Stage-Dependent Expression of Protein Gene Product 9.5 in Donkey Testes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Testicular Sample Preparation
2.2. Western Blotting
2.3. Immunofluorescence
2.4. Imaging
3. Results
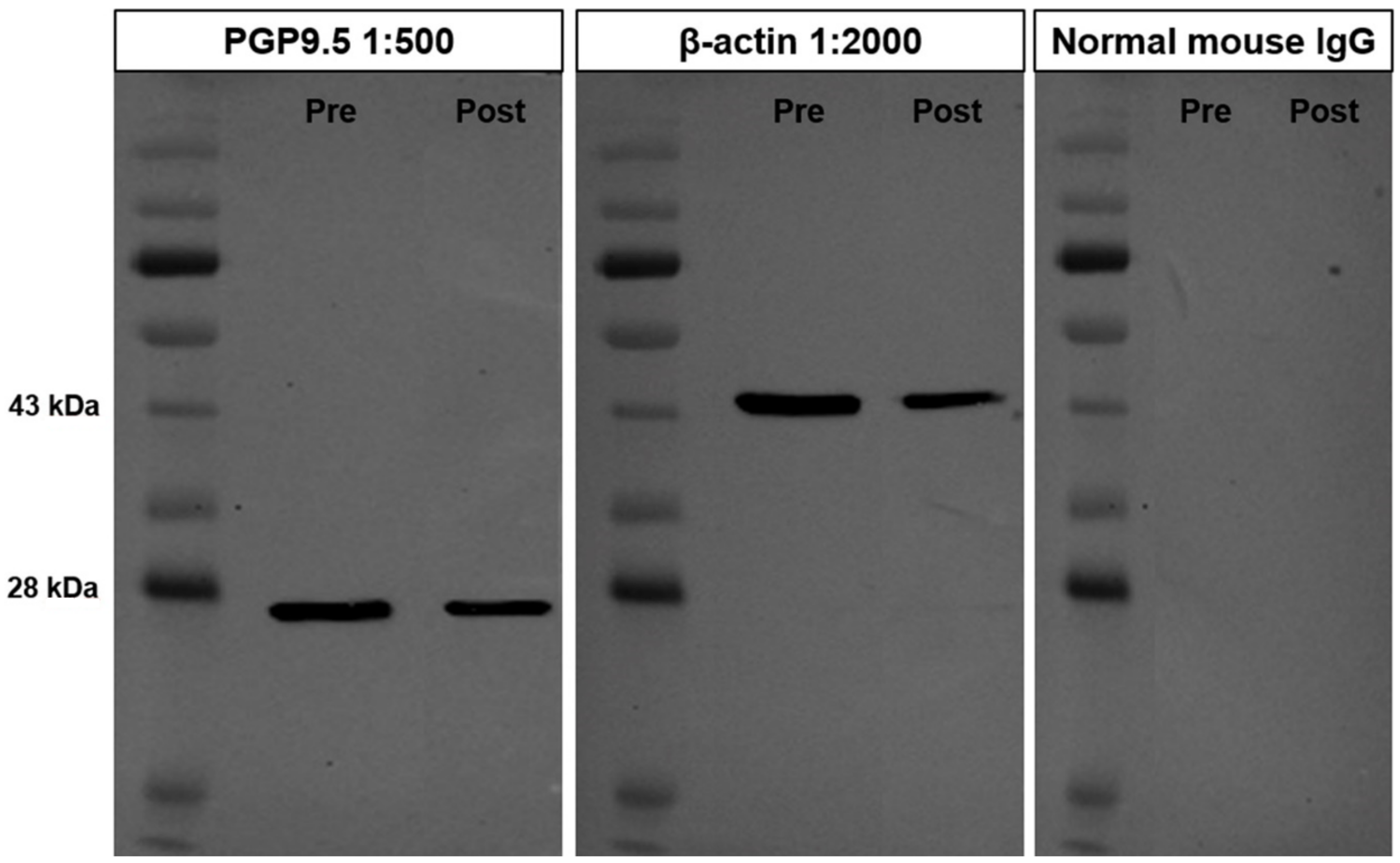
3.1. Cross-Reactivity of the PGP9.5 Antibody in Donkey Testes
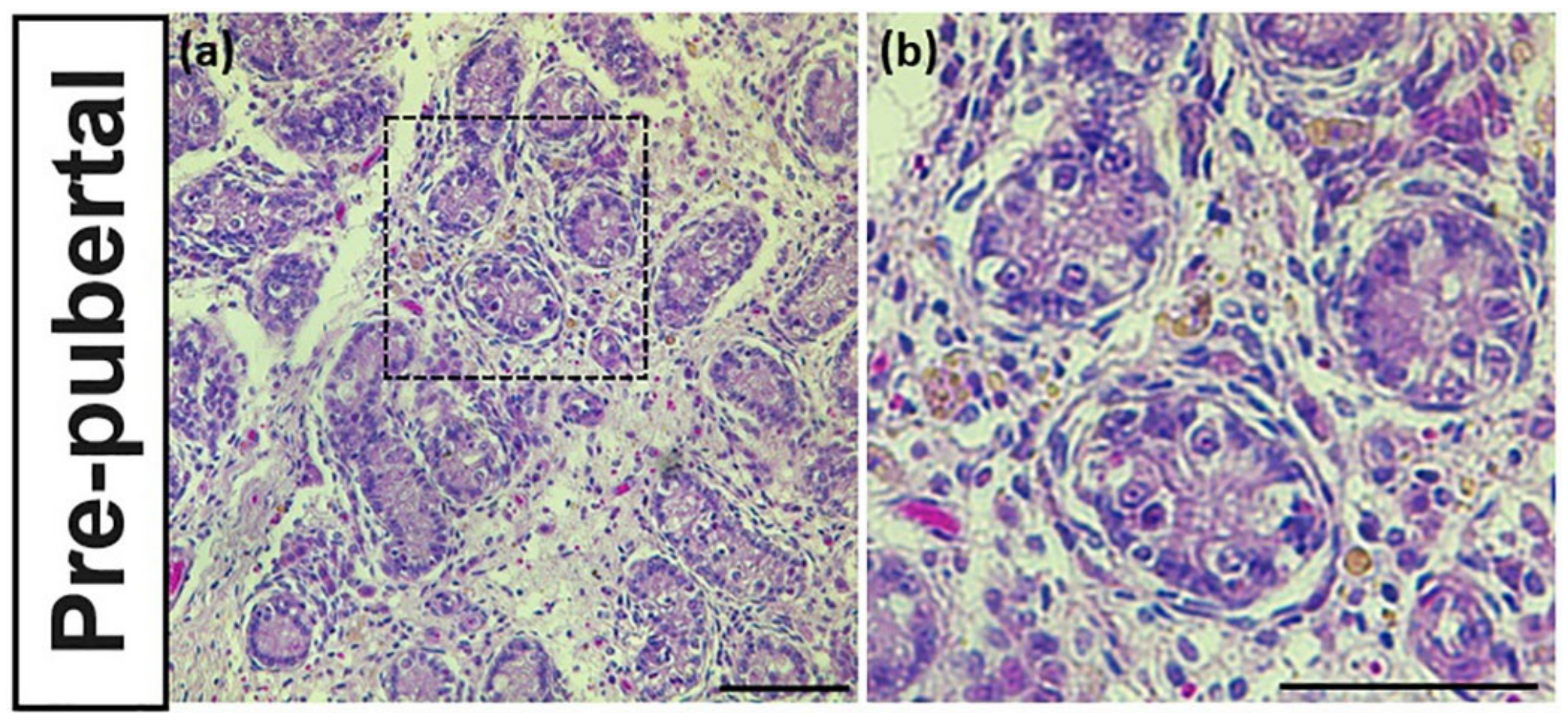
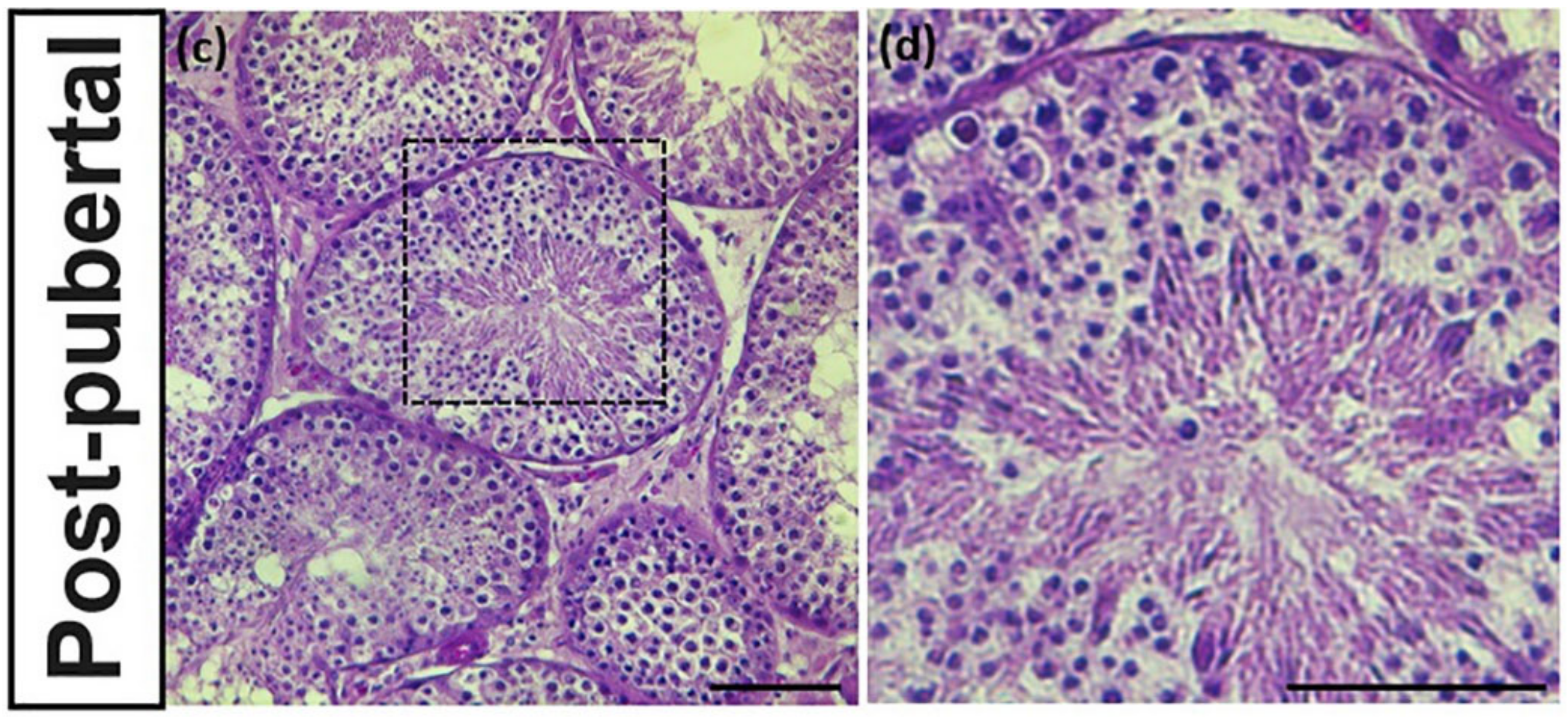
3.2. Identification of Reproductive Stageof Donkey Testes
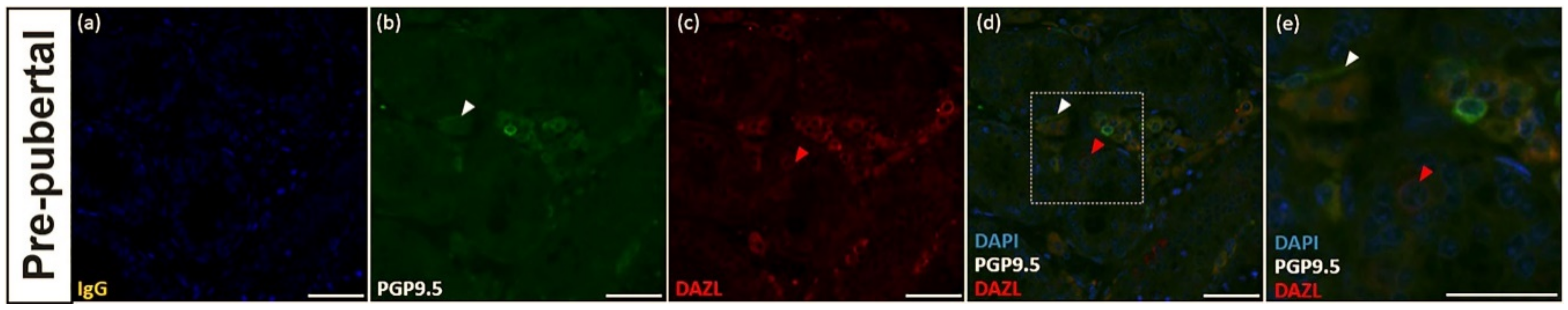
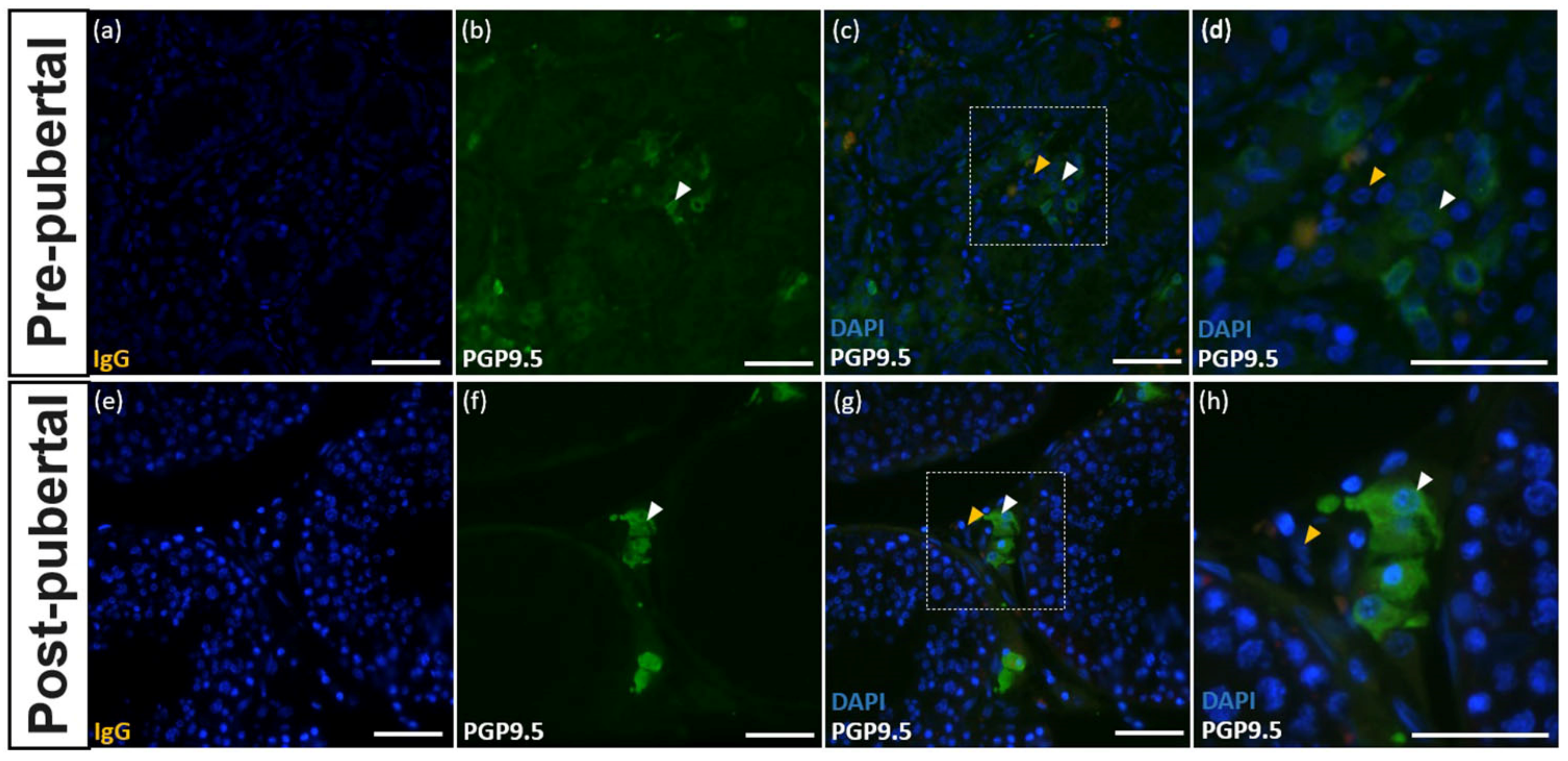
3.3. PGP9.5 and DAZL Expression in the Testes of Pre-Pubertal Donkeys
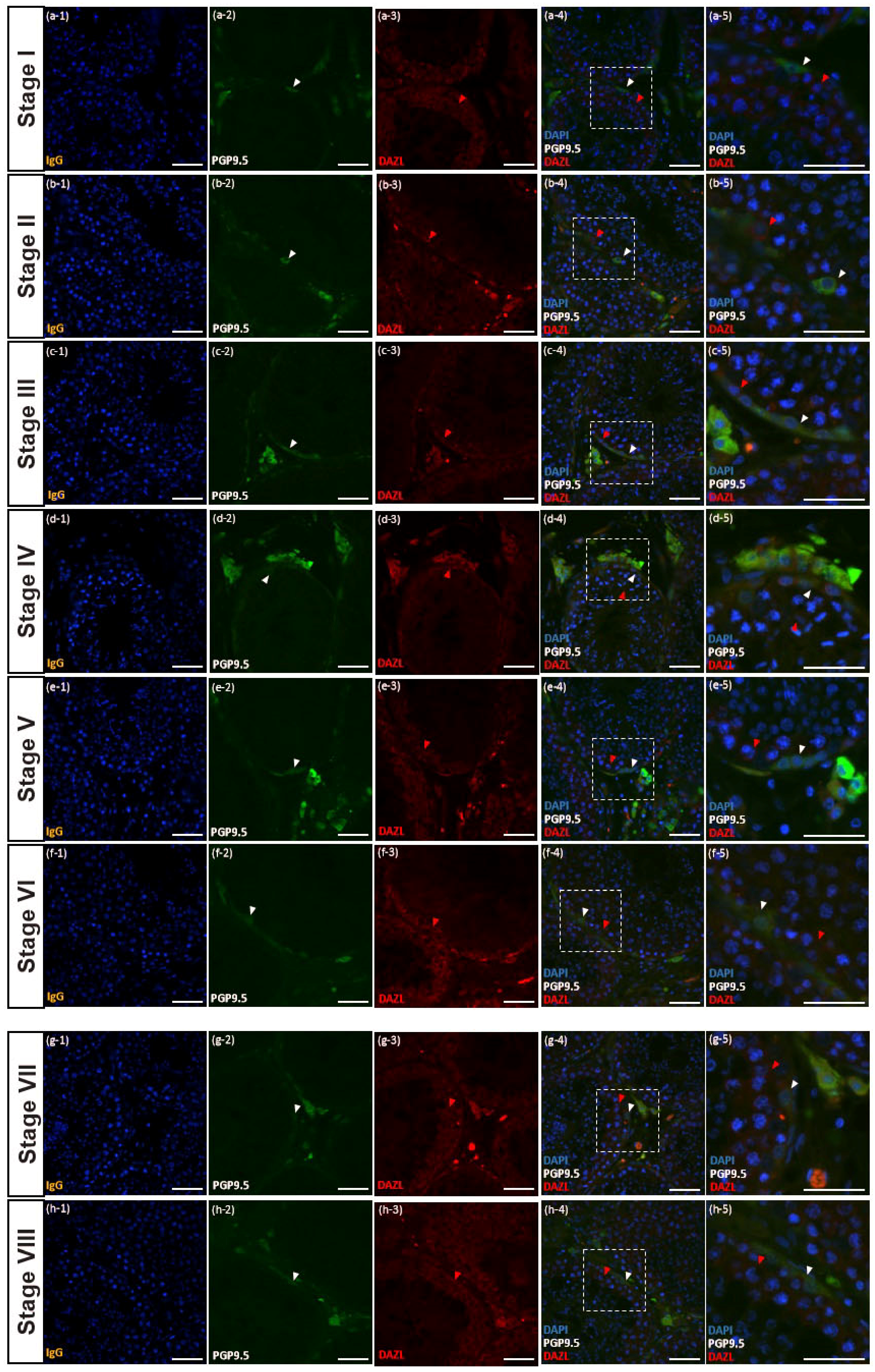
3.4. PGP9.5 and DAZL Expressions in Testes of Post-Pubertal Donkeys in Different Seminiferous Epithelium Cycle Stages
3.5. Expression of PGP9.5 in Leydig Cells of the Testes of Donkeys
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Costa, G.M.; Avelar, G.F.; Rezende-Neto, J.V.; Campos-Junior, P.H.; Lacerda, S.M.; Andrade, B.S.; Thomé, R.G.; Hofmann, M.C.; Franca, L.R. Spermatogonial stem cell markers and niche in equids. PLoS ONE 2012, 7, e44091. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Roser, J.F.; Yoon, M. UTF1, a putative marker for spermatogonial stem cells in stallions. PLoS ONE 2014, 9, e108825. [Google Scholar] [CrossRef]
- Thompson, R.J.; Doran, J.F.; Jackson, P.; Dhillon, A.P.; Rode, J. PGP 9.5—A new marker for vertebrate neurons and neuroendocrine cells. Brain Res. 1983, 278, 224–228. [Google Scholar] [CrossRef]
- Gong, B.; Cao, Z.; Zheng, P.; Vitolo, O.V.; Liu, S.; Staniszewski, A.; Moolman, D.; Zhang, H.; Shelanski, M.; Arancio, O.; et al. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell 2006, 126, 775–788. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.; Wang, Y.L.; Setsuie, R.; Sekiguchi, S.; Sakurai, M.; Sato, Y.; Lee, W.W.; Ishii, Y.; Kyuwa, S.; Noda, M.; et al. Developmental regulation of ubiquitin C-terminal hydrolase isozyme expression during spermatogenesis in mice. Biol. Reprod. 2004, 71, 515–521. [Google Scholar] [CrossRef]
- Wrobel, K.H. Prespermatogenesis and spermatogoniogenesis in the bovine testis. Anat. Embryol. (Berl.) 2000, 202, 209–222. [Google Scholar] [CrossRef]
- Luo, J.; Megee, S.; Rathi, R.; Dobrinski, I. Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: Application to enrichment and culture of porcine spermatogonia. Mol. Reprod. Dev. 2006, 73, 1531–1540. [Google Scholar] [CrossRef]
- Rodriguez-Sosa, J.R.; Dobson, H.; Hahnel, A. Isolation and transplantation of spermatogonia in sheep. Theriogenology 2006, 66, 2091–2103. [Google Scholar] [CrossRef]
- Heidari, B.; Rahmati-Ahmadabadi, M.; Akhondi, M.M.; Zarnani, A.H.; Jeddi-Tehrani, M.; Shirazi, A.; Naderi, M.M.; Behzadi, B. Isolation, identification, and culture of goat spermatogonial stem cells using c-kit and PGP9.5 markers. J. Assist. Reprod. Genet. 2012, 29, 1029–1038. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Jung, H.J.; Yoon, M.J. Undifferentiated embryonic cell transcription factor 1 (UTF1) and deleted in azoospermia-like (DAZL) expression in the testes of donkeys. Reprod. Domest. Anim. 2017, 52, 264–269. [Google Scholar] [CrossRef]
- Jung, H.; Song, H.; Yoon, M. Stage-dependent DAZL localization in stallion germ cells. Anim. Reprod. Sci. 2014, 147, 32–38. [Google Scholar] [CrossRef]
- Lee, G.; Jung, H.; Yoon, M. The Lin28 expression in stallion testes. PLoS ONE 2016, 11, e0165011. [Google Scholar] [CrossRef] [Green Version]
- Heninger, N.L.; Staub, C.; Blanchard, T.L.; Johnson, L.; Varner, D.D.; Forrest, D.W. Germ cell apoptosis in the testes of normal stallions. Theriogenology 2004, 62, 283–297. [Google Scholar] [CrossRef]
- Neves, E.S.; Chiarini-Garcia, H.; França, L.R. Comparative testis morphometry and seminiferous epithelium cycle length in donkeys and mules. Biol. Reprod. 2002, 67, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Yoon, M. Protein Gene Product 9.5 Expression in Stallion Testes. J. Equine Vet. Sci. 2016, 45, 1–7. [Google Scholar] [CrossRef]
- Myka, J.L.; Lear, T.L.; Houck, M.L.; Ryder, O.A.; Bailey, E. Homologous fission event(s) implicated for chromosomal polymorphisms among five species in the genus Equus. Cytogenet. Genome Res. 2003, 102, 217–221. [Google Scholar] [CrossRef]
- Kon, Y.; Endoh, D.; Iwanaga, T. Expression of protein gene product 9.5, a neuronal ubiquitin C-terminal hydrolase, and its developing change in Sertoli cells of mouse testis. Mol. Reprod. Dev. Inc. Gamete Res. 1999, 54, 333–341. [Google Scholar] [CrossRef]
- Herrid, M.; Davey, R.J.; Hill, J.R. Characterization of germ cells from pre-pubertal bull calves in preparation for germ cell transplantation. Cell Tissue Res. 2007, 330, 321–329. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Lee, K.-H.; Heo, Y.-T.; Kim, N.-H.; Kim, J.-H.; Kim, J.-H.; Moon, S.-H.; Chung, H.-J.; Yoon, M.-J.; Song, H. Transcriptional coactivator undifferentiated embryonic cell transcription factor 1 expressed in spermatogonial stem cells: A putative marker of boar spermatogonia. Anim. Reprod. Sci. 2014, 150, 115–124. [Google Scholar] [CrossRef]
- Kwon, J.; Mochida, K.; Wang, Y.-L.; Sekiguchi, S.; Sankai, T.; Aoki, S.; Ogura, A.; Yoshikawa, Y.; Wada, K. Ubiquitin C-terminal hydrolase L-1 is essential for the early apoptotic wave of germinal cells and for sperm quality control during spermatogenesis. Biol. Reprod. 2005, 73, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.; Wang, Y.-L.; Setsuie, R.; Sekiguchi, S.; Sato, Y.; Sakurai, M.; Noda, M.; Aoki, S.; Yoshikawa, Y.; Wada, K. Two closely related ubiquitin C-terminal hydrolase isozymes function as reciprocal modulators of germ cell apoptosis in cryptorchid testis. Am. J. Pathol. 2004, 165, 1367–1374. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J. The new function of two ubiquitin C-terminal hydrolase isozymes as reciprocal modulators of germ cell apoptosis. Exp. Anim. 2007, 56, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Matsui, Y. Regulation of germ cell death in mammalian gonads. APMIS 1998, 106, 142–148. [Google Scholar] [CrossRef]
- Print, C.G.; Loveland, K.L. Germ cell suicide: New insights into apoptosis during spermatogenesis. Bioessays 2000, 22, 423–430. [Google Scholar] [CrossRef]
- Culty, M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res. 2009, 87, 1–26. [Google Scholar] [CrossRef]
- Paniagua, R.; Nistal, M. Morphological and histometric study of human spermatogonia from birth to the onset of puberty. J. Anat. 1984, 139, 535. [Google Scholar]
- De Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wilson, P.; Barber, P.; Hamid, Q.; Power, B.; Dhillon, A.; Rode, J.; Day, I.; Thompson, R.; Polak, J. The immunolocalization of protein gene product 9.5 using rabbit polyclonal and mouse monoclonal antibodies. Br. J. Exp. Pathol. 1988, 69, 91. [Google Scholar]
- Lee, K.H.; Lee, R.; Lee, W.Y.; Kim, D.H.; Chung, H.J.; Kim, J.H.; Kim, N.H.; Choi, S.H.; Kim, J.H.; Song, H. Identification and in vitro derivation of spermatogonia in beagle testis. PLoS ONE 2014, 9, e109963. [Google Scholar] [CrossRef]
- Chen, H.; Ge, R.-S.; Zirkin, B. Leydig cells: From stem cells to aging. Mol. Cell. Endocrinol. 2009, 306, 9–16. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Jung, Y.; Kim, S.; Kim, J.; Jung, H.; Yoon, M. Stage-Dependent Expression of Protein Gene Product 9.5 in Donkey Testes. Animals 2020, 10, 2169. https://doi.org/10.3390/ani10112169
Choi Y, Jung Y, Kim S, Kim J, Jung H, Yoon M. Stage-Dependent Expression of Protein Gene Product 9.5 in Donkey Testes. Animals. 2020; 10(11):2169. https://doi.org/10.3390/ani10112169
Chicago/Turabian StyleChoi, Yeonju, Youngwook Jung, Seongmin Kim, Junyoung Kim, Heejun Jung, and Minjung Yoon. 2020. "Stage-Dependent Expression of Protein Gene Product 9.5 in Donkey Testes" Animals 10, no. 11: 2169. https://doi.org/10.3390/ani10112169
APA StyleChoi, Y., Jung, Y., Kim, S., Kim, J., Jung, H., & Yoon, M. (2020). Stage-Dependent Expression of Protein Gene Product 9.5 in Donkey Testes. Animals, 10(11), 2169. https://doi.org/10.3390/ani10112169




