Effects of Roughage Quality and Particle Size on Rumen Parameters and Fatty Acid Profiles of Longissimus Dorsi Fat of Lambs Fed Complete Feed
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals, Diets, and Management
2.2. Slaughtering and Sample Collection
2.3. Fatty Acid Composition Analysis of Lipid Deposits
2.4. Statistical Analysis
3. Results
3.1. Growth and Feed Efficiency of the Growing Lambs
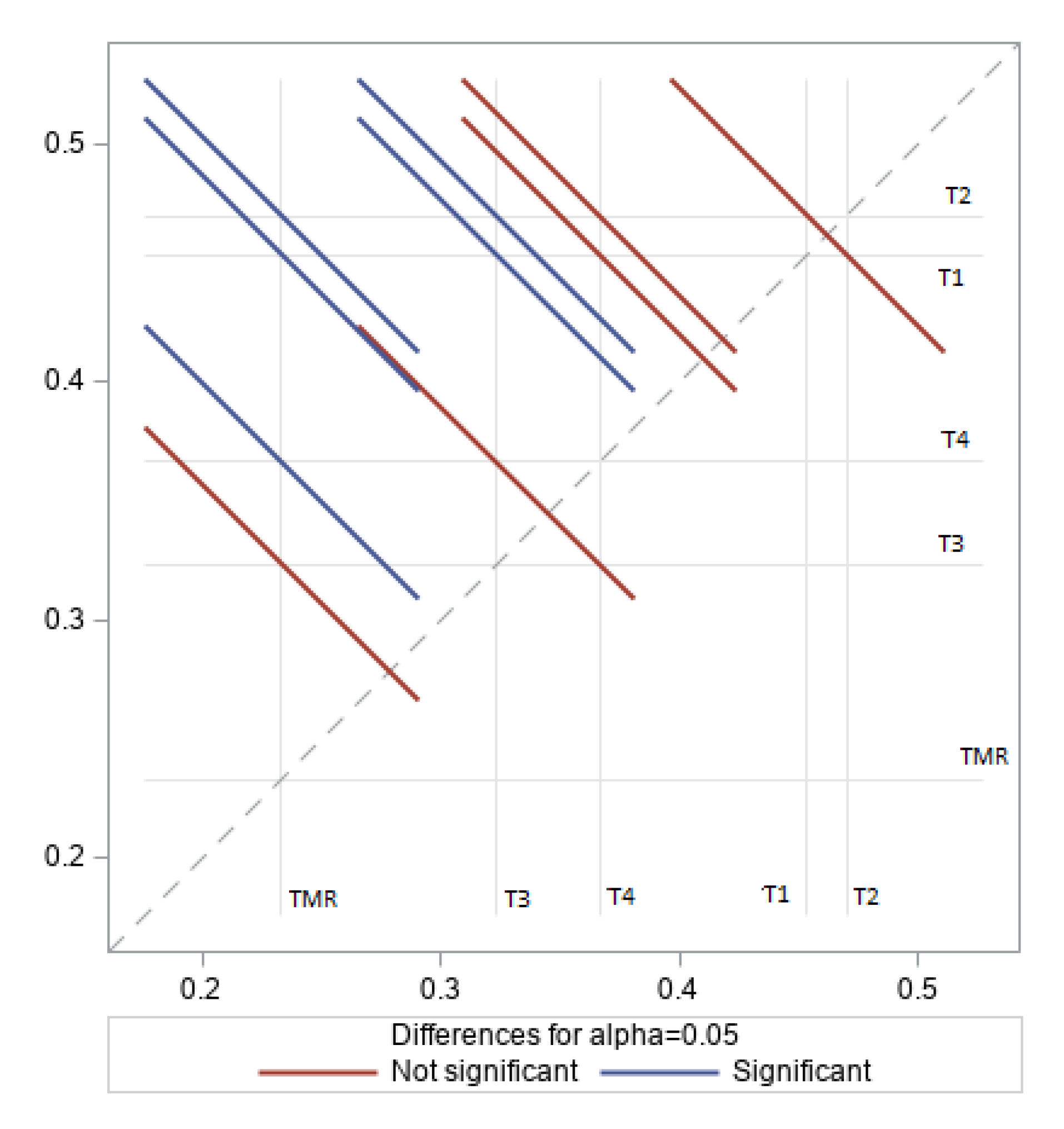
3.2. Rumen Volatile Fatty Acids
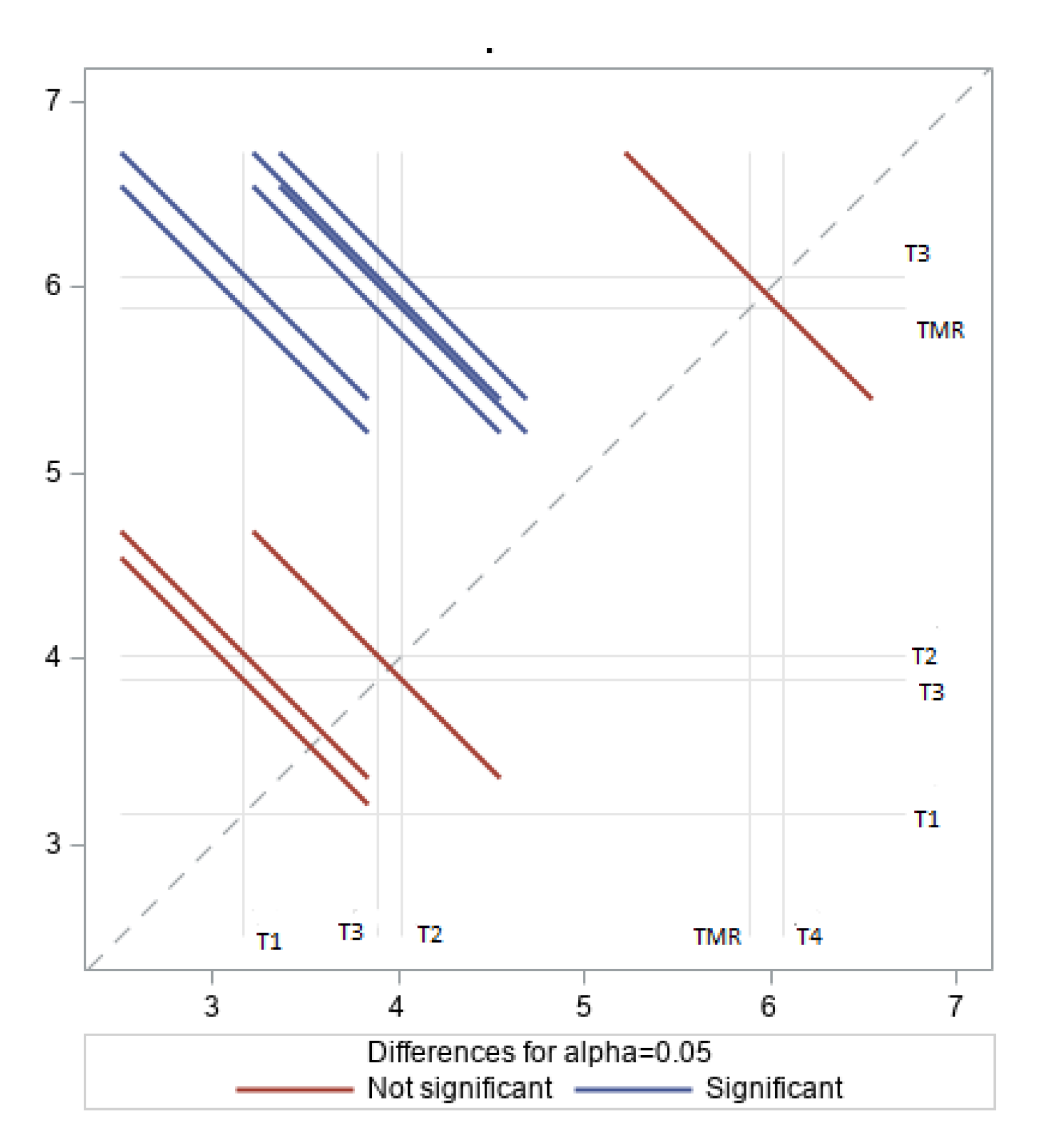
3.3. Fatty Acid Profiles of LD Muscle Fat
4. Discussion
4.1. Growth and Feed Efficiency of the Growing Lambs
4.2. Rumen Volatile Fatty Acids
4.3. Fatty Acid Profile of LD Fat
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gebauer, S.K.; Psota, T.L.; Harris, W.S.; Kris-Etherton, P.M. n-3 Fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am. J. Clin. Nutr. 2006, 83, 1526S–1535S. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; Enser, M. Manipulating the Fatty Acid Composition of Meat to Improve Nutritional Value and Meat Quality. In New Aspects of Meat Quality; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 501–535. [Google Scholar]
- Gaili, E.; Ali, A. Meat from Sudan desert sheep and goats: Part 2—Composition of the muscular and fatty tissues. Meat Sci. 1985, 13, 229–236. [Google Scholar] [CrossRef]
- Banskalieva, V. Effect of age, physiological state and nutrition on fatty acid composition in depot fat and ruminal volatile fatty acids in sheep. Small Rumin. Res. 1997, 24, 37–42. [Google Scholar] [CrossRef]
- Howes, N.L.; Bekhit, A.E.-D.A.; Burritt, D.J.; Campbell, A.W. Opportunities and Implications of Pasture-Based Lamb Fattening to Enhance the Long-Chain Fatty Acid Composition in Meat. Compr. Rev. Food Sci. Food Saf. 2014, 14, 22–36. [Google Scholar] [CrossRef]
- Williams, P. Nutritional composition of red meat. Nutr. Diet. 2007, 64, S113–S119. [Google Scholar] [CrossRef]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.; Bonham, M.P.; Fearon, A.M. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Vetter, W. Detection of 430 Fatty Acid Methyl Esters from a Transesterified Butter Sample. J. Am. Oil Chem. Soc. 2013, 90, 771–790. [Google Scholar] [CrossRef]
- Sampelayo, M.S.; Chilliard, Y.; Schmidely, P.; Boza, J. Influence of type of diet on the fat constituents of goat and sheep milk. Small Rumin. Res. 2007, 68, 42–63. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Bonnet, M.; Scollan, N.D. Recent developments in altering the fatty acid composition of ruminant-derived foods. Animal 2013, 7, 132–162. [Google Scholar] [CrossRef]
- Białek, M.; Czauderna, M. Composition of rumen-surrounding fat and fatty acid profile in selected tissues of lambs fed diets supplemented with fish and rapeseed oils, carnosic acid, and different chemical forms of selenium. Livest. Sci. 2019, 226, 122–132. [Google Scholar] [CrossRef]
- Enjalbert, F.; Combes, S.; Zened, A.; Meynadier, A. Rumen microbiota and dietary fat: A mutual shaping. J. Appl. Microbiol. 2017, 123, 782–797. [Google Scholar] [CrossRef] [PubMed]
- Sauvant, D.; Grenet, E.; Doreau, M. Dégradation Chimique des Aliments Dans le Réticulo-Rumen: Cinétique et Importance. In Nutrition des Ruminants Domestiques; Editions Quae: Versailles, France, 1995; pp. 383–406. [Google Scholar]
- Santos-Silva, J.; Mendes, I.; Portugal, P.; Bessa, R. Effect of particle size and soybean oil supplementation on growth performance, carcass and meat quality and fatty acid composition of intramuscular lipids of lambs. Livest. Prod. Sci. 2004, 90, 79–88. [Google Scholar] [CrossRef]
- Waghorn, G. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production—Progress and challenges. Anim. Feed. Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- NRC (National Research Council). Nutrient Requirements of Sheep, 6th ed.; National Academy Press: Washington, DC, USA, 1985. [Google Scholar]
- Vasta, V.; Ventura, V.; Luciano, G.; Andronico, V.; Pagano, R.I.; Scerra, M.; Biondi, L.; Avondo, M.; Priolo, A. The volatile compounds in lamb fat are affected by the time of grazing. Meat Sci. 2012, 90, 451–456. [Google Scholar] [CrossRef]
- Matar, A.M.; Ayadi, M.; Sbihi, H.M.; Nehdi, I.A.; Abdelrahman, M.M.; Aljumaah, R.S. Effect of Diet Composition on Milk Composition and Fatty Acid Profile of Najdi Ewes. Pak. J. Zool. 2019, 52, 87–96. [Google Scholar] [CrossRef]
- Miltko, R.; Rozbicka-Wieczorek, J.A.; Więsyk, E.; Czauderna, M. The Influence of Different Chemical Forms of Selenium Added to the Diet Including Carnosic Acid, Fish Oil and Rapeseed Oil on the Formation of Volatile Fatty Acids and Methane in the Rumen, and Fatty Acid Profiles in the Rumen Content and Muscles of Lambs. Acta Vet. 2016, 66, 373–391. [Google Scholar] [CrossRef]
- Wood, J.; Enser, M.; Fisher, A.; Nute, G.; Sheard, P.; Richardson, R.; Hughes, S.; Whittington, F. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Zervas, G.; Tsiplakou, E. The effect of feeding systems on the characteristics of products from small ruminants. Small Rumin. Res. 2011, 101, 140–149. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Liu, M.; Zhao, X.; Luo, H. Effect of Breed on the Volatile Compound Precursors and Odor Profile Attributes of Lamb Meat. Foods 2020, 9, 1178. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Francisco, A.; Alves, S.P.; Portugal, P.; Dentinho, T.; Almeida, J.; Soldado, D.; Jerónimo, E.; Bessa, R.J. Effect of dietary neutral detergent fibre source on lambs growth, meat quality and biohydrogenation intermediates. Meat Sci. 2019, 147, 28–36. [Google Scholar] [CrossRef]
- Sung, K.-I.; Nejad, J.G.; Hong, S.-M.; Ohh, S.-J.; Lee, B.-H.; Peng, J.-L.; Ji, D.-H.; Kim, B.-W. Effects of forage level and chromium-methionine chelate supplementation on performance, carcass characteristics and blood metabolites in Korean native (Hanwoo) steers. J. Anim. Sci. Technol. 2015, 57, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zinn, R.A.; Plascencia, A. Effects of forage level on the comparative feeding value of supplemental fat in growing-finishing diets for feedlot cattle. J. Anim. Sci. 1996, 74, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Zinn, R.A.; Plascencia, A.; Barajas, R. Interaction of forage level and monensin in diets for feedlot cattle on growth performance and digestive function. J. Anim. Sci. 1994, 72, 2209–2215. [Google Scholar] [CrossRef] [PubMed]
- Romero-Bernal, J.; Almaraz, E.M.; Ortega, O.A.C.; Salas, N.P.; González-Ronquillo, M. Chemical composition and fatty acid profile in meat from grazing lamb diets supplemented with ryegrass hay, fishmeal and soya bean meal as PUFA sources. Ciência Rural 2017, 47, 47. [Google Scholar] [CrossRef][Green Version]
- Lopes, L.S.; Ladeira, M.M.; Neto, O.R.M.; Ramos, E.M.; Paulino, P.V.R.; Chizzotti, M.L.; Guerreiro, M.C. Composição química e de ácidos graxos do músculo longissimus dorsi e da gordura subcutânea de tourinhos Red Norte e Nelore. Rev. Bras. Zootec. 2012, 41, 978–985. [Google Scholar] [CrossRef][Green Version]
- Chikwanha, O.C.; Vahmani, P.; Muchenje, V.; Dugan, M.E.; Mapiye, C. Nutritional enhancement of sheep meat fatty acid profile for human health and wellbeing. Food Res. Int. 2018, 104, 25–38. [Google Scholar] [CrossRef]
- Sinclair, L.A.; Cooper, S.L.; Chikunya, S.; Wilkinson, R.G.; Hallett, K.G.; Enser, M.; Wood, J.D. Biohydrogenation of n-3 polyunsaturated fatty acids in the rumen and their effects on microbial metabolism and plasma fatty acid concentrations in sheep. Anim. Sci. 2005, 81, 239–248. [Google Scholar] [CrossRef]
- Anderson, B.M.; Ma, D. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 2009, 8, 33. [Google Scholar] [CrossRef]
- O’Connell, T.D.; Block, R.C.; Huang, S.P.; Shearer, G.C. ω3-Polyunsaturated fatty acids for heart failure: Effects of dose on efficacy and novel signaling through free fatty acid receptor 4. J. Mol. Cell. Cardiol. 2017, 103, 74–92. [Google Scholar] [CrossRef]
- Nute, G.; Richardson, R.; Wood, J.; Hughes, S.; Wilkinson, R.; Cooper, S.; Sinclair, L. Effect of dietary oil source on the flavour and the colour and lipid stability of lamb meat. Meat Sci. 2007, 77, 547–555. [Google Scholar] [CrossRef]
- Chikunya, S.; Demirel, G.; Enser, M.; Wood, J.D.; Wilkinson, R.G.; Sinclair, L.A. Biohydrogenation of dietaryn-3 PUFA and stability of ingested vitamin E in the rumen, and their effects on microbial activity in sheep. Br. J. Nutr. 2004, 91, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.L.; Sinclair, L.A.; Wilkinson, R.G.; Hallett, K.G.; Enser, M.; Wood, J.D. Manipulation of the n-3 polyunsaturated fatty acid content of muscle and adipose tissue in lambs. J. Anim. Sci. 2004, 82, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Aurousseau, B.; Bauchart, D.; Calichon, E.; Micol, D.; Priolo, A. Effect of grass or concentrate feeding systems and rate of growth on triglyceride and phospholipid and their fatty acids in the M. longissimus thoracis of lambs. Meat Sci. 2004, 66, 531–541. [Google Scholar] [CrossRef]
- Daniel, Z.; Wynn, R.; Salter, A.; Buttery, P. Differing effects of forage and concentrate diets on the oleic acid and conjugated linoleic acid content of sheep tissues: The role of stearoyl-CoA desaturase. J. Anim. Sci. 2004, 82, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Nuernberg, K.; Nuernberg, G.; Ender, K.; Dannenberger, D.; Schabbel, W.; Grumbach, S.; Zupp, W.; Steinhart, H. Effect of grassvs. concentrate feeding on the fatty acid profile of different fat depots in lambs. Eur. J. Lipid Sci. Technol. 2005, 107, 737–745. [Google Scholar] [CrossRef]
- Kuhnt, K.; Degen, C.; Jahreis, G. Evaluation of the Impact of Ruminant trans Fatty Acids on Human Health: Important Aspects to Consider. Crit. Rev. Food Sci. Nutr. 2015, 56, 1964–1980. [Google Scholar] [CrossRef]
- Griinari, J.M.; Corl, B.A.; Lacy, S.H.; Chouinard, P.Y.; Nurmela, K.V.V.; Bauman, D.E. Conjugated Linoleic Acid Is Synthesized Endogenously in Lactating Dairy Cows by Δ9-Desaturase. J. Nutr. 2000, 130, 2285–2291. [Google Scholar] [CrossRef]
- Christophersen, O.A.; Haug, A. Animal products, diseases and drugs: A plea for better integration between agricultural sciences, human nutrition and human pharmacology. Lipids Health Dis. 2011, 10, 16. [Google Scholar] [CrossRef]
- Van Tran, L.; Malla, B.A.; Kumar, S.; Tyagi, A.K. Polyunsaturated Fatty Acids in Male Ruminant Reproduction—A Review. Asian Australas. J. Anim. Sci. 2016, 30, 622–637. [Google Scholar] [CrossRef]
- Enser, M. The Chemistry, Biochemistry and Nutritional Importance of Animal Fats. In Fats in Animal Nutrition; Elsevier BV: Amsterdam, The Netherlands, 1984; pp. 23–51. [Google Scholar]
- French, P.; Stanton, C.; Lawless, F.; O’Riordan, E.G.; Monahan, F.J.; Caffrey, P.J.; Moloney, A.P. Fatty acid composition, including conjugated linoleic acid, of intramuscular fat from steers offered grazed grass, grass silage, or concentrate-based diets. J. Anim. Sci. 2000, 78, 2849–2855. [Google Scholar] [CrossRef]
- Melo, H.M.; Santos, L.E.; Ferreira, S.T. Diet-Derived Fatty Acids, Brain Inflammation, and Mental Health. Front. Neurosci. 2019, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Bas, P.; Morand-Fehr, P. Effect of nutritional factors on fatty acid composition of lamb fat deposits. Livest. Prod. Sci. 2000, 64, 61–79. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated Fatty Acids and Risk of Coronary Heart Disease: Modulation by Replacement Nutrients. Curr. Atheroscler. Rep. 2010, 12, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Campo, M.; Muela, E.; Resconi, V.; Barahona, M.; Sañudo, C. Influence of commercial cut on proximate composition and fatty acid profile of Rasa Aragonesa light lamb. J. Food Compos. Anal. 2016, 53, 7–12. [Google Scholar] [CrossRef]
- Madruga, M.; Bezerra, T.K.A.; Guerra, I.C.D.; Batista, A.S.M.; Silva, A.M.D.A.; Fernandes, R.D.P.P. The effect of feed restriction on the fat profile of Santa Inês lamb meat. Acta Sci. Anim. Sci. 2020, 42, e48229. [Google Scholar] [CrossRef]
- Costa, R.G.; Batista, A.S.M.; De Azevedo, P.S.; Queiroga, R.D.C.R.D.E.; Madruga, M.S.; Filho, J.T.D.A. Lipid profile of lamb meat from different genotypes submitted to diets with different energy levels. Rev. Bras. Zootec. 2009, 38, 532–538. [Google Scholar] [CrossRef]

| Item | Alfalfa Hay | Wheat Straw | TMR |
|---|---|---|---|
| Chemical Composition (Dry Matter Basis) | |||
| Dry matter | 90.4 | 90.3 | 90.14 |
| Crude fiber | 24.1 | 41.6 | 11.98 |
| Crude protein | 16.2 | 3.90 | 12.8 |
| Neutral detergent fiber (NDF) | 45.2 | 18.0 | 41.95 |
| Acid detergent fiber (ADF) | 32.9 | 50.0 | 26.10 |
| Ether extract | 2.50 | 1.40 | 2.75 |
| ME, Mcal/kg 1 | 2.10 | 1.50 | 2.86 |
| Fatty Acids Composition (% by weight) | |||
| C8:0 | 7.82 | 7.08 | 0.67 |
| C10:0 | - | - | 0.78 |
| C12:0 | 1.55 | 0.66 | 12.97 |
| C14:0 | 2.21 | 5.14 | 5.18 |
| C16:0 | 21.20 | 28.28 | 10.95 |
| C16:1 cis 9 | - | - | 0.10 |
| C17:0 | - | - | 0.08 |
| C18:0 | 4.13 | 4.18 | 2.79 |
| C18:1 cis 9 | 3.64 | 8.23 | 26.65 |
| C18:1 cis 11 | - | - | 0.73 |
| C18:2 cis 9, 12 | 19.05 | 15.48 | 36.16 |
| C20:0 | 1.80 | 5.96 | 0.43 |
| C18:3 cis 9, 12, 15 | 37.30 | 10.03 | 2.01 |
| C22:0 | 0.88 | 9.91 | 0.21 |
| C24:0 | 0.41 | 5.06 | 0.29 |
| SFA | 34.92 | 63.69 | 34.35 |
| USFA | 65.08 | 36.31 | 65.65 |
| MUFA | 3.95 | 8.85 | 27.48 |
| PUFA | 61.13 | 27.46 | 38.17 |
| Performance Measurements | C | T1 | T2 | T3 | T4 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| BW gain, kg | 19.9 b | 22.0 a | 21.5 a | 17.4 b | 20.5 b | 1.72 | 0.07 |
| Total DMI, kg | 145.7 b | 155.5 a | 154.6 a | 133.2 b | 146.3 b | 9.71 | 0.005 |
| FCR | 8.05 | 7.12 | 7.29 | 7.93 | 7.15 | 0.25 | 0.18 |
| VFA | Acetic | Propionic | Butyric | Acetic: Propionic |
|---|---|---|---|---|
| C (mol%) % | 15.94 b 0.45 | 14.57 a 0.39 | 63.3 a 0.17 | 1.09 c |
| T1 (mol%) % | 16.79 a 0.47 | 13.14 a 0.37 | 55.2 a,b 0.16 | 1.29 b |
| T2 (mol%) % | 17.28 a 0.50 | 11.04 b 0.32 | 59.01 a 0.17 | 1.58 a |
| T3 (mol%) % | 17.48 a 0.48 | 13.90 a 0.38 | 47.78 b 0.13 | 1.27 b |
| T4 (mol%) % | 17.53 a 0.50 | 12.61 a 0.36 | 51.55 b 0.15 | 1.39 b |
| SEM | 1.93 | 1.56 | 5.16 | 0.05 |
| p-value | 0.09 | 0.40 | 0.01 | 0.04 |
| Diets | |||||||
|---|---|---|---|---|---|---|---|
| Fatty Acid (g/100 g) | C | T1 | T2 | T3 | T4 | SE | p-Value |
| C10:0 | 0.16 | 0.19 | 0.12 | 0.17 | 0.13 | 0.02 | 0.33 |
| C11:0 | 0.08 | 0.12 | 0.05 | 0.07 | 0.04 | 0.03 | 0.81 |
| C12:0 | 0.40 | 0.39 | 0.30 | 0.37 | 0.53 | 0.09 | 0.66 |
| C13:0 | 0.10 | 0.09 | 0.06 | 0.08 | 0.03 | 0.03 | 0.82 |
| C14:0 iso | - | 0.06 | 0.04 | 0.04 | 0.04 | 0.01 | 0.75 |
| C14:0 | 5.17 | 4.86 | 4.71 | 5.07 | 5.28 | 0.61 | 0.74 |
| C15:0 antiso | 0.06 b | 0.13 ab | 0.10 ab | 0.13 ab | 0.16 a | 0.02 | 0.04 |
| C15:0 iso | 0.36 | 0.28 | 0.26 | 0.31 | 0.30 | 0.04 | 0.52 |
| C14:1 cis9 | 0.13 | 0.14 | 0.16 | 0.18 | 0.12 | 0.02 | 0.49 |
| C15:0 | 0.98 | 0.89 | 0.80 | 0.90 | 0.68 | 0.11 | 0.52 |
| C16:1 cis7 | 0.13 | 0.15 | 0.09 | 0.11 | 0.13 | 0.05 | 0.79 |
| C16:0 iso | 0.16 | 0.20 | 0.18 | 0.19 | 0.18 | 0.02 | 0.81 |
| C17:0 iso | 0.24 | 0.17 | 0.14 | 0.20 | 0.15 | 0.04 | 0.35 |
| C16:0 | 25.57 | 26.73 | 26.12 | 26.31 | 24.70 | 1.21 | 0.85 |
| C17:0 antiso | 1.80 | 1.53 | 1.39 | 1.71 | 1.26 | 0.23 | 0.55 |
| C17:0 | 2.70 | 2.55 | 2.50 | 2.74 | 2.33 | 0.24 | 0.82 |
| C16:1cis 9 | 1.32 | 1.93 | 1.71 | 1.88 | 1.35 | 0.18 | 0.15 |
| C18:0 iso | 0.12 | 0.15 | 0.14 | 0.15 | 0.12 | 0.02 | 0.46 |
| C17:1 cis10 | 0.90 | 1.16 | 0.97 | 1.27 | 0.71 | 0.25 | 0.64 |
| C18:0 | 14.75 | 15.31 | 15.21 | 14.05 | 19.57 | 1.72 | 0.39 |
| C18:1 trans9 | 0.52 a | 0.41 ab | 0.30 ab | 0.16 b | 0.19 b | 0.08 | 0.04 |
| C19:0 | 7.90 a | 1.94 b | 2.86 b | 3.39 b | 4.31 ab | 1.23 | 0.04 |
| C18:1 cis9 | 30.74 | 34.90 | 35.67 | 34.81 | 30.82 | 1.72 | 0.22 |
| C18:1cis11 | 1.17 | 1.09 | 0.99 | 1.25 | 1.14 | 0.10 | 0.54 |
| C18:1 cis13 | 0.29 b | 0.48 ab | 0.56 a | 0.39 ab | 0.51 ab | 0.07 | 0.02 |
| C19:0 iso | 0.13 | 0.14 | 0.12 | 0.12 | 0.20 | 0.02 | 0.31 |
| C18:2 trans 9,12 | 0.18 c | 0.29 b | 0.33 a | 0.28 bc | 0.21 bc | 0.03 | 0.04 |
| C18:2 trans 12, 15 | 0.08 | 0.13 | 0.15 | 0.15 | 0.11 | 0.03 | 0.64 |
| C18:2 cis9 trans12 | 3.01 ab | 2.33 c | 3.33 a | 2.41 b | 3.62 a | 0.24 | 0.02 |
| C20:2 trans 11,13 | 0.07 | 0.13 | 0.11 | 0.12 | 0.11 | 0.03 | 0.75 |
| C20:0 | 0.10 | 0.11 | 0.11 | 0.11 | 0.13 | 0.01 | 0.92 |
| C18:3 cis9,12,15 | 0.23 c | 0.45 a | 0.47 a | 0.31 b | 0.37 ab | 0.03 | 0.005 |
| C18:2 cis9 trans11 | 0.34 | 0.60 | 0.56 | 0.51 | 0.53 | 0.08 | 0.30 |
| C22:0 | - | 0.07 | - | 0.02 | - | - | - |
| C20:4 cis5,8,11,4 | 0.04 | 0.03 | 0.03 | 0.05 | 0.07 | 0.01 | 0.33 |
| C22:5 cis7,10,13,16,19 | 0.02 | 0.06 | 0.02 | 0.04 | 0.03 | 0.01 | 0.41 |
| Parameter | Diet | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| C | T1 | T2 | T3 | T4 | |||
| SFA | 60.78 | 55.80 | 54.55 | 56.12 | 60.05 | 1.95 | 0.20 |
| USFA | 39.22 | 44.19 | 45.45 | 43.88 | 39.95 | 1.95 | 0.20 |
| MUSFA | 35.21 | 40.17 | 40.42 | 40.02 | 34.86 | 2.01 | 0.22 |
| PUSFA | 4.01 a,b | 4.03 a,b | 5.03 a,b | 3.86 b | 5.10 a | 0.36 | 0.04 |
| n3 | 0.36 b | 0.62 a | 0.64 a | 0.50 a,b | 0.55 a,b | 0.07 | 0.02 |
| n6 | 3.29 a,b | 2.77 b | 3.80 a | 2.84 b | 3.97 a | 0.26 | 0.04 |
| n3/n6 | 9.21 a | 4.48 c | 6.27 b | 5.84 b,c | 7.19 a,b | 0.67 | 0.01 |
| Δ9C18 | 69.18 | 70.66 | 71.18 | 70.14 | 62.57 | 3.09 | 0.40 |
| AI | 1.19 | 1.08 | 0.95 | 1.09 | 1.16 | 0.12 | 0.70 |
| TI | 1.39 | 1.55 | 1.37 | 1.43 | 1.59 | 0.13 | 0.77 |
| hFA | 34.75 b | 38.92 a,b | 40.70 a | 38.66 a,b | 35.92 a,b | 1.78 | 0.02 |
| HFA | 31.15 | 31.97 | 30.59 | 31.75 | 30.51 | 1.74 | 0.97 |
| h/H | 1.12 | 1.23 | 1.33 | 1.25 | 1.18 | 0.11 | 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matar, A.M.; Abdelrahman, M.M.; Alhidary, I.A.; Ayadi, M.A.; Alobre, M.M.; Aljumaah, R.S. Effects of Roughage Quality and Particle Size on Rumen Parameters and Fatty Acid Profiles of Longissimus Dorsi Fat of Lambs Fed Complete Feed. Animals 2020, 10, 2182. https://doi.org/10.3390/ani10112182
Matar AM, Abdelrahman MM, Alhidary IA, Ayadi MA, Alobre MM, Aljumaah RS. Effects of Roughage Quality and Particle Size on Rumen Parameters and Fatty Acid Profiles of Longissimus Dorsi Fat of Lambs Fed Complete Feed. Animals. 2020; 10(11):2182. https://doi.org/10.3390/ani10112182
Chicago/Turabian StyleMatar, Abdulkareem M., Mutassim M. Abdelrahman, Ibrahim A. Alhidary, Moez A. Ayadi, Mohsen M. Alobre, and Riyadh S. Aljumaah. 2020. "Effects of Roughage Quality and Particle Size on Rumen Parameters and Fatty Acid Profiles of Longissimus Dorsi Fat of Lambs Fed Complete Feed" Animals 10, no. 11: 2182. https://doi.org/10.3390/ani10112182
APA StyleMatar, A. M., Abdelrahman, M. M., Alhidary, I. A., Ayadi, M. A., Alobre, M. M., & Aljumaah, R. S. (2020). Effects of Roughage Quality and Particle Size on Rumen Parameters and Fatty Acid Profiles of Longissimus Dorsi Fat of Lambs Fed Complete Feed. Animals, 10(11), 2182. https://doi.org/10.3390/ani10112182







