Validation of a New Immunoenzymatic Method for the Measurement of Canine Parathyroid Hormone" Animals 10, no. 12: 2411. https://doi.org/10.3390/ani10122411
APA StyleZambarbieri, J., Tagliasacchi, F., Moretti, P., Giordano, A., & Scarpa, P. (2020). Analytical and Clinical Validation of a New Immunoenzymatic Method for the Measurement of Canine Parathyroid Hormone. Animals, 10(12), 2411. https://doi.org/10.3390/ani10122411





