Genome-Wide Association Analysis Identified BMPR1A as a Novel Candidate Gene Affecting the Number of Thoracic Vertebrae in a Large White × Minzhu Intercross Pig Population
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement/Sample Description
2.2. Phenotypic Data, Heritability, and Genetic Correlation between the NTV and NV
2.3. Genotyping for VRTN g.19034 A>C 37, VRTN g.20311_20312ins291, LTBP2 c.4481A>C, and Missense or Splice Variants on SSC7 and 147 SNPs on SSC1
2.4. Genome-Wide Association Study Based on the SNPs and Variants That Merged with Porcine SNP60K Genotyping BeadChip Assays
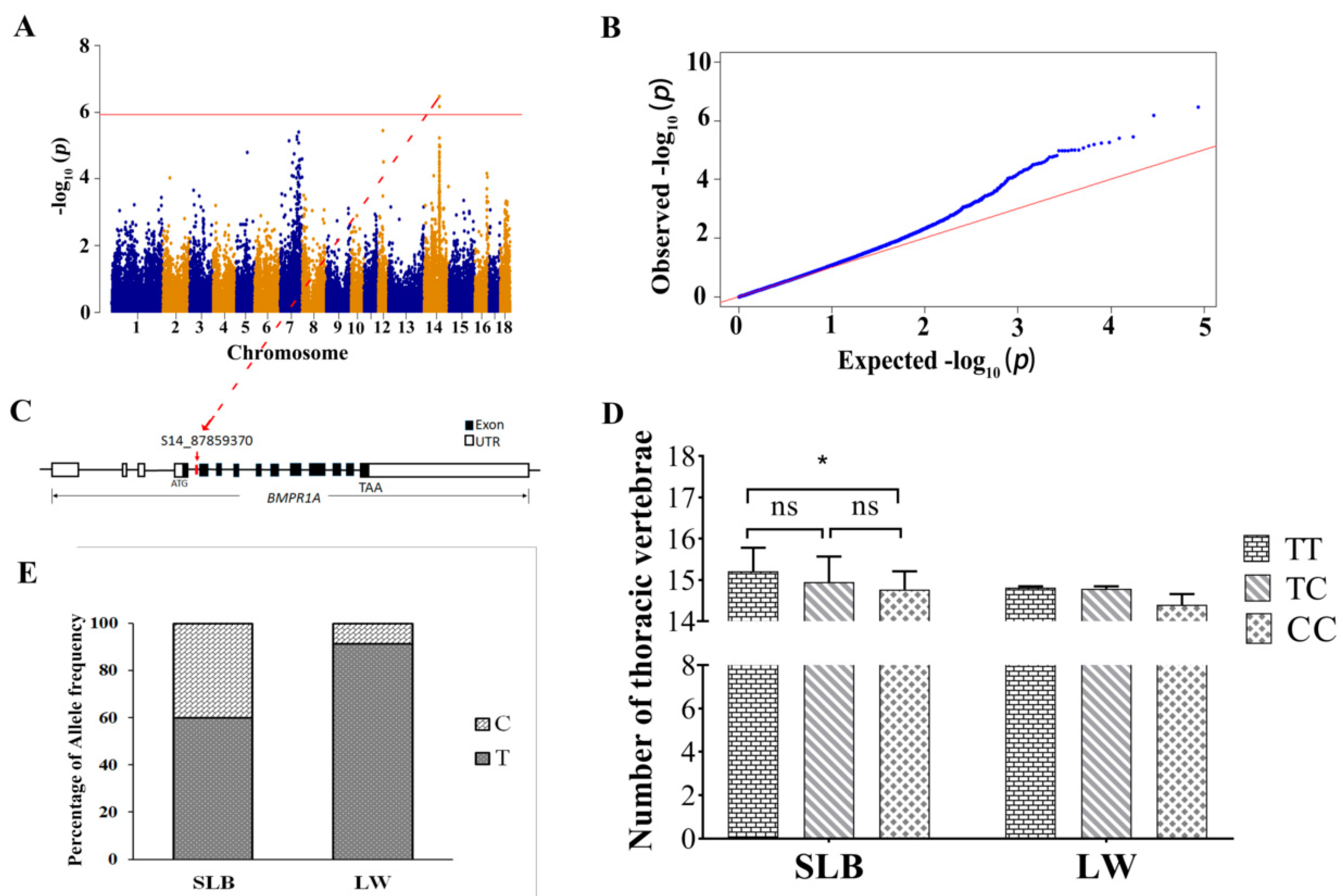
2.5. Identification of the Effect of S14_87859370 on the NTV in Large White and Songliao Black Pigs
2.6. Linkage Disequilibrium Analysis
3. Results
3.1. Phenotypes and Genetic Parameters Pertaining to the NTV and NV
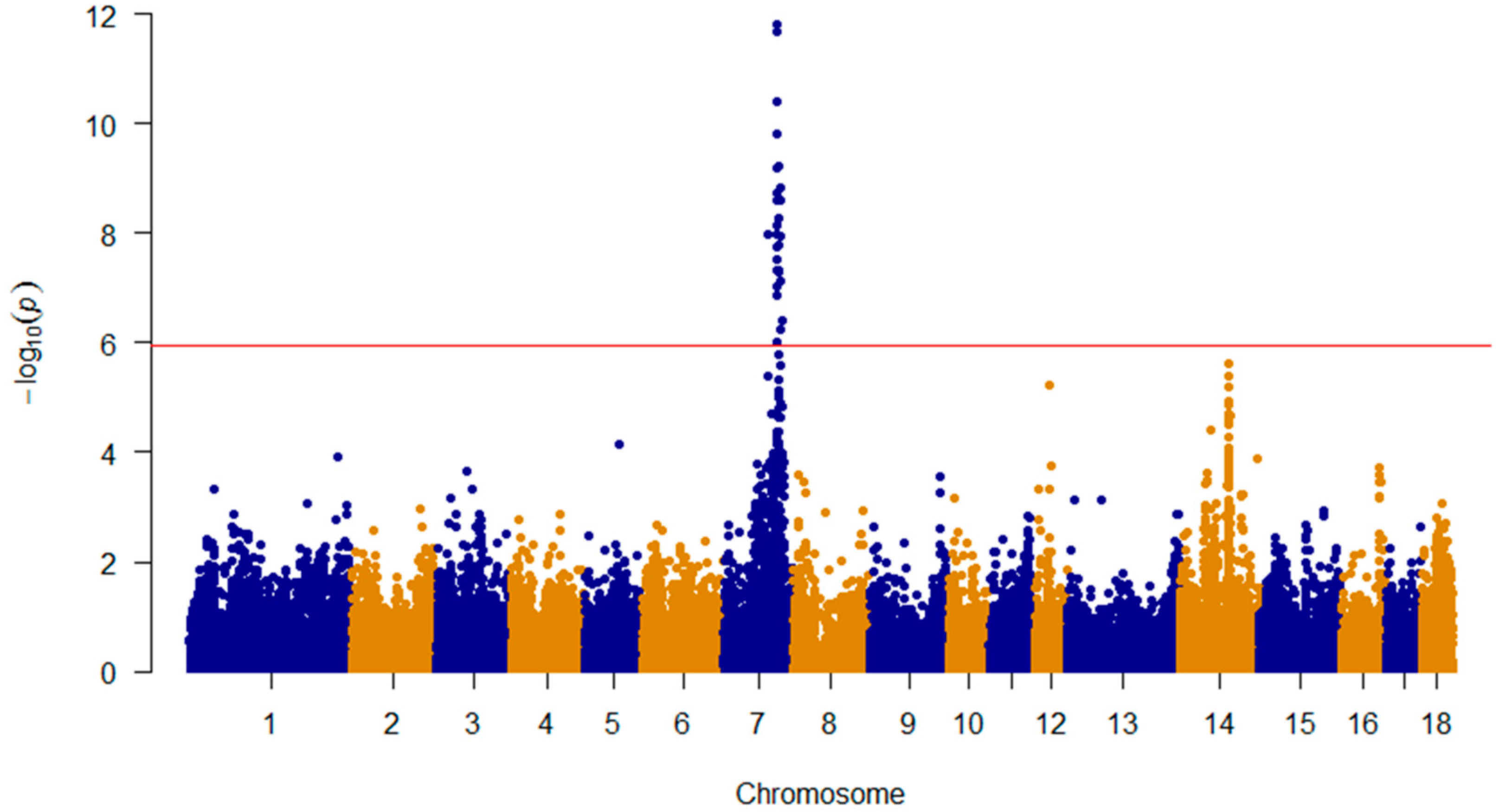
3.2. GWAS with the NTV by Using the Merged SNP Data from 542 F2 Animals Revealed Significant Variants
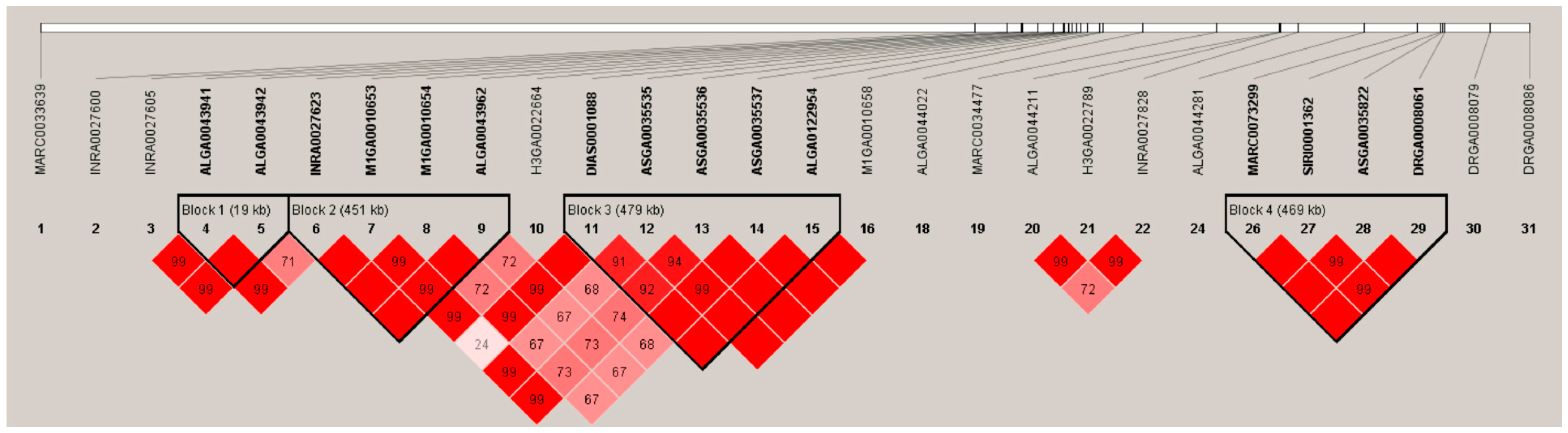
3.3. Linkage Disequilibrium Analysis Suggested Candidate Genes Are Contained in a 479-Kb Region on SSC7
3.4. Conditional GWAS and Haplotype Block Analysis Revealed BMPR1A as a Novel Candidate Gene Affecting the NTV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galis, F. Why do almost all mammals have seven cervical vertebrae? Developmental constraints, Hox genes, and cancer. Exp. Zool. 1999, 285, 19–26. [Google Scholar] [CrossRef]
- Narita, Y.; Kuratani, S. Evolution of the vertebral formulae in mammals; a perspective on developmental constraints. Exp. Zool. B Mol. Dev. Evol. 2005, 304, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Li, B.D.; Chen, X.H. Pig Breeds in China; Shanghai Scientific and Technical Publisher: Shanghai, China, 1986. [Google Scholar]
- King, J.W.B.; Roberts, R.C. Carcass length in the bacon pig: Its association with vertebrae numbers and prediction from radiographs of the young pig. Anim. Prod. Sci. 1960, 2, 59–65. [Google Scholar] [CrossRef]
- Van Son, M.; Lopes, M.S.; Martell, H.J.; Derks, M.F.L.; Gangsei, L.E.; Kongsro, J.; Wass, M.N.; Grindflek, E.H.; Harlizius, B. A QTL for number of teats shows breed specific effects on number of vertebrae in pigs: Bridging the gap between molecular and quantitative genetics. Front. Genet. 2019, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, M.; Yuan, J.; Zhou, X.; Xu, S.; Liu, B. Association of polymorphisms in NR6A1, PLAG1 and VRTN with the number of vertebrae in Chinese Tongcheng × Large White crossbred pigs. Anim. Genet. 2018, 49, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiong, Y.; Zuo, B.; Lei, M.; Jiang, S.; Li, F.; Zheng, R.; Li, J.; Xu, D. Detection of quantitative trait loci associated with several internal organ traits and teat number trait in a pig population. Genet. Genomics 2007, 34, 307–314. [Google Scholar] [CrossRef]
- Edwards, D.B.; Ernst, C.W.; Raney, N.E.; Doumit, M.E.; Hoge, M.D.; Bates, R.O. Quantitative trait locus mapping in an F2 Duroc × Pietrain resource population: II. Carcass and meat quality traits. Anim. Sci. 2008, 86, 254–266. [Google Scholar] [CrossRef]
- Choi, I.; Steibel, J.P.; Bates, R.O.; Raney, N.E.; Rumph, J.M.; Ernst, C.W. Identification of carcass and meat quality QTL in an F(2) Duroc × Pietrain pig resource population using different least-squares analysis models. Front. Genet. 2011, 2, 18. [Google Scholar] [CrossRef]
- Casiró, S.; Velez-Irizarry, D.; Ernst, C.W.; Raney, N.E.; Bates, R.O.; Charles, M.G.; Steibel, J.P. Genome-wide association study in an F(2) Duroc × Pietrain resource population for economically important meat quality and carcass traits. Anim. Sci. 2017, 95, 545–558. [Google Scholar] [CrossRef]
- Velez-Irizarry, D.; Casiro, S.; Daza, K.R.; Bates, R.O.; Raney, N.E.; Steibel, J.P.; Ernst, C.W. Genetic control of longissimus dorsi muscle gene expression variation and joint analysis with phenotypic quantitative trait loci in pigs. BMC Genomics 2019, 20, 3. [Google Scholar] [CrossRef]
- Harmegnies, N.; Davin, F.; De Smet, S.; Buys, N.; Georges, M.; Coppieters, W. Results of a whole-genome quantitative trait locus scan for growth, carcass composition and meat quality in a porcine four-way cross. Anim. Genet. 2006, 37, 543–553. [Google Scholar] [CrossRef]
- Mikawa, S.; Sato, S.; Nii, M.; Morozumi, T.; Yoshioka, G.; Imaeda, N.; Yamaguchi, T.; Awata, T. Identification of a second gene associated with variation in vertebral number in domestic pigs. BMC Genet. 2011, 12, 5. [Google Scholar] [CrossRef]
- Yang, J.; Huang, L.; Yang, M.; Fan, Y.; Li, L.; Fang, S.; Deng, W.; Cui, L.; Zhang, Z.; Ai, H.; et al. Possible introgression of the VRTN mutation increasing vertebral number, carcass length and teat number from Chinese pigs into European pigs. Sci. Rep. 2016, 6, 19240. [Google Scholar] [CrossRef]
- Park, H.B.; Han, S.H.; Lee, J.B.; Cho, I.C. Rapid Communication: High-resolution quantitative trait loci analysis identifies LTBP2 encoding latent transforming growth factor beta binding protein 2 associated with thoracic vertebrae number in a large F2 intercross between Landrace and Korean native pigs. Anim. Sci. 2017, 95, 1957–1962. [Google Scholar]
- Zhang, L.C.; Yue, J.W.; Pu, L.; Wang, L.G.; Liu, X.; Liang, J.; Yan, H.; Zhao, K.B.; Li, N.; Shi, H.B.; et al. Genome-wide study refines the quantitative trait locus for number of ribs in a Large White × Minzhu intercross pig population and reveals a new candidate gene. Mol. Genet. Genom. 2016, 291, 1885–1890. [Google Scholar] [CrossRef]
- Fan, Y.; Xing, Y.; Zhang, Z.; Ai, H.; Ouyang, Z.; Ouyang, J.; Yang, M.; Li, P.; Chen, Y.; Gao, J.; et al. A further look at porcine chromosome 7 reveals VRTN variants associated with vertebral number in Chinese and Western pigs. PLoS ONE 2013, 8, e62534. [Google Scholar] [CrossRef]
- Gilbert, S.F. Developmental Biology, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Borchers, N.N.; Reinsch, N.; Kalm, E. The number of ribs and vertebrae in a Piétrain cross: Variation, heritability and effects on performance traits. Anim. Breed. Genet. 2004, 121, 392–403. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Liang, J.; Yan, H.; Zhao, K.; Li, N.; Pu, L.; Shi, H.; Zhang, Y.; Wang, L.; et al. Quantitative trait loci for the number of vertebrae on Sus scrofa chromosomes 1 and 7 independently influence the numbers of thoracic and lumbar vertebrae in pigs. J. Integrat. Agri. 2015, 14, 2027–2033. [Google Scholar] [CrossRef]
- Madsen, P.; Jensen, J. DMU: A User’s Guide. A Package for Analysing Ultivariate Mixed 253 Models. 2007, Version 6, Release 4.7. Available online: http://dmu.agrsci.dk/dmuv6_guide-R4-6-7.Pdf (accessed on 1 December 2014).
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Endelman, J.B.; Jannink, J.L. Shrinkage estimation of the realized relationship matrix. G3 (Bethesda) 2012, 2, 1405–1413. [Google Scholar] [CrossRef]
- Ren, D.R.; Ren, J.; Ruan, G.F.; Guo, Y.M.; Wu, L.H.; Yang, G.C.; Zhou, L.H.; Li, L.; Zhang, Z.Y.; Huang, L.S. Mapping and fine mapping of quantitative trait loci for the number of vertebrae in a White Duroc × Chinese Erhualian intercross resource population. Anim. Genet. 2012, 43, 545–551. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, H.; Zhang, Z.; Gao, J.; Yang, J.; Wu, Z.; Fan, Y.; Xing, Y.; Li, L.; Xiao, S.; et al. VRTN is required for the development of thoracic vertebrae in mammals. Int. J. Biol. Sci. 2018, 14, 667–681. [Google Scholar] [CrossRef]
- Guerreiro, I.; Casaca, A.; Nunes, A.; Monteiro, S.; Nóvoa, A.; Ferreira, R.B.; Bom, J.; Mallo, M. Regulatory role for a conserved motif adjacent to the homeodomain of Hox10 proteins. Development 2012, 139, 2703–2710. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, N. A 10-gene expression signature of Notch pathway predicts recurrence in ovarian carcinoma. Oncol. Lett. 2015, 10, 1704–1708. [Google Scholar] [CrossRef]
- Portanova, P.; Notaro, A.; Pellerito, O.; Sabella, S.; Giuliano, M.; Calvaruso, G. Notch inhibition restores TRAIL-mediated apoptosis via AP1-dependent upregulation of DR4 and DR5 TRAIL receptors in MDA-MB-231 breast cancer cells. Int. J. Oncol. 2013, 43, 121–130. [Google Scholar] [CrossRef][Green Version]
- Liao, B.K.; Oates, A.C. Delta-Notch signalling in segmentation. Arthropod Struct. Dev. 2017, 46, 429–447. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Ovitt, C.; Grigoriadis, A.E.; Möhle-Steinlein, U.; Rüther, U.; Wagner, E.F. Bone and haematopoietic defects in mice lacking c-fos. Nature 1992, 360, 741–745. [Google Scholar] [CrossRef]
- Jing, J.; Hinton, R.J.; Feng, J.Q. Bmpr1a signaling in cartilage development and endochondral bone formation. Vitam. Horm. 2015, 99, 273–291. [Google Scholar]
- Mishina, Y.; Suzuki, A.; Ueno, N.; Behringer, R.R. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes. Dev. 1995, 9, 3027–3037. [Google Scholar] [CrossRef]
- Danesh, S.M.; Villasenor, A.; Chong, D.; Soukup, C.; Cleaver, O. BMP and BMP receptor expression during murine organogenesis. Gene Expr. Patterns 2009, 9, 255–265. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef]
- Kishigami, S.; Mishina, Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005, 16, 265–278. [Google Scholar] [CrossRef]
- Miyazono, K. Signal transduction by bone morphogenetic protein receptors: Functional roles of Smad proteins. Bone 1999, 25, 91–93. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Otsuka, F.; Hino, J.; Miyoshi, T.; Takano, M.; Miyazato, M.; Makino, H.; Kangawa, K. Bone morphogenetic protein-3b (BMP-3b) inhibits osteoblast differentiation via Smad2/3 pathway by counteracting Smad1/5/8 signaling. Mol. Cell. Endocrinol. 2012, 350, 78–86. [Google Scholar] [CrossRef]
- Li, X.; Nie, S.; Chang, C.; Qiu, T.; Cao, X. Smads oppose Hox transcriptional activities. Exp. Cell Res. 2006, 312, 854–864. [Google Scholar] [CrossRef]
- Wellik, D.M. Hox genes and vertebrate axial pattern. Curr. Top. Dev. Biol. 2009, 88, 257–278. [Google Scholar]
- Alexander, T.; Nolte, C.; Krumlauf, R. Hox genes and segmentation of the hindbrain and axial skeleton. Annu. Rev. Cell Dev. Biol. 2009, 25, 431–456. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, J.; Zhou, W.; Li, H.; Zhou, R.; Zhang, L.; Zhao, H.; Cao, J.; Zhu, X.; Hu, H.; et al. Ndrg2 regulates vertebral specification in differentiating somites. Dev. Biol. 2012, 369, 308–318. [Google Scholar] [CrossRef][Green Version]
- Nifuji, A.; Kellermann, O.; Kuboki, Y.; Wozney, J.M.; Noda, M. Perturbation of BMP signaling in somitogenesis resulted in vertebral and rib malformations in the axial skeletal formation. J. Bone Miner. Res. 1997, 12, 332–342. [Google Scholar] [CrossRef]
| Traits | NTV | |||
|---|---|---|---|---|
| 14 | 15 | 16 | ||
| NV | 20 | 146 | 88 | 0 |
| 21 | 47 | 209 | 19 | |
| 22 | 0 | 11 | 22 | |
| Marker | Chr 2 | Position 3 | Rs 4 | p-Value | Add_p 5 | Dom_p 6 | Nearest Gene | Var (%) 7 |
|---|---|---|---|---|---|---|---|---|
| MARC0033639 | 7 | 80264245 | rs80875505 | 1.06 × 10−8 | 2.00 × 10−12 | 1.02 × 10−2 | SLC12A6 | 5.18 |
| INRA0027600 | 7 | 96435072 | rs333341186 | 1.33 × 10−7 | 2.38 × 10−12 | 9.66 × 10−3 | RBM25 | 4.44 |
| INRA0027605 | 7 | 96994146 | rs337851273 | 9.12 × 10−8 | 4.69 × 10−12 | 3.78 × 10−2 | ENSSSCG00000002348 | 4.55 |
| ALGA0043941 | 7 | 97247184 | rs81396045 | 2.56 × 10−9 | 2.05 × 10−11 | 7.49 × 10−2 | FAM161B | 5.59 |
| ALGA0043942 | 7 | 97266221 | rs80856304 | 4.84 × 10−8 | 7.41 × 10−11 | 6.97 × 10−3 | COQ6 | 4.74 |
| INRA0027623 | 7 | 97521999 | rs321816080 | 1.03 × 10−8 | 3.53 × 10−10 | 1.14 × 10−2 | VSX2 | 5.18 |
| M1GA0010653 | 7 | 97795697 | rs81396078 | 1.72 × 10−8 | 4.32 × 10−10 | 8.41 × 10−4 | LTBP2 | 5.037 |
| M1GA0010654 | 7 | 97954258 | rs80864705 | 1.87 × 10−9 | 2.37 × 10−10 | 8.79 × 10−2 | FCF1 | 5.68 |
| ALGA0043962 | 7 | 97973860 | rs80929215 | 1.87 × 10−9 | 2.37 × 10−10 | 8.79 × 10−2 | YLPM1 | 5.68 |
| H3GA0022664 | 7 | 98066911 | rs80813473 | 3.11 × 10−8 | 8.24 × 10−10 | 1.09 × 10−3 | PROX2 | 4.863 |
| DIAS0001088 | 7 | 98116120 | rs336641062 | 6.52 × 10−10 | 3.30 × 10−10 | 1.11 × 10−1 | RPS6KL1 | 5.99 |
| ASGA0035535 | 7 | 98186259 | rs80963494 | 1.49 × 10−10 | 4.32 × 10−9 | 1.45 × 10−2 | EIF2B2 | 7.01 |
| ASGA0035536 | 7 | 98264173 | rs80846252 | 3.92 × 10−11 | 9.72 × 10−10 | 3.34 × 10−2 | ACYP1 | 6.91 |
| ASGA0035537 | 7 | 98374939 | rs80854726 | 7.21 × 10−9 | 4.42 × 10−9 | 1.97 × 10−1 | TMED10 | 5.30 |
| ALGA0122954 | 7 | 98595714 | rs81317665 | 2.03 × 10−12 | 2.17 × 10−9 | 7.21 × 10−4 | JDP2 | 7.74 |
| M1GA0010658 | 7 | 98648325 | rs80804788 | 1.55 × 10−12 | 9.66 × 10−9 | 2.23 × 10−2 | PGF | 7.80 |
| ALGA0108658 | 7 | 98648325 | rs81336593 | 9.67 × 10−7 | 4.26 × 10−9 | 1.22 × 10−2 | JDP2 | 3.88 |
| ALGA0044022 | 7 | 99337831 | rs80919617 | 5.78 × 10−10 | 2.22 × 10−9 | 4.40 × 10−3 | GPATCH2L | 6.04 |
| MARC0034477 | 7 | 100621452 | rs80954820 | 4.91 × 10−8 | 2.16 × 10−6 | 2.02 × 10−1 | SPTLC2 | 4.74 |
| ALGA0044211 | 7 | 101703098 | rs80950372 | 5.03 × 10−8 | 1.01 × 10−8 | 3.80 × 10−3 | NRXN3 | 4.72 |
| H3GA0022789 | 7 | 101728012 | rs80933409 | 5.52 × 10−9 | 1.03 × 10−8 | 6.25 × 10−2 | NRXN3 | 5.37 |
| INRA0027828 | 7 | 102031355 | rs323989598 | 1.60 × 10−8 | 7.11 × 10−9 | 2.05 × 10−2 | NA | 5.06 |
| ASGA0035786 | 7 | 103002983 | rs80802872 | 2.46 × 10−9 | 1.71 × 10−8 | 6.72 × 10−3 | DIO2 | 5.63 |
| ALGA0044281 | 7 | 103164950 | rs80808662 | 1.17 × 10−8 | 1.15 × 10−8 | 8.36 × 10−3 | NA | 5.15 |
| H3GA0022821 | 7 | 103189827 | rs80970878 | 1.45 × 10−9 | 1.17 × 10−8 | 8.56 × 10−3 | DIO2 | 5.76 |
| MARC0073299 | 7 | 104087830 | rs80786139 | 1.15 × 10−8 | 1.17 × 10−8 | 8.56 × 10−3 | STON2 | 5.15 |
| SIRI0001362 | 7 | 104480447 | rs320949387 | 7.60 × 10−8 | 7.80 × 10−7 | 7.64 × 10−2 | ENSSSCG00000049060 | 4.60 |
| ASGA0035822 | 7 | 104525604 | rs80936448 | 7.68 × 10−8 | 4.22 × 10−7 | 1.40 × 10−1 | ENSSSCG00000049060 | 4.60 |
| DRGA0008061 | 7 | 104557781 | rs80813073 | 7.68 × 10−8 | 9.73 × 10−6 | 4.92 × 10−1 | ENSSSCG00000049060 | 4.60 |
| DRGA0008079 | 7 | 105341213 | rs80962000 | 5.53 × 10−7 | 8.99 × 10−8 | 1.66 × 10−2 | ENSSSCG00000047467 | 4.03 |
| DRGA0008086 | 7 | 106025579 | rs81295294 | 3.95 × 10−7 | 3.22 × 10−7 | 1.99 × 10−1 | ENSSSCG00000045920 | 4.13 |
| Marker | Chr 1 | Position 2 | Rs 3 | Var% 4 | p-Value |
|---|---|---|---|---|---|
| S7_97537758 | 7 | 97537758 | rs336742966 | 0.67 | 8.99 × 10−2 |
| VRTN g.19034 A > C | 7 | 97614602 | rs709317845 | 2.99 | 2.41 × 10−5 |
| S7_97622681 | 7 | 97622681 | rs787326242 | 1.31 | 1.01 × 10−2 |
| S7_97623045 | 7 | 97623045 | rs1108261998 | 1.42 | 6.57 × 10−3 |
| S7_97662010 | 7 | 97662010 | rs696186042 | 0.52 | 1.58 × 10−1 |
| S7_97662082 | 7 | 97662082 | rs345827854 | 2.02 | 9.68 × 10−4 |
| S7_97662535 | 7 | 97662535 | rs332888554 | 1.19 | 1.51 × 10−2 |
| S7_97750084 | 7 | 97750084 | rs322330509 | 2.91 | 3.91 × 10−5 |
| LTBP2 c.4481 A > C | 7 | 97751432 | rs322260921 | 2.95 | 2.83 × 10−5 |
| S7_97765472 | 7 | 97765472 | rs339379718 | 1.6 | 3.58 × 10−3 |
| S7_97771260 | 7 | 97771260 | rs337082599 | 1.69 | 2.92 × 10−3 |
| S7_97775923 | 7 | 97775923 | rs335686067 | 0.24 | 4.45 × 10−1 |
| S7_97777490 | 7 | 97777490 | rs331228271 | 0.49 | 1.88 × 10−1 |
| S7_97895559 | 7 | 97895559 | rs341911129 | 1.49 | 5.30 × 10−3 |
| S7_97899571 | 7 | 97899571 | rs325918746 | 1.21 | 1.24 × 10−2 |
| S7_97901617 | 7 | 97901617 | rs322374710 | 2.63 | 8.55 × 10−5 |
| S7_97901619 | 7 | 97901619 | rs331788516 | 2.63 | 8.55 × 10−5 |
| S7_98073512 | 7 | 98073512 | rs80930259 | 2.78 | 5.24 × 10−5 |
| S7_98073927 | 7 | 98073927 | rs329005836 | 0.97 | 3.36 × 10−2 |
| S7_98074140 | 7 | 98074140 | rs339766519 | 1.5 | 4.63 × 10−3 |
| S7_98074438 | 7 | 98074438 | rs322346679 | 2.73 | 8.41 × 10−5 |
| S7_98116877 | 7 | 98116877 | rs344681928 | 0.18 | 5.21 × 10−1 |
| S7_98130124 | 7 | 98130124 | rs323664885 | 0.03 | 9.07 × 10−1 |
| S7_98203930 | 7 | 98203930 | rs787271115 | 0.36 | 2.77 × 10−1 |
| S7_98219169 | 7 | 98219169 | rs323701300 | 0.1 | 7.04 × 10−1 |
| S7_98219967 | 7 | 98219967 | rs344167352 | 0.29 | 3.52 × 10−1 |
| S7_98242037 | 7 | 98242037 | rs338693270 | 0.11 | 6.67 × 10−1 |
| S7_98242461 | 7 | 98242461 | rs694346166 | 0.56 | 1.29 × 10−1 |
| S7_98242725 | 7 | 98242725 | rs340407061 | 0.92 | 3.68 × 10−2 |
| S7_98243724 | 7 | 98243724 | rs713439416 | 1.08 | 2.03 × 10−2 |
| S7_98244079 | 7 | 98244079 | rs333141847 | 0.19 | 5.08 × 10−1 |
| S7_98266495 | 7 | 98266495 | rs329334983 | 1.98 | 1.57 × 10−4 |
| S7_98266534 | 7 | 98266534 | rs324580288 | 1.76 | 3.68 × 10−4 |
| S7_98266749 | 7 | 98266749 | rs342214814 | 1.58 | 3.35 × 10−3 |
| S7_98266963 | 7 | 98266963 | rs693150674 | 1.72 | 1.98 × 10−3 |
| S7_98279107 | 7 | 98279107 | rs323090151 | 1.84 | 1.36 × 10−3 |
| S7_98300295 | 7 | 98300295 | rs341533265 | 0.96 | 3.05 × 10−2 |
| S7_98451235 | 7 | 98451235 | rs319445329 | 0.28 | 3.68 × 10−1 |
| S7_98451601 | 7 | 98451601 | rs80846787 | 0.43 | 2.25 × 10−1 |
| Marker | Chr 2 | Position 3 | p-Value | Add_p 4 | Dom_p 5 | Nearest Gene | Position in Gene | Var (%) 6 |
|---|---|---|---|---|---|---|---|---|
| S14_87859370 | 14 | 87859370 | 3.40 × 10−7 | 2.38 × 10−3 | 6.71 × 10−8 | BMPR1A | Intron | 3.86 |
| S14_87859377 | 14 | 87859377 | 6.79 × 10−7 | 2.28 × 10−3 | 1.51 × 10−7 | BMPR1A | Intron | 3.65 |
| Breed | Number | Genotype | Number of Thoracic Vertebrae 2 |
|---|---|---|---|
| Songliao Black | 39 | TT | 15.21 ± 0.57 a |
| 77 | TC | 14.95 ± 0.62 ab | |
| 13 | CC | 14.77 ± 0.44 b | |
| Large White | 179 | TT | 14.82 ± 0.03 |
| 33 | TC | 14.79 ± 0.06 | |
| 2 | CC | 14.40 ± 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Yue, J.; Niu, N.; Liu, X.; Yan, H.; Zhao, F.; Hou, X.; Gao, H.; Shi, L.; Wang, L.; et al. Genome-Wide Association Analysis Identified BMPR1A as a Novel Candidate Gene Affecting the Number of Thoracic Vertebrae in a Large White × Minzhu Intercross Pig Population. Animals 2020, 10, 2186. https://doi.org/10.3390/ani10112186
Liu Q, Yue J, Niu N, Liu X, Yan H, Zhao F, Hou X, Gao H, Shi L, Wang L, et al. Genome-Wide Association Analysis Identified BMPR1A as a Novel Candidate Gene Affecting the Number of Thoracic Vertebrae in a Large White × Minzhu Intercross Pig Population. Animals. 2020; 10(11):2186. https://doi.org/10.3390/ani10112186
Chicago/Turabian StyleLiu, Qian, Jingwei Yue, Naiqi Niu, Xin Liu, Hua Yan, Fuping Zhao, Xinhua Hou, Hongmei Gao, Lijun Shi, Lixian Wang, and et al. 2020. "Genome-Wide Association Analysis Identified BMPR1A as a Novel Candidate Gene Affecting the Number of Thoracic Vertebrae in a Large White × Minzhu Intercross Pig Population" Animals 10, no. 11: 2186. https://doi.org/10.3390/ani10112186
APA StyleLiu, Q., Yue, J., Niu, N., Liu, X., Yan, H., Zhao, F., Hou, X., Gao, H., Shi, L., Wang, L., Wang, L., & Zhang, L. (2020). Genome-Wide Association Analysis Identified BMPR1A as a Novel Candidate Gene Affecting the Number of Thoracic Vertebrae in a Large White × Minzhu Intercross Pig Population. Animals, 10(11), 2186. https://doi.org/10.3390/ani10112186






