A High Dietary Incorporation Level of Chlorella vulgaris Improves the Nutritional Value of Pork Fat without Impairing the Performance of Finishing Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Recombinant Four-CAZyme Mixture
2.2. Animal Care, Experimental Design and Experimental Diets
2.3. Animal Performance, Slaughter, and Sampling
2.4. Microalga and Experimental Diets Analyses
2.5. Meat Quality Traits
2.6. Cooking Loss and Shear Force Measurements
2.7. Trained Sensory Panel Analysis
2.8. Determination of Total Cholesterol and Diterpene Profile in Meat
2.9. Determination of Pigments in Meat
2.10. Determination of Total Lipid Content and Fatty Acid Composition
2.11. Determination of Meat Lipid Oxidation
2.12. Statistical Analysis
3. Results and Discussion
3.1. Feed Intake, Growth Performance and Carcass Characteristics of Pigs
3.2. Pork Quality Traits and Sensory Evaluation
3.3. Vitamin E Profile and Pigments of Pork
3.4. Total Lipids, Cholesterol and Fatty Acid Composition of Pork
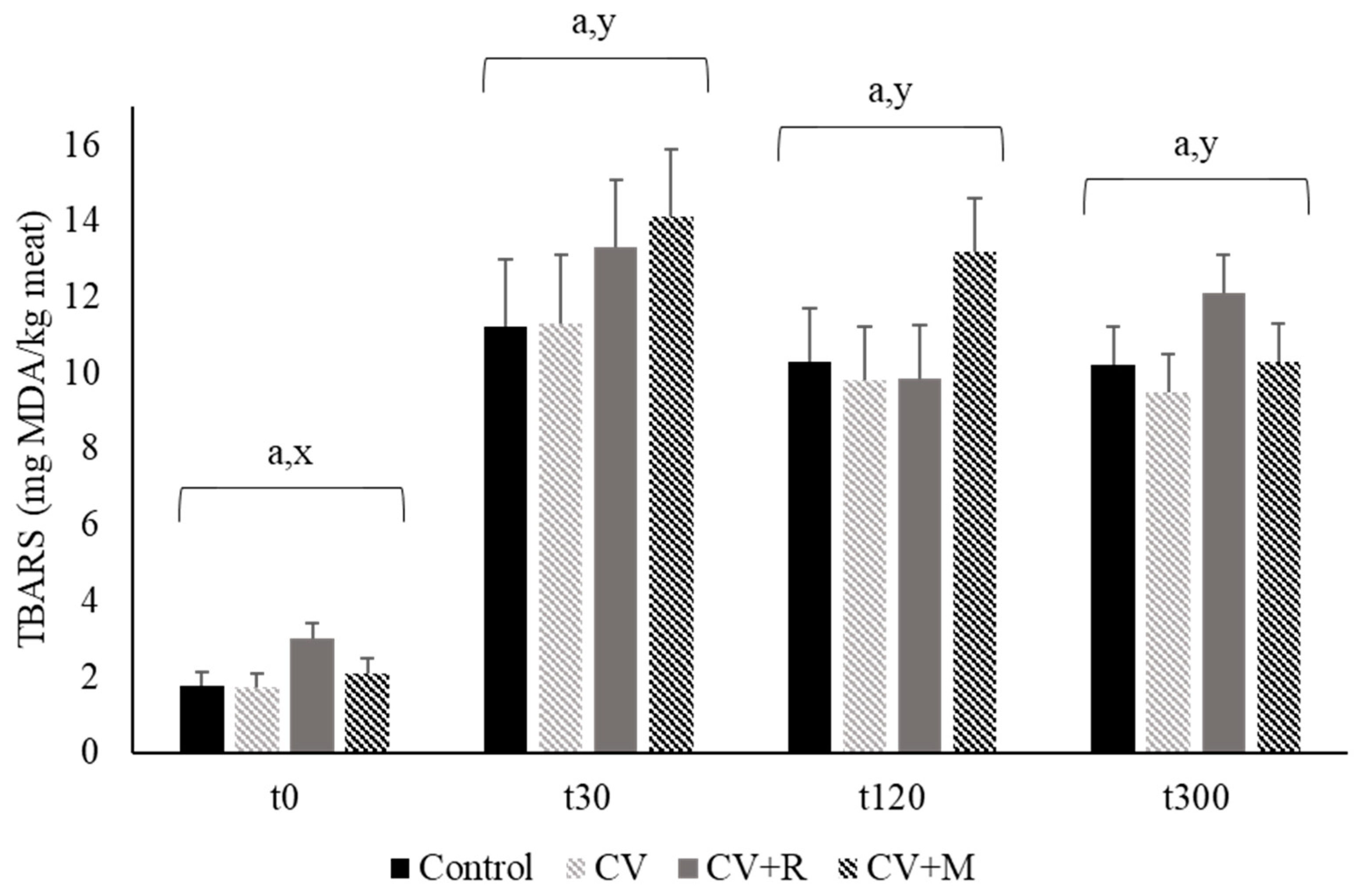
3.5. Oxidative Stability of Pork
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, M.; Zou, X.; Zhao, D.; Zhao, F.; Li, C. Pork Meat Proteins Alter Gut Microbiota and Lipid Metabolism Genes in the Colon of Adaptive Immune-Deficient Mice. Mol. Nutr. Food Res. 2020, 64. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture. 2007. Available online: http://www.fao.org (accessed on 9 September 2020).
- FAO. The State of Food Insecurity in the World. 2011. Available online: http://www.fao.org (accessed on 9 September 2020).
- Ekmay, R.; Gatrell, S.; Lum, K.; Kim, J.; Lei, X.G. Nutritional and Metabolic Impacts of a Defatted Green Marine Microalgal (Desmodesmussp.) Biomass in Diets for Weanling Pigs and Broiler Chickens. J. Agric. Food Chem. 2014, 62, 9783–9791. [Google Scholar] [CrossRef] [PubMed]
- Taelman, S.E.; De Meester, S.; Van Dijk, W.; Da Silva, V.; Dewulf, J. Environmental sustainability analysis of a protein-rich livestock feed ingredient in The Netherlands: Microalgae production versus soybean import. Resour. Conserv. Recycl. 2015, 101, 61–72. [Google Scholar] [CrossRef]
- Morgan, C.A.; Noble, R.C.; Cocchi, M.; McCartney, R. Manipulation of the fatty acid composition of pig meat lipids by dietary means. J. Sci. Food Agric. 1992, 58, 357–368. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M. Factors influencing fatty acids in meat and the role of antioxidants in improving meat quality. Br. J. Nutr. 1997, 78, S49–S60. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Cameron-Smith, D.; Garg, M.; Sinclair, A.J. Docosapentaenoic acid (22:5n-3): A review of its biological effects. Prog. Lipid Res. 2011, 50, 28–34. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3: The good oil. Nutr. Bull. 2017, 42, 132–140. [Google Scholar] [CrossRef]
- Liu, J.; Chen, F. Biology and Industrial Applications of Chlorella: Advances and Prospects. Adv. Biochem. Eng. Biotechnol. 2014, 153, 1–35. [Google Scholar] [CrossRef]
- Kotrbáček, V.; Doubek, J.; Doucha, J. The chlorococcalean alga Chlorella in animal nutrition: A review. Environ. Boil. Fishes 2015, 27, 2173–2180. [Google Scholar] [CrossRef]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Baudelet, P.-H.; Ricochon, G.; Linder, M.; Muniglia, L. A new insight into cell walls of Chlorophyta. Algal Res. 2017, 25, 333–371. [Google Scholar] [CrossRef]
- Teuling, E.; Wierenga, P.A.; Agboola, J.O.; Gruppen, H.; Schrama, J.W. Cell wall disruption increases bioavailability of Nannochloropsis gaditana nutrients for juvenile Nile tilapia (Oreochromis niloticus). Aquaculture 2019, 499, 269–282. [Google Scholar] [CrossRef]
- Son, J.-H.; Ravindran, V. Feed enzyme technology: Present status and future developments. Recent Patents Food Nutr. Agric. 2011, 3, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Sander, K.B.; Murthy, G.S. Enzymatic Degradation of Microalgal Cell Walls; American Society of Agricultural and Biological Engineers (ASABE): San Jose, MI, USA, 2009; p. 1. [Google Scholar]
- Zheng, H.; Yin, J.; Gao, Z.; Huang, H.; Ji, X.; Dou, C. Disruption of Chlorella vulgaris Cells for the Release of Biodiesel-Producing Lipids: A Comparison of Grinding, Ultrasonication, Bead Milling, Enzymatic Lysis, and Microwaves. Appl. Biochem. Biotechnol. 2011, 164, 1215–1224. [Google Scholar] [CrossRef]
- Cho, H.-S.; Oh, Y.-K.; Park, S.-C.; Lee, J.-W.; Park, J.-Y. Effects of enzymatic hydrolysis on lipid extraction from Chlorella vulgaris. Renew. Energy 2013, 54, 156–160. [Google Scholar] [CrossRef]
- Gerken, H.G.; Donohoe, B.; Knoshaug, E.P. Enzymatic cell wall degradation of Chlorella vulgaris and other microalgae for biofuels production. Planta 2013, 237, 239–253. [Google Scholar] [CrossRef]
- Coelho, D.; Lopes, P.A.; Cardoso, V.; Ponte, P.; Brás, J.; Madeira, M.S.; Alfaia, C.M.; Bandarra, N.M.; Gerken, H.G.; Fontes, C.M.G.A.; et al. Novel combination of feed enzymes to improve the degradation of Chlorella vulgaris recalcitrant cell wall. Sci. Rep. 2019, 9, 5382. [Google Scholar] [CrossRef]
- Frederick, K.R. Pork Carcass Evaluation and Procedures; Oklahoma Coop. Ext. Service: Stillwater, OK, USA, 1972. [Google Scholar]
- Association of Official Analytical Chemists International. Official Methods of Analysis, 17th ed.; AOAC International: Arlington, VA, USA, 2000. [Google Scholar]
- Clegg, K.M. The application of the anthrone reagent to the estimation of starch in cereals. J. Sci. Food Agric. 1956, 7, 40–44. [Google Scholar] [CrossRef]
- Noblet, J.; Fortune, H.; Dubois, S.; Henry, Y. Nouvelles Bases D’estimation des Teneurs en Énergie Digestible Métabolisable et Nette des Aliments Pour le Porc; INRA: Paris, France, 1989. [Google Scholar]
- Association of Official Analytical Chemists International. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Henderson, J.W.; Ricker, R.D.; Bidlingmeyer, B.A.; Woodward, C. Rapid, Accurate, Sensitive and Reproducible Analysis of Amino Acids; Agilent Publication Number 5980-1193EN; Agilent Technologies: Palo Alto, CA, USA, 2000. [Google Scholar]
- Sukhija, P.S.; Palmquist, D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- Prates, J.; Quaresma, M.A.G.; Bessa, R.J.B.; Fontes, C.M.A.; Alfaia, C.M.M. Simultaneous HPLC quantification of total cholesterol, tocopherols and β-carotene in Barrosã-PDO veal. Food Chem. 2006, 94, 469–477. [Google Scholar] [CrossRef]
- Teimouri, M.; Amirkolaie, A.K.; Yeganeh, S. The effects of Spirulina platensis meal as a feed supplement on growth performance and pigmentation of rainbow trout (Oncorhynchus mykiss). Aquaculture 2013, 396, 14–19. [Google Scholar] [CrossRef]
- Pestana, J.; Puerta, B.; Santos, H.; Madeira, M.; Alfaia, C.; Lopes, P.; Pinto, R.; Lemos, J.; Fontes, C.; Lordelo, M.; et al. Impact of dietary incorporation of Spirulina (Arthrospira platensis) and exogenous enzymes on broiler performance, carcass traits, and meat quality. Poult. Sci. 2020, 99, 2519–2532. [Google Scholar] [CrossRef] [PubMed]
- Hynstova, V.; Sterbova, D.; Klejdus, B.; Hedbavny, J.; Huska, D.; Adamab, V. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using High Performance Thin Layer Chromatography. J. Pharm. Biomed. Anal. 2018, 148, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Madeira, M.S.M.D.S.; Costa, P.; Alfaia, C.M.; Lopes, P.A.A.B.; Bessa, R.J.B.; Lemos, J.P.; Prates, J. The increased intramuscular fat promoted by dietary lysine restriction in lean but not in fatty pig genotypes improves pork sensory attributes1. J. Anim. Sci. 2013, 91, 3177–3187. [Google Scholar] [CrossRef] [PubMed]
- Hope-Jones, M.; Strydom, P.E.; Frylinck, L.; Webb, E. Effect of dietary beta-agonist treatment, vitamin D3 supplementation and electrical stimulation of carcasses on colour and drip loss of steaks from feedlot steers. Meat Sci. 2012, 90, 607–612. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Oillic, S.; Lemoine, E.; Gros, J.-B.; Kondjoyan, A. Kinetic analysis of cooking losses from beef and other animal muscles heated in a water bath—Effect of sample dimensions and prior freezing and ageing. Meat Sci. 2011, 88, 338–346. [Google Scholar] [CrossRef]
- American Meat Science Association. Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Meat, 2nd ed.; AMSA: Champaign, IL, USA, 2016. [Google Scholar]
- Cross, H.R.; Moen, R.; Stanfield, M.S. Training and testing of judges for sensory analysis of meat quality. Food Technol. 1979, 32, 48–54. [Google Scholar]
- Almeida, J.M.; Bressan, M.C.; Santos-Silva, J.; Moreira, O.; Bettencourt, C.; Da Gama, L.T. Physicochemical characteristics and sensory attributes of meat from heavy-weight Iberian and F1 Large White × Landrace pigs finished intensively or in free-range conditions. J. Anim. Sci. 2018, 96, 2734–2746. [Google Scholar] [CrossRef]
- Belk, K.E.; Dikeman, M.E.; Calkins, C.R.; Andy King, D.; Shackelford, S.D.; Hale, D. Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Meat; American Meat Science Association: Champaign, IL, USA, 2015; pp. 6–104. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Raes, K.; De Smet, S.; Demeyer, D. Effect of double-muscling in Belgian Blue young bulls on the intramuscular fatty acid composition with emphasis on conjugated linoleic acid and polyunsaturated fatty acids. Anim. Sci. 2001, 73, 253–260. [Google Scholar] [CrossRef]
- Madeira, M.S.M.D.S.; Pires, V.M.R.; Alfaia, C.M.; Luxton, R.; Doran, O.; Bessa, R.J.B.; Prates, J. Combined effects of dietary arginine, leucine and protein levels on fatty acid composition and gene expression in the muscle and subcutaneous adipose tissue of crossbred pigs. Br. J. Nutr. 2014, 111, 1521–1535. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grau, A.; Guardiola, F.; Boatella, J.; Barroeta, A.C.; Codony, R. Measurement of 2-thiobarbituric acid values in dark chicken meat through derivative spectrophotometry: Influence of various parameters. J. Agric. Food Chem. 2000, 48, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Mercier, Y.; Gatellier, P.; Renerre, M. Lipid and protein oxidation in vitro, and antioxidant potential in meat from Charolais cows finished on pasture or mixed diet. Meat Sci. 2004, 66, 467–473. [Google Scholar] [CrossRef]
- Yan, L.; Lim, S.U.; Kim, I. Effect of Fermented Chlorella Supplementation on Growth Performance, Nutrient Digestibility, Blood Characteristics, Fecal Microbial and Fecal Noxious Gas Content in Growing Pigs. Asian Australas. J. Anim. Sci. 2012, 25, 1742–1747. [Google Scholar] [CrossRef]
- Baňoch, T.; Svoboda, M.; Kuta, J.; Saláková, A.; Fajt, Z. The effect of iodine from iodine-enriched alga Chlorella spp. on the pork iodine content and meat quality in finisher pigs. Acta Veter Brno 2012, 81, 339–346. [Google Scholar] [CrossRef][Green Version]
- Furbeyre, H.; Van Milgen, J.; Mener, T.; Gloaguen, M.; Labussière, E. Effects of dietary supplementation with freshwater microalgae on growth performance, nutrient digestibility and gut health in weaned piglets. Animal 2017, 11, 183–192. [Google Scholar] [CrossRef]
- Furbeyre, H.; Van Milgen, J.; Mener, T.; Gloaguen, M.; Labussière, E. Effects of oral supplementation with Spirulina and Chlorella on growth and digestive health in piglets around weaning. Animal 2018, 12, 2264–2273. [Google Scholar] [CrossRef]
- Oh, S.T.; Zheng, L.; Kwon, H.J.; Choo, Y.K.; Lee, K.W.; Kang, C.W.; An, B.K. Effects of Dietary Fermented Chlorella vulgaris (CBT®) on Growth Performance, Relative Organ Weights, Cecal Microflora, Tibia Bone Characteristics, and Meat Qualities in Pekin Ducks. Asian Australas. J. Anim. Sci. 2014, 28, 95–101. [Google Scholar] [CrossRef]
- Nogareda, C.; Moreno, J.A.; Angulo, E.; Sandmann, G.; Portero, M.; Capell, T.; Zhu, C.; Christou, P. Carotenoid-enriched transgenic corn delivers bioavailable carotenoids to poultry and protects them against coccidiosis. Plant. Biotechnol. J. 2016, 14, 160–168. [Google Scholar] [CrossRef]
- Gouveia, L.; Veloso, V.; Reis, A.; Fernandas, H.; Novais, J.; Empis, J. Chlorella vulgaris used to colour egg yolk. J. Sci. Food Agric. 1996, 70, 167–172. [Google Scholar] [CrossRef]
- Lemahieu, C.; Bruneel, C.; Termote-Verhalle, R.; Muylaert, K.; Buyse, J.; Foubert, I. Impact of feed supplementation with different omega-3 rich microalgae species on enrichment of eggs of laying hens. Food Chem. 2013, 141, 4051–4059. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P. New Insights into Mechanisms of Action for Omega-3 Fatty Acids in Atherothrombotic Cardiovascular Disease. Curr. Atheroscler. Rep. 2019, 21, 1–11. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Agence Nationale de Sécurité Sanitaire de L’alimentation, de L’environnement et du Travail. Actualisation des Apports Nutritionnels Conseillés Pour les Acides Gras; ANSES: Maisons-Alfort, France, 2011.
- Kris-Etherton, P.M.; Grieger, J.A.; Etherton, T.D. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 99–104. [Google Scholar] [CrossRef]
- Molendi-Coste, O.; Legry, V.; Leclercq, I.A. Why and How Meet n-3 PUFA Dietary Recommendations? Gastroenterol. Res. Pr. 2010, 2011, 1–11. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- El-Bahr, S.M.; Shousha, S.; Shehab, A.; Khattab, W.; Ahmed-Farid, O.; Sabike, I.; El-Garhy, O.; Albokhadaim, I.; Al-Busada, K.A. Effect of Dietary Microalgae on Growth Performance, Profiles of Amino and Fatty Acids, Antioxidant Status, and Meat Quality of Broiler Chickens. Animal 2020, 10, 761. [Google Scholar] [CrossRef]
- HMSO, UK. Department of Health. Nutritional aspects of cardio-vascular disease. Rep. Health Soc. Subj. 1994, 46, 37–46. [Google Scholar]
- Jayasingh, P.; Cornforth, D.P. Comparison of antioxidant effects of milk mineral, butylated hydroxytoluene and sodium tripolyphosphate in raw and cooked ground pork. Meat Sci. 2004, 66, 83–89. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Wojtasik-Kalinowska, I.; Guzek, D.; Górska-Horczyczak, E.; Brodowska, M.; Sunabcd, D.-W.; Wierzbicka, A. Diet with linseed oil and organic selenium yields low n-6/n-3 ratio pork Semimembranosus meat with unchanged volatile compound profiles. Int. J. Food Sci. Technol. 2018, 53, 1838–1846. [Google Scholar] [CrossRef]
- Martini, S.; Tagliazucchi, D.; Minelli, G.; Fiego, D.P.L. Influence of linseed and antioxidant-rich diets in pig nutrition on lipid oxidation during cooking and in vitro digestion of pork. Food Res. Int. 2020, 137, 109528. [Google Scholar] [CrossRef]
- Vossen, E.; Raes, K.; Van Mullem, D.; De Smet, S. Production of docosahexaenoic acid (DHA) enriched loin and dry cured ham from pigs fed algae: Nutritional and sensory quality. Eur. J. Lipid Sci. Technol. 2017, 119, 1600144. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. Environ. Boil. Fishes 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
| Ingredients (%) | Experimental Diets | |||
|---|---|---|---|---|
| Control | CV | CV + R | CV + M | |
| Corn | 56 | 56 | 56 | 56 |
| Soybean meal | 19.3 | 11.7 | 11.6 | 11.7 |
| Barley | 10 | 10 | 10 | 10 |
| Sunflower meal | 5.4 | 6.8 | 6.8 | 6.8 |
| Wheat | 5 | 5 | 5 | 5 |
| Calcium carbonate | 1.3 | 1.2 | 1.2 | 1.2 |
| Soybean oil | 0.5 | 0.2 | 0.2 | 0.2 |
| Wheat bran | 0.4 | 1.7 | 1.66 | 1.65 |
| Salt | 0.4 | 0.4 | 0.4 | 0.4 |
| Vitamin–trace mineral premix 1 | 0.4 | 0.4 | 0.4 | 0.4 |
| Dicalcium phosphate | 0.26 | 0.36 | 0.36 | 0.36 |
| Sodium bicarbonate | 0.1 | 0.1 | 0.1 | 0.1 |
| Betaine-HCl | 0.15 | 0.15 | 0.15 | 0.15 |
| Mold inhibitor mixture 2 | 0.075 | 0.075 | 0.075 | 0.075 |
| Fatty acid mixture 3 | 0.075 | 0.075 | 0.075 | 0.075 |
| L-Lysine | 0.41 | 0.57 | 0.57 | 0.57 |
| L-Threonine | 0.1180 | 0.2000 | 0.2000 | 0.2000 |
| DL-Methionine | 0.0712 | 0.1080 | 0.1080 | 0.1080 |
| L-Tryptophan | 0.0064 | - | - | - |
| Chlorella vulgaris | - | 5 | 5 | 5 |
| Mix of 4 CAZymes | - | - | - | 0.01 |
| Rovabio® Excel AP | - | - | 0.005 | - |
| Item | Microalga C. vulgaris | Experimental Diets | |||
|---|---|---|---|---|---|
| Control | CV | CV + R | CV + M | ||
| ME, kcal/kg DM 1 | 3557 | 3576 | 3540 | 3644 | 3547 |
| Proximate composition, % | |||||
| Dry matter | 93.1 | 90.0 | 89.7 | 89.5 | 90.0 |
| Crude protein | 42.8 | 14.0 | 15.9 | 15.2 | 15.2 |
| Starch | 1.86 | 45.5 | 45.3 | 44.7 | 47.4 |
| Crude fat | 8.73 | 2.60 | 3.00 | 3.10 | 3.10 |
| Crude fiber | 1.52 | 4.60 | 5.00 | 5.30 | 5.20 |
| NDF | 1.05 | 13.7 | 13.9 | 12.7 | 13.7 |
| ADF | 0.286 | 4.90 | 5.50 | 5.50 | 5.90 |
| Ash | 11.8 | 4.03 | 4.70 | 4.60 | 4.60 |
| Amino acid composition, % | |||||
| Alanine | 2.77 | 0.682 | 0.848 | 0.806 | 0.776 |
| Arginine | 3.89 | 0.890 | 1.11 | 1.03 | 0.969 |
| Asparagine | 0.062 | 0.023 | 0.022 | 0.015 | 0.018 |
| Aspartate | 3.04 | 1.00 | 1.08 | 1.11 | 1.01 |
| Cysteine | 0.665 | 0.292 | 0.268 | 0.237 | 0.248 |
| Glutamate | 4.07 | 2.33 | 2.22 | 2.21 | 2.10 |
| Glutamine | 0.016 | nd | nd | nd | nd |
| Glycine | 1.72 | 0.544 | 0.687 | 0.614 | 0.584 |
| Histidine | 0.654 | 0.512 | 0.593 | 0.528 | 0.489 |
| Hydroxyproline | 0.741 | 0.880 | 1.33 | 1.19 | 1.16 |
| Isoleucine | 1.26 | 0.478 | 0.536 | 0.521 | 0.482 |
| Leucine | 2.45 | 0.942 | 1.05 | 1.03 | 0.984 |
| Lysine | 2.63 | 1.04 | 1.43 | 1.42 | 1.32 |
| Methionine | 0.451 | 0.116 | 0.124 | 0.144 | 0.088 |
| Phenylalanine | 1.49 | 0.578 | 0.634 | 0.621 | 0.587 |
| Proline | 1.87 | 1.06 | 1.04 | 1.04 | 1.01 |
| Serine | 1.56 | 0.689 | 0.771 | 0.727 | 0.679 |
| Threonine | 2.32 | 0.761 | 0.989 | 1.00 | 0.943 |
| Tryptophan | 0.471 | 0.156 | 0.172 | 0.147 | 0.133 |
| Tyrosine | 1.18 | 0.429 | 0.495 | 0.470 | 0.437 |
| Valine | 3.52 | 1.20 | 1.43 | 1.32 | 1.26 |
| Fatty acid profile, % total fatty acids | |||||
| 14:0 | 1.13 | 0.150 | 0.218 | 0.190 | 0.190 |
| 16:0 | 17.2 | 16.3 | 16.6 | 16.3 | 16.5 |
| 16:1c9 | 3.90 | 0.228 | 1.14 | 0.989 | 0.972 |
| 17:0 | 0.234 | 0.189 | 0.182 | 0.153 | 0.154 |
| 17:1c9 | 0.610 | 0.038 | 0.704 | 0.739 | 0.732 |
| 18:0 | 3.00 | 2.89 | 3.29 | 3.11 | 3.08 |
| 18:1c9 | 11.7 | 27.4 | 27.4 | 27.6 | 27.5 |
| 18:1c11 | nd | 0.885 | 1.70 | 1.38 | 1.42 |
| 18:2n-6 | 11.2 | 48.1 | 44.1 | 45.1 | 44.9 |
| 18:3n-3 | 10.1 | 2.57 | 3.47 | 3.28 | 3.28 |
| 20:0 | 0.174 | 0.528 | 0.513 | 0.517 | 0.500 |
| 20:1c11 | 0.127 | 0.292 | 0.288 | 0.320 | 0.320 |
| 20:5n-3 | nd | nd | nd | nd | nd |
| 22:0 | 0.060 | 0.304 | 0.294 | 0.262 | 0.266 |
| 22:1n-9 | nd | 0.155 | 0.155 | 0.131 | 0.149 |
| 22:6n-3 | nd | nd | nd | nd | nd |
| Diterpene profile, μg/g | |||||
| α-Tocopherol | 19.2 | 16.5 | 18.7 | 19.4 | 16.5 |
| α-Tocotrienol | nd | 4.84 | 3.70 | 3.88 | 4.36 |
| β-Tocopherol | 0.340 | 0.380 | 0.268 | 0.244 | 0.258 |
| γ -Tocopherol | 0.520 | 3.53 | 2.74 | 2.35 | 2.65 |
| γ-Tocotrienol | 0.560 | 7.23 | 5.93 | 7.30 | 6.02 |
| δ-Tocopherol | 0.360 | 0.340 | 0.331 | 0.312 | 0.314 |
| δ-Tocotrienol | nd | 0.287 | 0.230 | 0.246 | 0.247 |
| Pigments, μg/g | |||||
| β-Carotene | 198 | 1.19 | 7.10 | 7.40 | 6.49 |
| Chlorophyll a 2 | 906 | 4.31 | 127 | 139 | 126 |
| Chlorophyll b 3 | 171 | 7.46 | 33.9 | 36.6 | 34.2 |
| Total Chlorophylls 4 | 1077 | 11.8 | 161 | 176 | 160 |
| Total Carotenoids 5 | 228 | 3.97 | 36.5 | 39.5 | 34.9 |
| Total Chlorophylls+ carotenoids 6 | 1305 | 15.7 | 198 | 215 | 195 |
| Item | Control | CV | CV + R | CV + M | SEM | p-Value |
|---|---|---|---|---|---|---|
| Growth performance | ||||||
| Initial weight, kg | 62.8 | 56.1 | 58.4 | 59.4 | 1.79 | 0.075 |
| Final weight, kg | 101 | 101 | 101 | 101 | 0.643 | 0.927 |
| ADFI, kg | 2.56 | 2.67 | 2.65 | 2.60 | 0.052 | 0.409 |
| ADG, kg | 0.959 | 1.08 | 1.01 | 1.04 | 0.037 | 0.141 |
| FCR | 2.69 | 2.49 | 2.63 | 2.55 | 0.079 | 0.286 |
| G:F, kg/kg | 0.374 | 0.404 | 0.382 | 0.398 | 0.011 | 0.244 |
| Carcass characteristics | ||||||
| HCW, kg | 80.1 | 79.5 | 79.3 | 78.9 | 0.735 | 0.703 |
| Carcass yield, % | 77.4 | 77.1 | 76.9 | 76.8 | 0.430 | 0.749 |
| Perirenal fat, kg | 0.897 b | 0.666 a | 0.806 ab | 0.711 ab | 0.055 | 0.026 |
| Mesenteric fat, kg | 0.525 | 0.530 | 0.572 | 0.583 | 0.024 | 0.231 |
| P2 backfat thickness, mm | 6.38 | 5.54 | 7.17 | 6.40 | 0.633 | 0.359 |
| L6 backfat thickness, mm | 9.33 | 10.1 | 10.8 | 9.64 | 0.758 | 0.535 |
| S2 backfat thickness, mm | 4.98 | 5.22 | 5.42 | 5.77 | 0.737 | 0.891 |
| Loin weight, kg | 2.14 | 2.11 | 2.10 | 2.18 | 0.066 | 0.850 |
| Drip loss % 1 | 5.82 | 5.63 | 7.27 | 6.51 | 0.460 | 0.065 |
| Item | Control | CV | CV + R | CV + M | SEM | p-Value |
|---|---|---|---|---|---|---|
| Temperature, °C | ||||||
| 45 min | 39.9 | 39.8 | 39.7 | 40.0 | 0.246 | 0.911 |
| pH | ||||||
| 45 min | 6.11 | 6.34 | 6.12 | 6.28 | 0.109 | 0.351 |
| 24 h | 5.49 | 5.54 | 5.50 | 5.51 | 0.016 | 0.260 |
| Color measurements | ||||||
| Lightness (L*) | 57.0 | 56.5 | 57.9 | 56.9 | 0.976 | 0.791 |
| Redness (a*) | 6.50 | 5.68 | 6.28 | 6.39 | 0.600 | 0.770 |
| Yellowness (b*) | 7.26 | 6.46 | 7.24 | 7.07 | 0.526 | 0.679 |
| Other traits | ||||||
| WBSF, kg | 6.92 | 7.17 | 6.44 | 6.95 | 0.373 | 0.574 |
| Cooking loss, % | 30.8 | 30.7 | 31.0 | 30.1 | 0.605 | 0.740 |
| Item | Control | CV | CV + R | CV + M | SEM | p-Value |
|---|---|---|---|---|---|---|
| Tenderness | 4.45 | 4.61 | 4.57 | 4.54 | 0.117 | 0.788 |
| Juiciness | 3.72 | 3.85 | 3.74 | 3.84 | 0.111 | 0.760 |
| Flavor | 4.09 | 4.20 | 4.29 | 4.20 | 0.109 | 0.649 |
| Off-flavor | 0.061 | 0.111 | 0.171 | 0.131 | 0.029 | 0.064 |
| Flavor acceptability | 5.55 | 5.29 | 5.36 | 5.32 | 0.104 | 0.260 |
| Overall acceptability | 5.23 | 5.22 | 5.13 | 5.10 | 0.101 | 0.756 |
| Item | Control | CV | CV + R | CV + M | SEM | p-Value |
|---|---|---|---|---|---|---|
| Diterpene profile, µg/100 g | ||||||
| α-Tocopherol | 95.4 | 73.6 | 74.9 | 79.4 | 6.2 | 0.062 |
| γ-Tocopherol | 3.5 | 3.7 | 3.5 | 3.2 | 0.2 | 0.441 |
| γ-Tocotrienol | 10.2 | 9.0 | 10.4 | 8.2 | 1.9 | 0.821 |
| Pigments, µg/100 g | ||||||
| β-Carotene | nd | nd | nd | nd | - | - |
| Chlorophyll a | 14.7 | 23.9 | 31.3 | 28.0 | 4.75 | 0.094 |
| Chlorophyll b | 27.7 | 47.2 | 56.9 | 54.7 | 9.00 | 0.109 |
| Total chlorophylls | 42.4 | 71.2 | 88.1 | 82.8 | 13.7 | 0.103 |
| Total carotenoids | 7.18 a | 16.4 b | 16.4 b | 15.1 b | 2.55 | 0.042 |
| Total chlorophylls and carotenoids | 49.6 a | 87.6 ab | 104 b | 97.9 ab | 13.9 | 0.038 |
| Item | Control | CV | CV + R | CV + M | SEM | p-Value |
|---|---|---|---|---|---|---|
| Total lipids, g/100 g | 1.18 | 1.03 | 1.05 | 0.933 | 0.073 | 0.141 |
| Cholesterol, mg/g | 0.363 | 0.363 | 0.361 | 0.367 | 0.015 | 0.993 |
| FA composition, g/100 g FA | ||||||
| 10:0 | 0.053 b | 0.023 a | 0.042 ab | 0.023 a | 0.007 | 0.013 |
| 12:0 | 0.056 | 0.045 | 0.053 | 0.051 | 0.006 | 0.536 |
| 14:0 | 1.05 | 0.952 | 0.994 | 0.904 | 0.045 | 0.126 |
| 14:1c9 | 0.034 a | 0.062 ab | 0.064 ab | 0.068 b | 0.008 | 0.021 |
| 15:0 | 0.081 | 0.072 | 0.067 | 0.069 | 0.007 | 0.519 |
| DMA 16:0 | 0.089 | 0.047 | 0.054 | 0.140 | 0.029 | 0.107 |
| 16:0 | 23.4 | 22.8 | 23.2 | 22.5 | 0.279 | 0.119 |
| 16:1c7 | 0.335 | 0.352 | 0.338 | 0.388 | 0.015 | 0.065 |
| 16:1c9 | 2.94 | 2.67 | 2.79 | 2.42 | 0.131 | 0.054 |
| 17:0 | 0.432 | 0.435 | 0.417 | 0.460 | 0.038 | 0.882 |
| 17:1c9 | 0.340 | 0.369 | 0.363 | 0.334 | 0.023 | 0.647 |
| DMA 18:0 | 0.045 | 0.019 | 0.067 | 0.076 | 0.032 | 0.597 |
| DMA 18:1 | 0.023 | 0.006 | 0.034 | 0.039 | 0.020 | 0.637 |
| 18:0 | 11.9 | 11.6 | 11.9 | 12.2 | 0.373 | 0.698 |
| 18:1c9 | 37.3 | 36.1 | 36.7 | 34.8 | 0.933 | 0.270 |
| 18:1c11 | 3.99 | 3.94 | 3.91 | 3.79 | 0.072 | 0.260 |
| 18:2n-6 | 11.8 | 13.4 | 12.4 | 13.9 | 0.846 | 0.291 |
| 18:2t9t12 | 0.039 | 0.034 | 0.026 | 0.032 | 0.006 | 0.494 |
| 18:3n-6 | 0.121 | 0.129 | 0.123 | 0.133 | 0.014 | 0.934 |
| 18:3n-3 | 0.279 a | 0.408 b | 0.377 b | 0.381 b | 0.020 | >0.001 |
| 18:4n-3 | 0.027 a | 0.050 b | 0.041 ab | 0.058 b | 0.006 | 0.004 |
| 20:0 | 0.167 | 0.154 | 0.161 | 0.171 | 0.007 | 0.422 |
| 20:1c11 | 0.604 | 0.593 | 0.594 | 0.595 | 0.033 | 0.996 |
| 20:2n-6 | 0.341 | 0.358 | 0.326 | 0.336 | 0.018 | 0.675 |
| 20:3n-6 | 0.362 | 0.415 | 0.383 | 0.457 | 0.035 | 0.270 |
| 20:4n-6 | 2.30 | 2.72 | 2.40 | 2.93 | 0.280 | 0.368 |
| 20:3n-3 | 0.056 a | 0.080 ab | 0.089 b | 0.092 b | 0.008 | 0.008 |
| 20:5n-3 | 0.064 a | 0.119 b | 0.114 b | 0.112 b | 0.015 | 0.042 |
| 22:0 | 0.068 | 0.070 | 0.069 | 0.088 | 0.008 | 0.240 |
| 22:1n-9 | 0.047 | 0.049 | 0.055 | 0.043 | 0.008 | 0.740 |
| 22:5n-3 | 0.266 a | 0.385 ab | 0.356 ab | 0.428 b | 0.040 | 0.036 |
| 22:6n-3 | 0.241 a | 0.328 ab | 0.342 ab | 0.393 b | 0.038 | 0.035 |
| 23:0 | 0.162 | 0.189 | 0.170 | 0.211 | 0.021 | 0.366 |
| Others | 0.946 | 1.03 | 1.02 | 1.35 | 0.227 | 0.615 |
| Partial sums of FA, g/100 g FA | ||||||
| SFA | 37.4 | 36.4 | 37.1 | 36.7 | 0.534 | 0.564 |
| MUFA | 45.6 | 44.2 | 44.8 | 42.4 | 1.09 | 0.213 |
| PUFA | 15.9 | 18.4 | 17.0 | 19.3 | 1.25 | 0.243 |
| n-6 PUFA | 14.9 | 17.0 | 15.6 | 17.8 | 1.17 | 0.306 |
| n-3 PUFA | 0.932 a | 1.37 b | 1.32 b | 1.46 b | 0.093 | 0.001 |
| Ratios of FA | ||||||
| PUFA:SFA | 0.427 | 0.508 | 0.461 | 0.530 | 0.038 | 0.232 |
| n-6:n-3 | 16.1 b | 12.3 a | 11.9 a | 12.3 a | 0.395 | <0.001 |
| TBARS, mg MDA/kg meat | Control | CV | CV + R | CV + M | SEM | p-Value |
|---|---|---|---|---|---|---|
| Day 0 | nd | nd | nd | nd | - | - |
| Day 4 | 0.027 | 0.047 | nd | 0.031 | 0.017 | 0.604 |
| Day 8 | 0.186 | 0.174 | 0.517 | 0.160 | 0.142 | 0.234 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, D.; Pestana, J.; Almeida, J.M.; Alfaia, C.M.; Fontes, C.M.G.A.; Moreira, O.; Prates, J.A.M. A High Dietary Incorporation Level of Chlorella vulgaris Improves the Nutritional Value of Pork Fat without Impairing the Performance of Finishing Pigs. Animals 2020, 10, 2384. https://doi.org/10.3390/ani10122384
Coelho D, Pestana J, Almeida JM, Alfaia CM, Fontes CMGA, Moreira O, Prates JAM. A High Dietary Incorporation Level of Chlorella vulgaris Improves the Nutritional Value of Pork Fat without Impairing the Performance of Finishing Pigs. Animals. 2020; 10(12):2384. https://doi.org/10.3390/ani10122384
Chicago/Turabian StyleCoelho, Diogo, José Pestana, João M. Almeida, Cristina M. Alfaia, Carlos M. G. A. Fontes, Olga Moreira, and José A. M. Prates. 2020. "A High Dietary Incorporation Level of Chlorella vulgaris Improves the Nutritional Value of Pork Fat without Impairing the Performance of Finishing Pigs" Animals 10, no. 12: 2384. https://doi.org/10.3390/ani10122384
APA StyleCoelho, D., Pestana, J., Almeida, J. M., Alfaia, C. M., Fontes, C. M. G. A., Moreira, O., & Prates, J. A. M. (2020). A High Dietary Incorporation Level of Chlorella vulgaris Improves the Nutritional Value of Pork Fat without Impairing the Performance of Finishing Pigs. Animals, 10(12), 2384. https://doi.org/10.3390/ani10122384







