Effect of Type of Pregnancy on Transcriptional and Plasma Metabolic Response in Sheep and Its Further Effect on Progeny Lambs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioethics
2.2. Location
2.3. Animals and Sampling
2.4. qRT-PCR Analysis
2.5. Animal Performance
2.6. Metabolic Determining
2.7. Statistical Analysis
3. Results
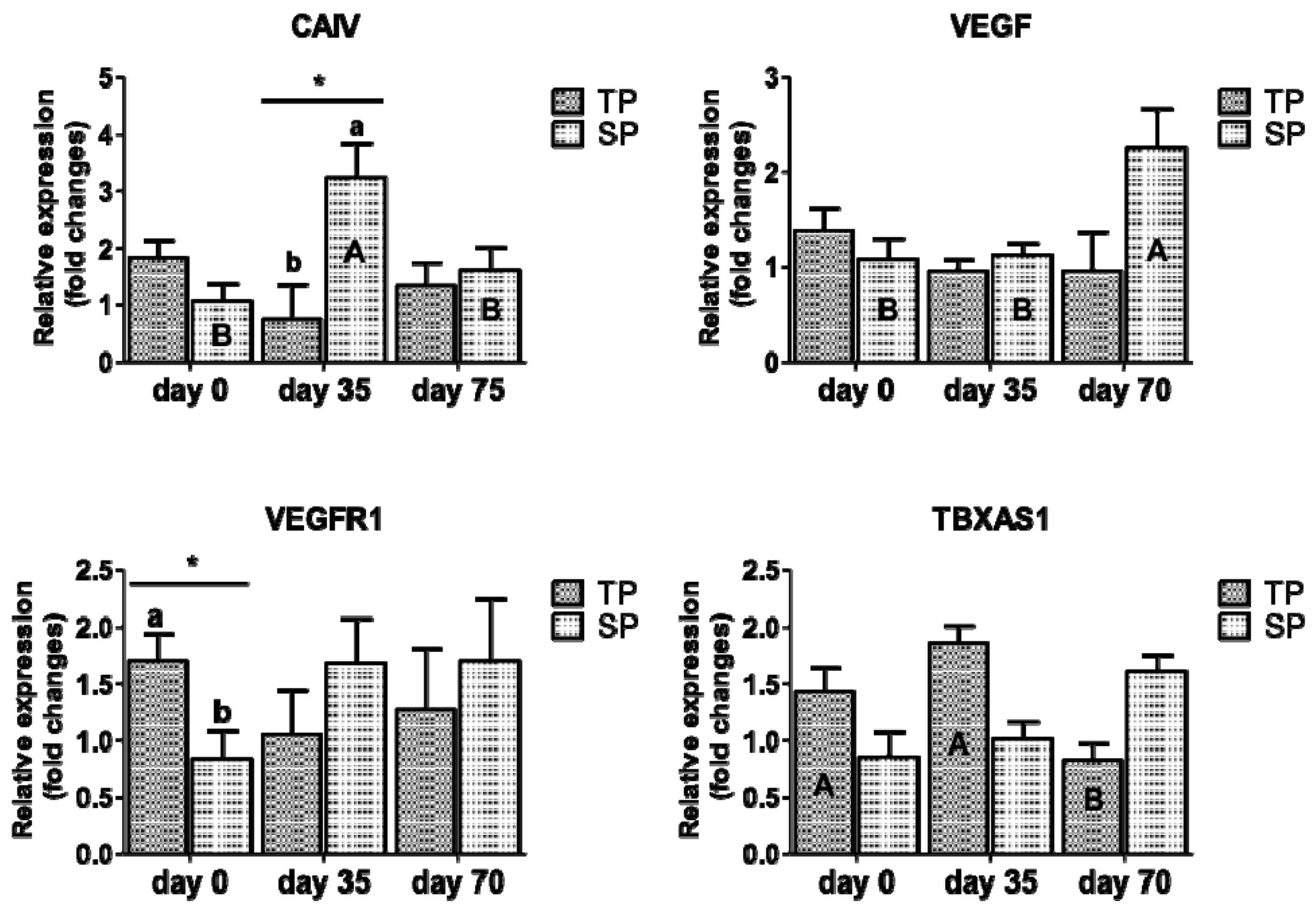
3.1. Relative mRNA Expression
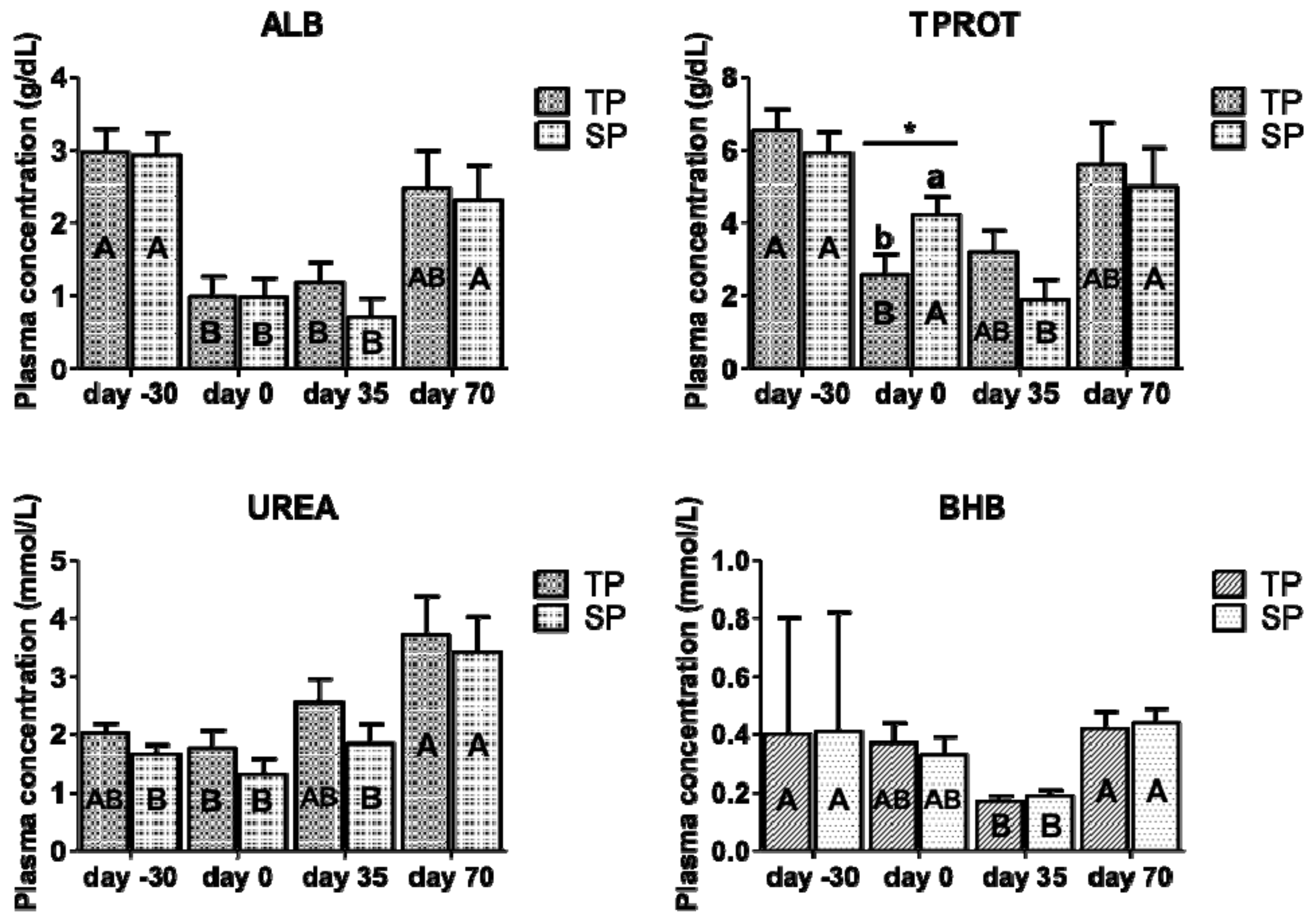
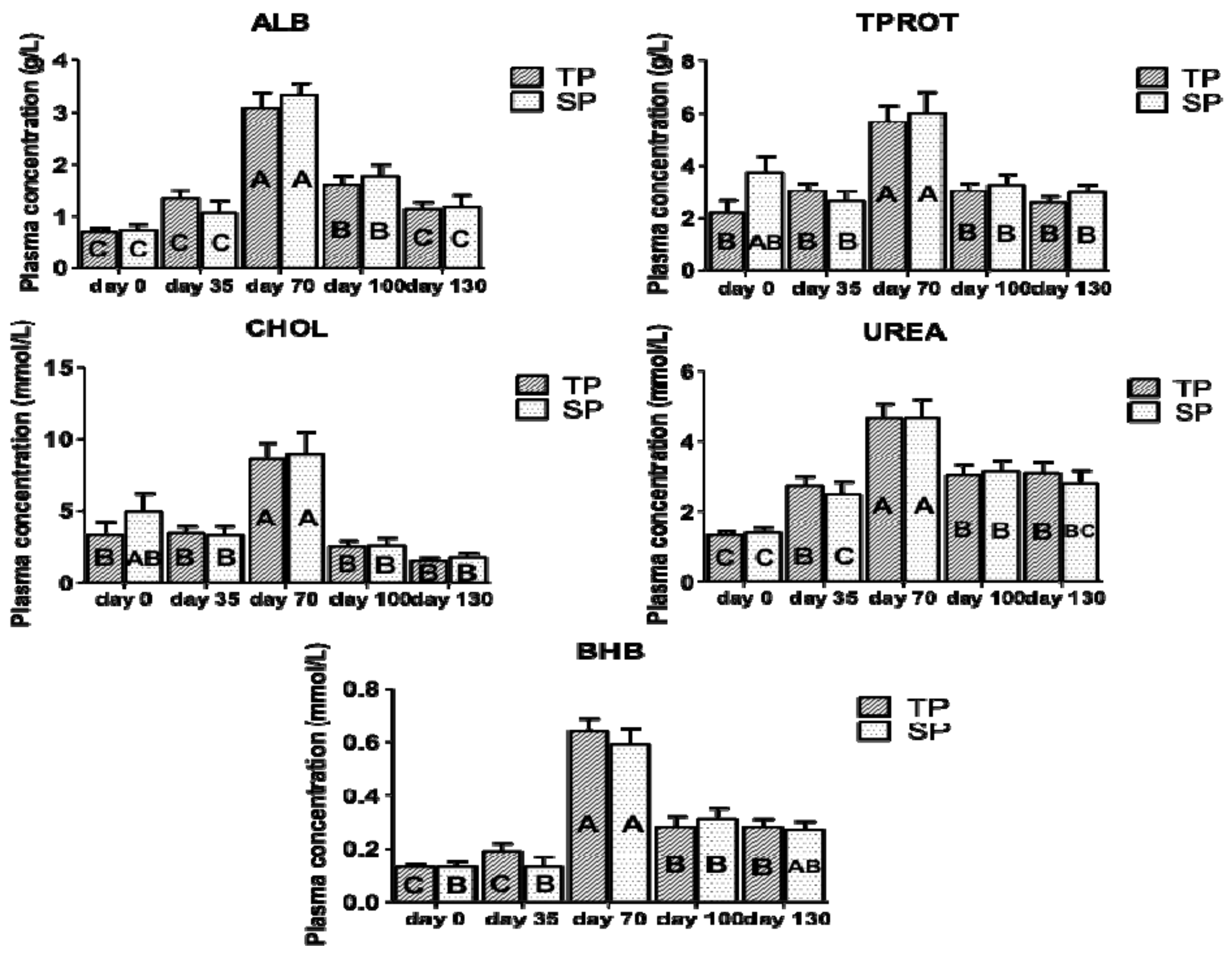
3.2. Effect on Metabolic Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cal-Pereyra, L.; Benech, A.; Da Silva, S.; González-Montaña, J.-R.; Martin, A. Metabolismo energético en ovejas gestantes esquiladas y no esquiladas sometidas a dos planos nutricionales. Efecto sobre las reservas energéticas de sus corderos. Arch. Med. Vet. 2011, 43, 277–285. [Google Scholar] [CrossRef][Green Version]
- Danso, A.S.; Morel, P.C.H.; Kenyon, P.R.; Blair, H.T. Relationships between prenatal ewe traits, milk production, and preweaning performance of twin lambs. J. Anim. Sci. 2016, 94, 3527–3539. [Google Scholar] [CrossRef] [PubMed]
- Rook, J.S. Pregnancy toxemia of ewes, does, and beef cows. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 293–317. [Google Scholar] [CrossRef]
- Hamann, H.; Horstick, A.; Wessels, A.; Distl, O. Estimation of genetic parameters for test day milk production, somatic cell score and litter size at birth in East Friesian ewes. Livest. Prod. Sci. 2004, 87, 153–160. [Google Scholar] [CrossRef]
- Agenäs, S.; Nielsen, M.O.; Safayi, S.; Knight, C. Timming of mammary angiogenesis: A posible explanation for post partum apoptosis in the bovine udder? Lactation Research in mammals and humans: The mammary gland in health and disease. In Proceedings of the CRU Symposium, Uppsala, Sweden, 7–8 December 2010. [Google Scholar]
- Djonov, V.; Andres, A.C.; Ziemiecki, A. Vascular remodelling during the normal and malignant life cycle of the mammary gland. Microsc. Res. Tech. 2001, 52, 182–189. [Google Scholar] [CrossRef]
- Shibuya, M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct. Funct. 2001, 26, 25–35. [Google Scholar] [CrossRef]
- Thurston, G. Role of Angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell Tissue Res. 2003, 314, 61–68. [Google Scholar] [CrossRef]
- Zidi, A.; Casas, E.; Amills, M.; Jordana, J.; Carrizosa, J.; Urrutia, B.; Serradilla, J.M. Genetic variation at the caprine lactalbumin, alpha ( LALBA ) gene and its association with milk lactose concentration. Anim. Genet. 2014, 45, 609–613. [Google Scholar] [CrossRef]
- Safayi, S.; Theil, P.; Elbrønd, V.; Hou, L.; Engbæk, M.; Nørgaard, J.V.; Sejrsen, K.; Nielsen, M. Mammary remodeling in primiparous and multiparous dairy goats during lactation. J. Dairy Sci. 2010, 93, 1478–1490. [Google Scholar] [CrossRef]
- Chen, J.; Kok, A.; Remmelink, G.J.; Gross, J.J.; Bruckmaier, R.M.; Kemp, B.; Van Knegsel, A.T.M. Effects of dry period length and dietary energy source on lactation curve characteristics over 2 subsequent lactations. J. Dairy Sci. 2016, 99, 9287–9299. [Google Scholar] [CrossRef]
- Eckersall, P.D. Proteins, Proteomics, and the Dysproteinemias. In Clinical Biochemistry of Domestic Animals; Kaneko, J.J., Harvey, J.W., Bruss, M.L., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 117–155. [Google Scholar]
- Cavender, C.P.; Turley, S.D.; Dietschy, J.M. Sterol metabolism in fetal, newborn, and suckled lambs and their response to cholesterol after weaning. Am. J. Physiol. Endocrinol. Metab. 1995, 269, E331–E340. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, L.; Barberis, F.C.; Stangaferro, M.L.; Signorini, P.; Ruiz, M.; Zimmermann, R.; Bo, G.A.; Hein, G.J.; Ortega, H.H. Evaluation of metabolic and biochemical blood parameters in lactating cows with Cystic Ovarian Disease. InVet 2013, 15, 7–15. [Google Scholar]
- Hammond, A.C. Update on BUN and MUN as a Guide for Protein Supplementation in Cattle; US Department of Agriculture: Brooksville, FL, USA, 2006.
- Noro, M.; Vargas, V.; Pulido, R.G.; Wittwer, F. Efecto del tipo de concentrado sobre indicadores sanguíneos del metabolismo de energía y de proteínas en vacas lecheras en pastoreo primaveral. Arch. Med. Vet. 2006, 38, 227–232. [Google Scholar] [CrossRef]
- Le Blanc, S. Monitoring programs for transition dairy cows. In Proceedings of the 26th World Buiatrics Congress, Nice, France, 15 October 2006; pp. 460–472. [Google Scholar]
- Kaneko, J.J. Clinical Biochemistry of Domestic Animals; Academic Press: New York, NY, USA, 1989; p. 885. [Google Scholar]
- Mohammadi, V.; Anassori, E.; Jafari, S. Measure of energy related biochemical metabolites changes during peri-partum period in Makouei breed sheep. Vet. Res. Forum 2016, 7, 35–39. [Google Scholar]
- Wood, J.; Enser, M.; Fisher, A.; Nute, G.; Sheard, P.; Richardson, R.; Hughes, S.; Whittington, F. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Demirel, G.; Wachira, A.M.; Sinclair, L.A.; Wilkinson, R.G.; Wood, J.D.; Enser, M. Effects of dietary n-3 polyunsaturated fatty acids, breed and dietary vitamin E on the fatty acids of lamb muscle liver and adipose tissue. Br. J. Nutr. 2004, 91, 551–565. [Google Scholar] [CrossRef]
- Scollan, N.D.; Hocquette, J.-F.; Nuernberg, K.; Dannenberger, D.; Richardson, I.; Moloney, A. Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2006, 74, 17–33. [Google Scholar] [CrossRef]
- Islam, K.; Knight, B.; Frayn, K.; Patel, D.; Gibbons, G. Deficiency of PPARα disturbs the response of lipogenic flux and of lipogenic and cholesterogenic gene expression to dietary cholesterol in mouse white adipose tissue. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2005, 1734, 259–268. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Flemetakis, E.; Kalloniati, C.; Zervas, G. Differences in mRNA lipogenic gene expression in the subcutaneous adipose tissue of sheep and goats under the same dietary treatments. Small Rumin. Res. 2011, 99, 110–115. [Google Scholar] [CrossRef]
- Gallardo, M.A.; Cárcamo, J.G.; Hiller, B.; Nuernberg, G.; Nuernberg, K.; Dannenberger, D. Expression of lipid metabolism related genes in subcutaneous adipose tissue from Chilota lambs grazing on two different pasture types. Eur. J. Lipid Sci. Technol. 2015, 117, 23–30. [Google Scholar] [CrossRef]
- Dance, L.; Matthews, K.; Doran, O. Effect of breed on fatty acid composition and stearoyl-CoA desaturase protein expression in the Semimembranosus muscle and subcutaneous adipose tissue of cattle. Livest. Sci. 2009, 125, 291–297. [Google Scholar] [CrossRef]
- Herdmann, A.; Nuernberg, K.; Martin, J.; Doran, O. Effect of dietary fatty acids on expression of lipogenic enzymes and fatty acid profile in tissues of bulls. Animal 2010, 4, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Conte, G.; Jeronimo, E.; Serra, A.; Bessa, R.J.; Mele, M. Effect of dietary polyunsaturated fatty acids on Stearoyl CoA-Desaturase gene expression in intramuscular lipids of lamb. Ital. J. Anim. Sci. 2012, 11, e79. [Google Scholar] [CrossRef]
- Gallardo, M.A.; Pulido, R.; Gallo, C. Fatty Acid Composition of Longissimus dorsi Muscle of Suffolk down Lambs Fed on Different Dryland Forages. Chil. J. Agric. Res. 2011, 71, 566–571. [Google Scholar] [CrossRef][Green Version]
- Gallardo, M.; Cárcamo, J.G.; Arias-Darraz, L.; Alvear, C. Effect of Diet and Type of Pregnancy on Transcriptional Expression of Selected Genes in Sheep Mammary Gland. Animals 2019, 9, 589. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.; Cvek, K.; Dahlborn, K. Evolution of the mammary capillary network and carbonic anhydrase activity throughout lactation and during somatotropin treatment in goats. J. Dairy Res. 2010, 77, 368–375. [Google Scholar] [CrossRef]
- Gallardo, M.A.; Noro, M.; De La Barra, R.; Pulido, R. Metabolic profile in Chilota lambs grazing Calafatal. Trop. Anim. Health Prod. 2014, 46, 685–689. [Google Scholar] [CrossRef]
- Bateman, J.V. Nutrición Animal: Manual de Métodos Analíticos. Herrero Hermanos: México D.F., Mexico, 1970; p. 468. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis, 16th ed.; AOAC: Gaithersburg, VA, USA, 1996. [Google Scholar]
- Van Soest, P.; Robertson, J.; Lewis, B. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Tilley, J.M.A.; Terry, R.A. A two stages technique for the in vitro digestion of forages crops. J. Br. Grassl. Soc. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analysis; Agriculture Handbook no. 379; ARS-USDA: Washington, DC, USA, 1970; p. 19. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pepper, M.S.; Baetens, D.; Mandriota, S.J.; Di Sanza, C.; Oikemus, S.; Lane, T.F.; Soriano, J.V.; Montesano, R.; Iruela-Arispe, M.L. Regulation of VEGF and VEGF receptor expression in the rodent mammary gland during pregnancy, lactation, and involution. Dev. Dyn. 2000, 218, 507–524. [Google Scholar] [CrossRef]
- Nielsen, M.O.; Fleet, I.R.; Jakobsen, K.; Heap, R.B. The local differential effect of prostacyclin, prostaglandin E(2) and prostaglandin-F2-alpha on mammary blood-flow of lactating goats. J. Endocrinol. 1995, 145, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, J.V.; Nielsen, M.O.; Theil, P.; Sørensen, M.T.; Safayi, S.; Sejrsen, K. Development of mammary glands of fat sheep submitted to restricted feeding during late pregnancy. Small Rumin. Res. 2008, 76, 155–165. [Google Scholar] [CrossRef]
- Caja, G.; Salama, A.; Such, X. Omitting the Dry-Off Period Negatively Affects Colostrum and Milk Yield in Dairy Goats. J. Dairy Sci. 2006, 89, 4220–4228. [Google Scholar] [CrossRef]
- Lékó, A.H.; Cservenák, M.; Dobolyi, Á. Suckling induced insulin-like growth factor-1 (IGF-1) release in mother rats. Growth Horm. IGF Res. 2017, 37, 7–12. [Google Scholar] [CrossRef]
- Laban, C.; Bustin, S.A.; Jenkins, P.J. The GH-IGF-I axis and breast cancer. Trends Endocrinol. Metab. 2003, 14, 28–34. [Google Scholar] [CrossRef]
- Perry, J.K.; Emerald, B.S.; Mertani, H.C.; Lobie, P.E. The oncogenic potential of growth hormone. Growth Horm. IGF Res. 2006, 16, 277–289. [Google Scholar] [CrossRef]
- Reed, J.C. Bcl-2 family proteins. Oncogene 1998, 17, 3225–3236. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Flemetakis, E.; Kalloniati, C.; Papadomichelakis, G.; Katinakis, P.; Zervas, G. Sheep and goats differences in CLA and fatty acids milk fat content in relation with mRNA stearoyl-CoA desaturase and lipogenic genes expression in their mammary gland. J. Dairy Res. 2009, 76, 392–401. [Google Scholar] [CrossRef]
- Singh, M.; Thomson, P.C.; Sheehy, P.A.; Raadsma, H.W. Comparative transcriptome analyses reveal conserved and distinct mechanisms in ovine and bovine lactation. Funct. Integr. Genomics 2013, 13, 115–131. [Google Scholar] [CrossRef]
- Wittwer, F. Manual de Patología Clínica Veterinaria, 2nd ed.; Imprenta América: Valdivia, Chile, 2012. [Google Scholar]
- Carcamo, J.G.; Arias-Darraz, L.; Alvear, C.; Williams, P.; Gallardo, M. Effect of diet and type of pregnancy on plasma metabolic response in sheep and its further effect on lamb performance. Trop. Anim. Health Prod. 2019, 51, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Rios, C.; Marín, M.P.; Catafau, M.; Wittwer, F. Concentraciones sanguíneas de ß-hidroxibutirato, NEFA, colesterol y urea en cabras lecheras de tres rebaños con sistemas intensivos de producción y su relación con el balance nutricional. Arch. Med. Vet. 2006, 38, 19–23. [Google Scholar] [CrossRef]
- Kenyon, P.R.; Hickson, R.E.; Hutton, P.G.; Morris, S.T.; Stafford, K.J.; West, D.M. Effect of twin-bearing ewe body condition score and late pregnancy nutrition on lamb performance. Anim. Prod. Sci. 2012, 52, 483–490. [Google Scholar] [CrossRef]
- Moallem, U.; Rozov, A.; Gootwine, E.; Honig, H. Plasma concentrations of key metabolites and insulin in late-pregnant ewes carrying 1 to 5 fetuses. J. Anim. Sci. 2012, 90, 318–324. [Google Scholar] [CrossRef] [PubMed]

| Gene and Accession | Forward Primer Sequence | Amplicon |
|---|---|---|
| Angiogenesis | Reverse primer sequence | Length |
| CAIV | F: AGCGCTTTGCCATGGAGATACA | 148 |
| XM_012186664.1 | R: AGGGGCTGGAAGTTCACATTCTTG | |
| VEGF | F: TGCTCTACCTTCACCATGCCAA | 101 |
| NM_001025110.1 | R: GCGCTGGTAGACATCCATGAACTT | |
| VEGFR1 (FTL1) | F: AGGTGACCTGCTTCAAGCCAAT | 106 |
| XM_015098156.1 | R: GAAGGCAGGTGTCGAGTACGTAAA | |
| VEGFR2 (KDR) | F: AAGACGCTGACTTGCTTTGGGA | 150 |
| NM_001278565.1 | R: AAATGGGAAGAGCACGCAACCT | |
| ANGPT1 | F: GCACCCTCATGCATTCTTGTCA | 140 |
| XM_004011787.3 | R: ACCCTTTCCTCTACCCTATCTGCT | |
| ANGPT2 | F: GAGACCTGCTCCCAAAGCAGTAAA | 145 |
| XM_004021671.3 | R: TCACTGAGTGATGCGGGTTCAA | |
| MKI67 | F: TGCAGACTTTGGCACAAACGAC | 143 |
| XM_015103501.1 | R: AGTTTTAGCAGGACGCCTGGAA | |
| TBXAS1 | F: CATCTTCCTCATTGCTGGCTACGA | 143 |
| XM_012177234.2 | R: AGTACTCAGGGGCTGGATGTTTCT | |
| Cell turnover | ||
| LALBA | F: TGCCACCCAGGCTGAACAATTA | 106 |
| NM_001009797.1 | R: AAATGCGGTACAGACCCATTCAGG | |
| BAX | F: CTAAGACCTGGTGTAGCCAAGCAA | 103 |
| XM_015100639.1 | R: TCGAACCCATGTTCCCTGCATT | |
| BCL2 | F: ATGCGGCCCCTGTTTGATTTCT | 112 |
| XM_012103831.2 | R: GTGGACTTCACTTATGGCCCAGAT | |
| CCND1 | F: ACGACTTCATCGAGCACTTCCTCT | 127 |
| XM_015102997.1 | R: GGTGGGTTGGAAATGAACTTCACG | |
| IGF1 | F: CCAGACTTTGCACTTCAGAAGC | 106 |
| XM_012159642.2 | R: GATGTGACTGGCATCTTCACCT | |
| IGF1R | F: CGAGATCCTGTACATTCGCACCAA | 100 |
| XM_012098367.2 | R: GTTCCACTTCACGATCAGCTGAGA | |
| IGFBP1 | F: CAGCGATGAGGCCACAGATACAAA | 117 |
| NM_001145177.1 | R: CTGGACTCGGTCATCAAGTGGAAA | |
| IGFBP3 | F: AGGTTGACTACGAGTCTCAGAGCA | 122 |
| NM_001159276.1 | R: CAGGAACTTGAGGTCGTTCAGTGT | |
| IGFBP5 | F: TGCGTGGACAAGTATGGGATGAAG | 103 |
| NM_001129733.1 | R: AGGGGACGCATCACTCAACATT | |
| LPT | F: ATCCCACTCACCAGCATGCAAA | 145 |
| XM_004008038.3 | R: CTACCAAGTGCAAGCACAGTTAGC | |
| LPTR | F: TTGGATGGCCTAGGAATCTGGAGT | 105 |
| NM_001009763.1 | R: GTTAGACCCAACCGCTGTCAGAAT | |
| LTF | F: GGTTATTCTGGTGCCTTCAAGTGC | 119 |
| NM_001024862.1 | R: AGAAGCTCATACTGGTCCCTGTCA | |
| CYP19A | F: AACACGTCCACATAGCCCAAGT | 80 |
| NM_001123000.1 | R: ACCATCTGTGCTGATTCCATCACC | |
| TGFB1 | F: GCACGTGGAGCTGTACCAGAAATA | 116 |
| NM_001009400.1 | R: GCACAACTCCAGTGACGTCAAA | |
| TGFB1R1 | F: CCAAGGAAAACCAGCCATAGCTCA | 118 |
| XM_012120354.2 | R: TGTGGCCGAATCATGCCTTACT | |
| TGFB1R2 | F: CCTTACAAAGCATGTGGGCTTGAC | 132 |
| XM_012099307.2 | R: CCTGCACTGTAGGCGGATTCTTTA | |
| ACTIN | F: TGAAGTGTGACGTGGACATCCGTA | 108 |
| NM_001009784.1 | R: AGGTGATCTCCTTCTGCATCCTGT |
| Pastures | DM 6 | TA 7 | CF 8 | CP 9 | ME 10 | NDF 11 |
|---|---|---|---|---|---|---|
| NPH 1 | 87.68 ± 0.98 | 5.78 ± 0.30 | 1.75 ± 0.09 | 6.83 ± 0.10 | 1.90 ± 0.13 | 65.89 ± 2.20 |
| NP (at): | ||||||
| Birth 2 | 20.98 d ±0.03 | 8.78 c ± 0.05 | 2.56 c ± 0.01 | 27.91 a ± 9.31 | 3.07 a ± 0.02 | 46.88 c ± 0.01 |
| 35d a B. 3 | 20.28 e ± 0.03 | 8.66 c ± 0.09 | 2.52 c ± 0.02 | 22.23 b ± 0.06 | 3.07 a ± 0.02 | 45.71 e ± 0.02 |
| 70d (weaning) | 21.35 c ± 0.05 | 7.88 d ± 0.01 | 2.49 d ± 0.04 | 17.84 d ± 0.03 | 2.56 c ± 0.01 | 59.17 a ± 0.02 |
| 30d a. W. 4 | 28.10 b± 0.03 | 9.50 b ± 0.02 | 3.06 b ± 0.01 | 21.57 c ± 0.02 | 2.77 b ± 0.02 | 46.46 d ± 0.03 |
| 60d a. W. 5 | 28.25 a ± 0.02 | 9.95 a ± 0.01 | 4.26 a ± 0.01 | 15.48 e ± 0.04 | 2.55 c ± 0.01 | 55.17 b ± 0.02 |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallardo Paffetti, M.; Cárcamo, J.; Arias-Darraz, L.; Alvear, C.; Ojeda, J. Effect of Type of Pregnancy on Transcriptional and Plasma Metabolic Response in Sheep and Its Further Effect on Progeny Lambs. Animals 2020, 10, 2290. https://doi.org/10.3390/ani10122290
Gallardo Paffetti M, Cárcamo J, Arias-Darraz L, Alvear C, Ojeda J. Effect of Type of Pregnancy on Transcriptional and Plasma Metabolic Response in Sheep and Its Further Effect on Progeny Lambs. Animals. 2020; 10(12):2290. https://doi.org/10.3390/ani10122290
Chicago/Turabian StyleGallardo Paffetti, María, Juan Cárcamo, Luis Arias-Darraz, Carlos Alvear, and Javier Ojeda. 2020. "Effect of Type of Pregnancy on Transcriptional and Plasma Metabolic Response in Sheep and Its Further Effect on Progeny Lambs" Animals 10, no. 12: 2290. https://doi.org/10.3390/ani10122290
APA StyleGallardo Paffetti, M., Cárcamo, J., Arias-Darraz, L., Alvear, C., & Ojeda, J. (2020). Effect of Type of Pregnancy on Transcriptional and Plasma Metabolic Response in Sheep and Its Further Effect on Progeny Lambs. Animals, 10(12), 2290. https://doi.org/10.3390/ani10122290





