Management of Thermal Injuries in Donkeys: A Case Report
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
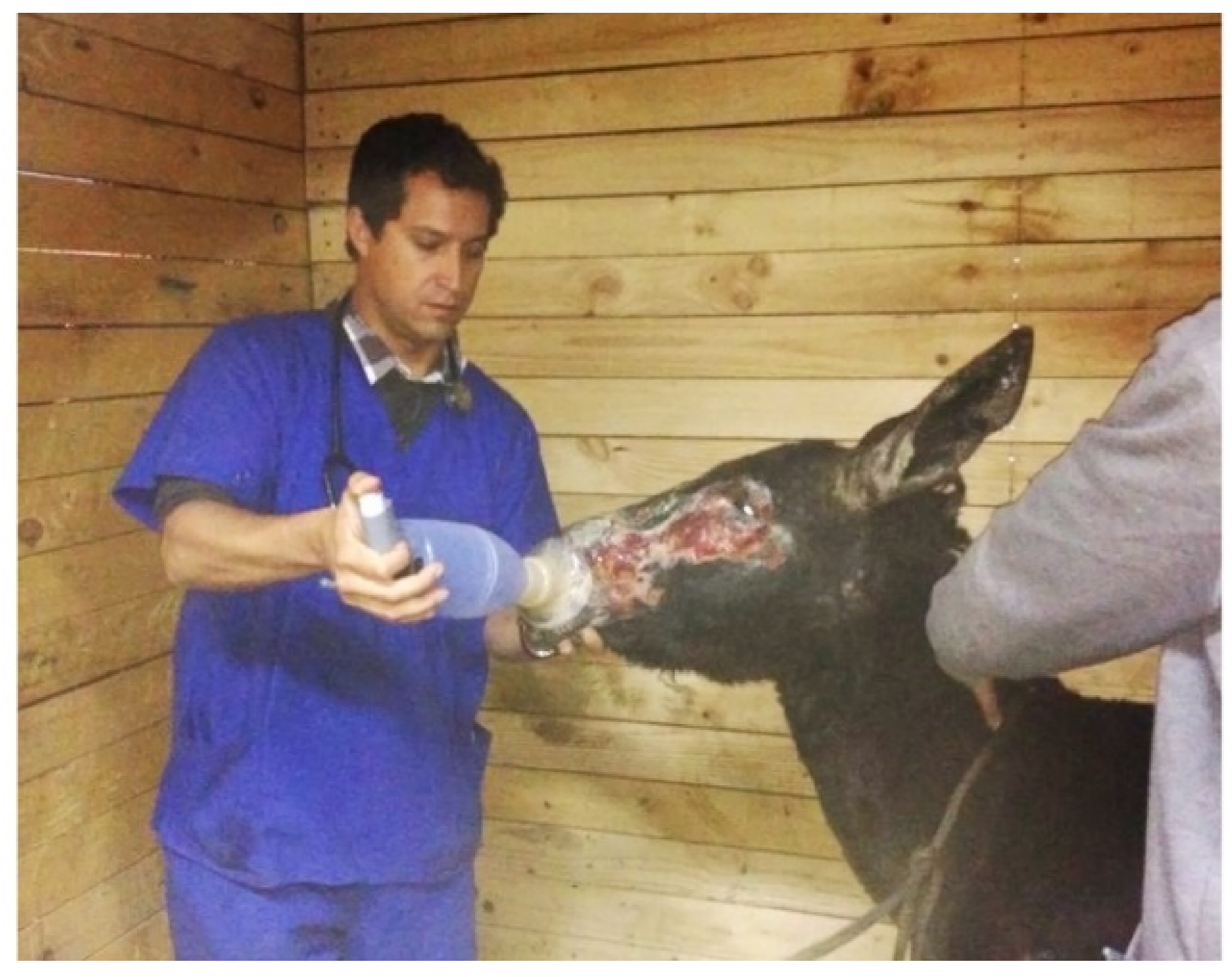
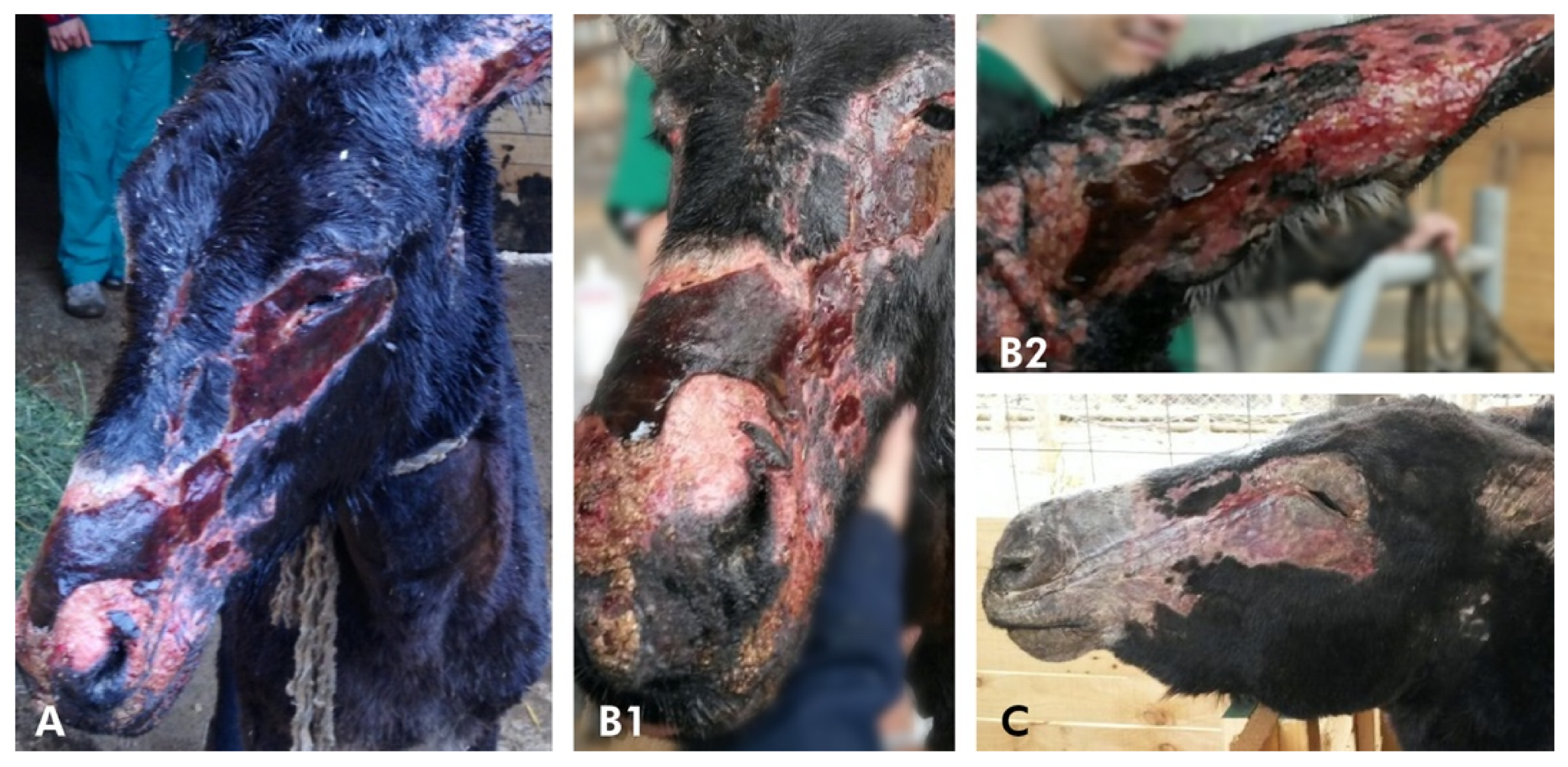
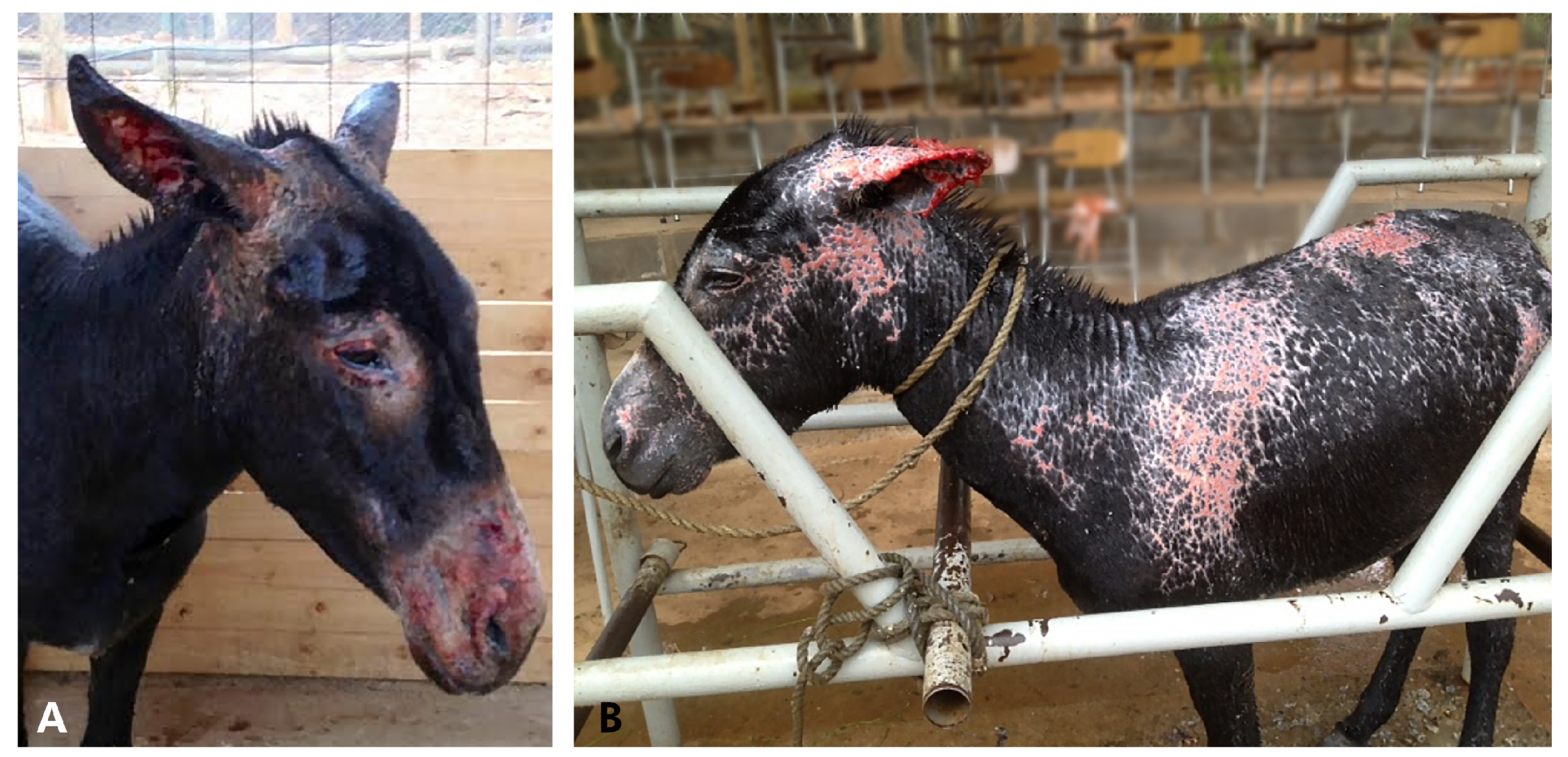
History and Case Presentation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Efimova, O.I.; Dimitriev, A.I.; Nesterova, O.P.; Aldyakov, A.V.; Obukhova, A.V.; Ivanova, T.N. Methods for the effective treatment of animal burns. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 5th International Conference on Agricultural and Biological Sciences (ABS), Macau, China, 21–24 July 2019; IOP Publishing: Bristol, UK, 2019; Volume 346, p. 012057. [Google Scholar] [CrossRef]
- Litt, J.S. Evaluation and Management of the Burn Patient: A Case Study and Review. Mo. Med. 2018, 115, 443–446. [Google Scholar] [PubMed]
- Geiser, D.R.; Walker, R.D. Management of thermal injuries in large animals. Vet. Clin. N. Am. Large. Anim. Prac. 1984, 6, 91–105. [Google Scholar] [CrossRef]
- Hanson, R.R. Management of burn injuries in the horse. Vet. Clin. N. Am. Equine Pract. 2005, 21, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Herbert, E.W. Findings and strategies for treating horses injured in open range fires. Equine. Vet. Educ. 2018, 30, 177–186. [Google Scholar] [CrossRef]
- Silva, S.; Ríspoli, V.; Graner, C.; Sá, L.R.; Belli, C.; De Zoppa, A.L. Using tilapia skin (Oreochromis niloticus) as an occlusive biological curative in equine wounds: Short communication. Braz. J. Vet. Res. Anim. Sci. 2019, 56, e154079. [Google Scholar] [CrossRef]
- Wohlsein, P.; Peters, M.; Schulze, C.; Baumgärtner, W. Thermal Injuries in Veterinary Forensic Pathology. Vet. Pathol. 2016, 53, 1001–1017. [Google Scholar] [CrossRef]
- Magdesian, G.; Watson, J. What Horse Owners Can Do To Monitor Horses Evacuated from Fire Areas. Guidelines UC Davis VMTH Large Animal Clinic. 2020. Available online: https://www.vetmed.ucdavis.edu/sites/g/files/dgvnsk491/files/inline-files/smoke-inhalation-quick-reference-guide.pdf (accessed on 21 October 2020).
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn Wound Infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef]
- Toussaint, J.; Singer, A.J. The evaluation and management of thermal injuries: 2014 update. Clin. Exp. Emerg. Med. 2014, 1, 8–18. [Google Scholar] [CrossRef]
- Hanson, R.R.; Munsterman, A.S. Treatment of Burn Injuries, Gunshot Wounds, and Dog-Bite Wound. In Equine Wound Management, 3rd ed.; Theoret, C., Schumacher, J., Eds.; John Wiley & Sons: Ames, IA, USA, 2017; Volume 21, pp. 476–490. [Google Scholar]
- Ministerio de Salud Gobierno de Chile. Gran Quemado. Guías Clínicas AUGE; Santiago, Chile, 2016; pp. 1–109. Available online: http://www.bibliotecaminsal.cl/wp/wp-content/uploads/2016/04/GPC-GRAN-QUEMADO-FINAL-18-MARZO-2016_DIAGRAMADA.pdf (accessed on 18 May 2020).
- Warden, G.D. Outpatient care of thermal injuries. Surg. Clin. N. Am. 1987, 67, 147–157. [Google Scholar] [CrossRef]
- The Donkey Sactuary. The Clinical Companion of the Donkey, 1st ed.; Matador, Troubador Ltd.: Leicestershire, UK, 2018; p. 267. [Google Scholar]
- Pavoni, P.; Gianesello, L.; Paparella, L.; Buoninsegni, L.T.; Barboni, E. Outcome predictors and quality of life of severe burn patients admitted to intensive care unit. Scand. J. Trauma Resusc. Emerg. Med. 2010, 18, 24. [Google Scholar] [CrossRef]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, N.C.; Lee, J.O.; Norbury, W.B.; Branski, L.K.; Wurzer, P.; Jimenez, C.J.; Benjamin, D.A.; Herndon, D.N. Accuracy of Currently Used Paper Burn Diagram vs. a Three-Dimensional Computerized Model. J. Burn Care Res. 2017, 38, e254–e260. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.F.; King, B.T.; Aden, J.K.; Serio-Melvin, M.; Chung, K.K.; Fenrich, C.A.; Salinas, J.; Renz, E.M.; Wolf, S.E.; Blackbourne, L.H.; et al. Comparison of traditional burn wound mapping with a computerized program. J. Burn Care Res. 2013, 34, e29–e35. [Google Scholar] [CrossRef] [PubMed]
- Wood, F.M.; Phillips, M.; Jovic, T.; Cassidy, J.T.; Cameron, P.; Edgar, D.W. Steering Committee of the Burn Registry of Australia and New Zealand (BRANZ) Water First Aid Is Beneficial In Humans Post-Burn: Evidence from a Bi-National Cohort Study. PLoS ONE 2016, 11, e0147259. [Google Scholar] [CrossRef] [PubMed]
- Hammett, E. The latest advice on burns. BDJ Team 2019, 6, 16–18. [Google Scholar] [CrossRef][Green Version]
- Cuttle, L.; Pearn, J.; McMillan, J.R.; Kimble, R.M. A review of first aid treatments for burn injuries. Burns 2009, 35, 768–775. [Google Scholar] [CrossRef]
- Gimenez, R.M.; Woods, J.A.; Dwyer, R.M.; Gimenez, T. A Review of Strategies to Prevent and Respond to Barn Fires Affecting the Horse Industry. AAEP Proc. 2008, 54, 160–179. [Google Scholar]
- Kim, J.Y.; Willard, J.J.; Supp, D.M.; Roy, S.; Gordillo, G.M.; Sen, C.K.; Powell, H.M. Burn Scar Biomechanics after Pressure Garment Therapy. Plast. Reconstr. Surg. 2015, 136, 572–581. [Google Scholar] [CrossRef]
- Coghlan, N.; Copley, J.; Aplin, T.; Strong, J. The experience of wearing compression garments after burn injury: “On the inside it is still me”. Burns 2019, 45, 1438–1446. [Google Scholar] [CrossRef]
- Somboonwong, J.; Thanamittramanee, S.; Jariyapongskul, A.; Patumraj, S. Therapeutic Effects of Aloe Vera on Cutaneous Microcirculation and Wound Healing in Second Degree Burn Model in Rats. J. Med. Assoc. Thai. 2000, 83, 417–425. [Google Scholar]
- Maenthaisong, R.; Chaiyakunapruk, N.; Niruntraporn, S.; Kongkaew, C. The Efficacy of Aloe Vera Used for Burn Wound Healing: A Systematic Review. Burns 2007, 33, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.Y.; Kim, H.B.; Jung, M.J.; Kang, S.Y.; Kwak, I.S.; Park, C.W.; Kim, H.O. Post-Burn Pruritus. Int. J. Mol. Sci. 2020, 21, 3880. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, M. Topical Application of Honey for Burn Wound Treatment-an Overview. Ann. Burns Fire Disasters 2007, 20, 137–139. [Google Scholar] [PubMed]
- Zbuchea, A. Up-to-date use of honey for burns treatment. Ann. Burns Fire Disasters 2014, 27, 22–30. [Google Scholar]
- Kuckelkorn, R.; Schrage, N.; Keller, G.; Redbrake, C. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmol. Scand. 2002, 80, 4–10. [Google Scholar] [CrossRef]
- The Brook. The Working Equid Veterinary Manual. Whittet Books. 2013, p. 528. Available online: https://www.thebrooke.org/our-work/working-equid-veterinary-manual (accessed on 16 October 2020).
- Dai, T.; Huang, Y.Y.; Sharma, S.K.; Hashmi, J.T.; Kurup, D.B.; Hamblin, M.R. Topical Antimicrobials for Burn Wound Infections. Recent. Pat. Antiinfect. Drug Discov. 2010, 5, 124–151. [Google Scholar] [CrossRef]
- Heyneman, A.; Hoeksema, H.; Vandekerckhove, D.; Pirayesh, A.; Monstrey, S. The Role of Silver Sulphadiazine in the Conservative Treatment of Partial Thickness Burn Wounds: A Systematic Review. Burns 2016, 42, 1377–1386. [Google Scholar] [CrossRef]
- Putra, O.N.; Saputro, I.D.; Nurrahman, N.D.; Herawati, E.D.; Dewi, L.K. Effects of empirical antibiotic administration on the level of C-Reactive protein and inflammatory markers in severe burn patients. Ann. Burns Fire Disasters 2020, 33, 20–26. [Google Scholar]
- Takashi, T.; Hiroki, M.; Kiyohide, F.; Hideo, Y. Prophylactic Antibiotics May Improve Outcome in Patients with Severe Burns Requiring Mechanical Ventilation: Propensity Score Analysis of a Japanese Nationwide Database. Clin. Infect. Dis. 2016, 62, 60–66. [Google Scholar] [CrossRef]
- Abdullahi, A.; Amini-Nik, S.; Jeschke, M.G. Animal Models in Burn Research. Cell. Mol. Life Sci. 2014, 71, 3241–3255. [Google Scholar] [CrossRef]
- Hu, R.H.; Yu, Y.M.; Costa, D.; Young, V.R.; Ryan, C.M.; Burke, J.F.; Tompkins, R.G. A rabbit model for metabolic studies after burn injury. J. Surg. Res. 1998, 75, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.L.; Volek, J.S. Guinea pigs: A suitable animal model to study lipoprotein metabolism, atherosclerosis and inflammation. Nutr. Metab. 2006, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, F.J.; Toribio, R.E.; Perez-Ecijaa, A. Donkey Internal Medicine—Part I: Metabolic, Endocrine, and Alimentary Tract Disturbances. J. Equine Vet. Sci. 2018, 65, 66–74. [Google Scholar] [CrossRef]
- Burden, F.; Thiemann, A. Donkeys Are Different. J. Equine Vet. Sci. 2015, 35, 376–382. [Google Scholar] [CrossRef]



| Parameters | D1 | D2 | D1 | D2 | D1 | D2 | RI LRL-URL |
|---|---|---|---|---|---|---|---|
| Day 2 | Day 8 | Day 15 | |||||
| RBC (×106/µL) | 4.6 | 5.5 | 6.6 | 6.6 | 6.8 | 7.0 | 4.4–7.1 |
| Hemoglobin (g/dL) | 11.1 | 9.3 | 11.4 | 11.4 | 12.2 | 13.9 | 8.9–14.7 |
| Hematocrit (%) | 30.0 | 29.0 | 32.0 | 36.0 | 32.0 | 38.0 | 27–42 |
| WBC (×109/L) | 15.1 | 15.8 | 14.8 | 14.2 | 11.1 | 13.4 | 6.2–15.0 |
| S. neutrophils (×109/L) | 8.4 | 7.1 | 6.2 | 6.0 | 6.0 | 5.5 | 2.4–6.3 |
| Lymphocytes (×109/L) | 4.1 | 1.5 | 1.9 | 3.0 | 2.6 | 2.7 | 2.9–9.6 |
| Eosinophils (×109/L) | 0.3 | 0.1 | 0 | 0,7 | 0.1 | 0.1 | 0.1–0.9 |
| Monocytes (×109/L) | 0.2 | 0 | 0.1 | 0 | 0 | 0 | 0–0.75 |
| Thrombocyte (×109/L) | 210 | 146 | 167 | 117 | 133 | 158 | 95–384 |
| Urea (mmol/L) | 4.0 | 3.0 | 4.2 | 3.6 | 1.9 | 2.5 | 1.5–5.2 |
| Creat (µmol/L) | 55 | 72 | 81 | 87 | 70 | 82 | 53–118 |
| ALP (U/L) | 328 | 293 | 291 | 270 | 123 | 201 | 98–252 |
| AST (U/L) | 829 | 712 | 621 | 589 | 399 | 512 | 238–536 |
| GGT (U/L) | 26 | 20 | 36 | 41 | 29 | 36 | 14–69 |
| CK (U/L) | 1024 | 619 | 319 | 375 | 326 | 208 | 128–525 |
| Trig (mmol/L) | 4.2 | 3.2 | 2.5 | 2.1 | 2.2 | 1.8 | 0.6–2.8 |
| Total Proteins (g/L) | 55 | 48 | 58 | 62 | 71 | 58 | 58–76 |
| Albumin (g/L) | 21 | 19 | 23 | 22 | 26 | 28 | 22–32 |
| Globulin (g/L) | 33 | 46 | 39 | 41 | 35 | 47 | 32–48 |
| Tbil (µmol/L) | 0.9 | 0.3 | 0.9 | 1.0 | 2.1 | 2.2 | 0.1–3.7 |
| Chol (mmol/L) | 2.9 | 3.0 | 2.5 | 2.8 | 1.9 | 2.3 | 1.4–2.9 |
| Glucose (mmol/L) | 5.1 | 4.0 | 4.5 | 4.6 | 4.1 | 4.5 | 3.9–4.7 |
| Calcium (mmol/L) | 2.1 | 1.9 | 2.5 | 2.9 | 3.0 | 3.2 | 2.2–3.4 |
| Na (mmol/L) | 130 | 131 | 129 | 135 | 129 | 133 | 128–138 |
| K (mmol/L) | 3.5 | 5.0 | 3.9 | 4.1 | 4.9 | 3.9 | 3.2–5.1 |
| Cl (mmol/L) | 95 | 100 | 98 | 104 | 99 | 104 | 96–106 |
| Parameters | D1 | D2 | RI LRL-URL |
|---|---|---|---|
| RBC (×106/µL) | 4.9 | 6.3 | 4.4–7.1 |
| Hemoglobin (g/dL) | 8.7 | 9.8 | 8.9–14.7 |
| Hematocrit (%) | 31 | 38 | 27–42 |
| WBC (×109/L) | 7.4 | 14.2 | 6.2–15.0 |
| S. neutrophils (×109/L) | 3.1 | 5.8 | 2.4–6.3 |
| Lymphocytes (×109/L) | 3.5 | 6.9 | 2.9–9.6 |
| Eosinophils (×109/L) | 0.1 | 0.3 | 0.1–0.9 |
| Monocytes (×109/L) | 0.2 | 0 | 0–0.75 |
| Thrombocyte (×109/L) | 128 | 305 | 95–384 |
| Urea (mmol/L) | 4.0 | 3.6 | 1.5–5.2 |
| Creat (µmol/L) | 53 | 92 | 53–118 |
| ALP (U/L) | 104 | 211 | 98–252 |
| AST (U/L) | 257 | 322 | 238–536 |
| GGT (U/L) | 26 | 49 | 14–69 |
| CK (U/L) | 130 | 513 | 128–525 |
| Tri (mmol/L) | 0.7 | 2.1 | 0.6–2.8 |
| Total Proteins (g/L) | 63 | 70 | 58–76 |
| Albumin (g/L) | 26 | 23 | 22–32 |
| Globulin (g/L) | 33 | 38 | 32–48 |
| Cho (mmol/L) | 2.2 | 2.7 | 1.4–2.9 |
| Tbil (µmol/L) | 0.4 | 1.5 | 0.1–3.7 |
| Glucose (mmol/L) | 3.9 | 4.1 | 3.9–4.7 |
| Calcium (mmol/L) | 2.3 | 2.6 | 2.2–3.4 |
| Na (mmol/L) | 130 | 134 | 128–138 |
| K (mmol/L) | 3.5 | 4.7 | 3.2–5.1 |
| Cl (mmol/L) | 98 | 104 | 96–106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohse, J.; Pietrantoni, P.; Tummers, C. Management of Thermal Injuries in Donkeys: A Case Report. Animals 2020, 10, 2131. https://doi.org/10.3390/ani10112131
Lohse J, Pietrantoni P, Tummers C. Management of Thermal Injuries in Donkeys: A Case Report. Animals. 2020; 10(11):2131. https://doi.org/10.3390/ani10112131
Chicago/Turabian StyleLohse, Jorge, Pierpaolo Pietrantoni, and Christian Tummers. 2020. "Management of Thermal Injuries in Donkeys: A Case Report" Animals 10, no. 11: 2131. https://doi.org/10.3390/ani10112131
APA StyleLohse, J., Pietrantoni, P., & Tummers, C. (2020). Management of Thermal Injuries in Donkeys: A Case Report. Animals, 10(11), 2131. https://doi.org/10.3390/ani10112131






