A Systematic Literature Review on Depopulation Methods for Swine
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
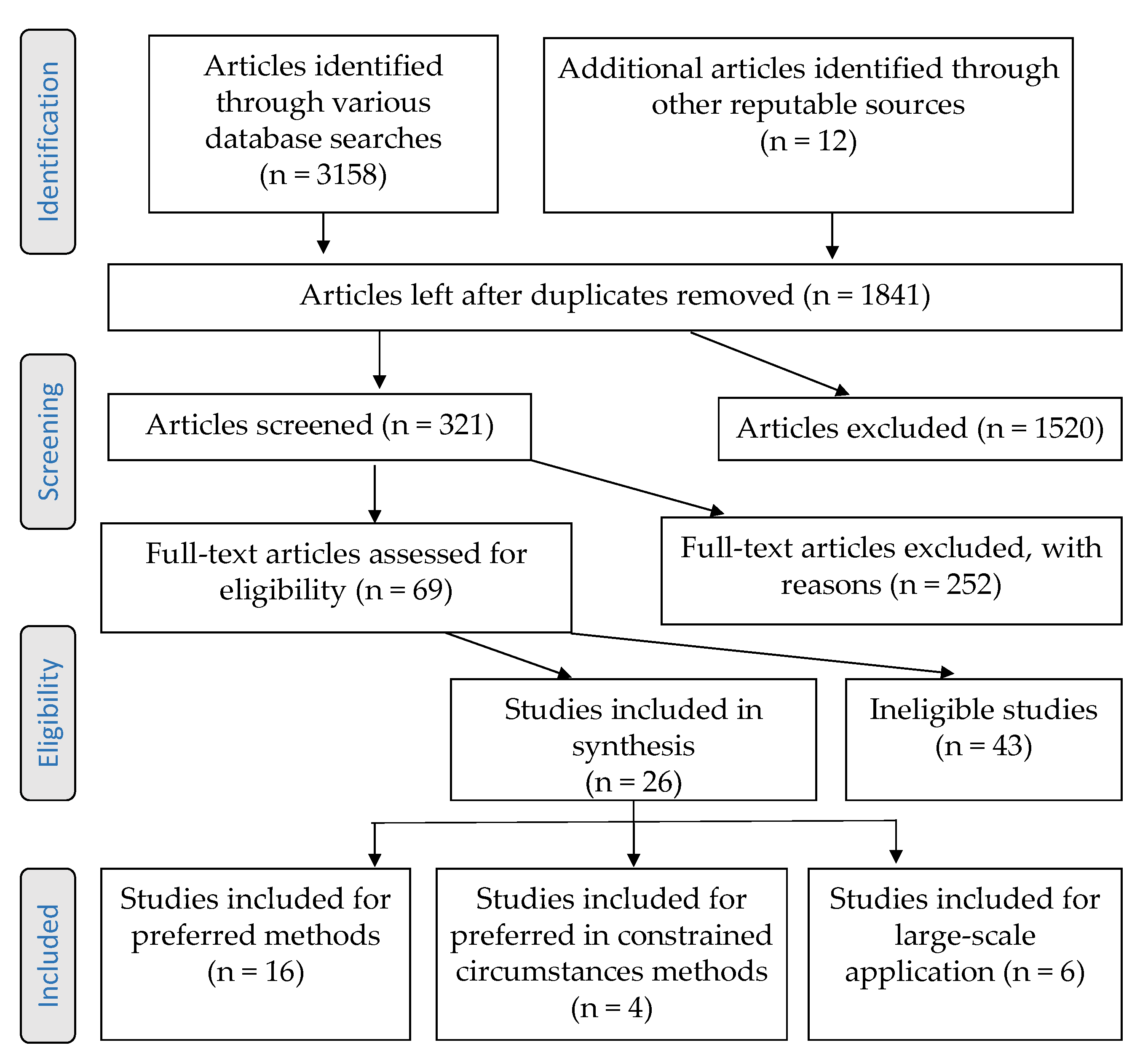
2.1. Article Search Strategy
2.2. Article Screening and Selection Criteria
- Type of study: descriptive and analytical (observational and experimental) studies
- Date of publication: articles published between January 1990 and May 2020
- Geographic focus: worldwide
- Population: applied to individual pigs or groups
- Outcomes/efficiency measurements: speed of unconsciousness, insensibility, and death
2.3. Article Assessment Process
3. Results
3.1. Overview of the Included Studies
3.2. Small-Scale Studies Using AVMA Preferred Depopulation Methods
3.2.1. Injectable Anesthetic Agents
3.2.2. Inhaled Agents/Gas
3.2.3. Physical Methods
3.3. Small-Scale Studies Using Methods Permitted in Constrained Circumstances
3.3.1. Ingested/Oral Formulations
3.3.2. Other: Ventilation Shutdown
3.4. Depopulation Methods Scalable to Large Populations
3.5. Important Considerations for Depopulation Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Veterinary Medical Association (AVMA) Guidelines for the Depopulation of Animals: 2019 Edition. Available online: https://www.avma.org/sites/default/files/resources/AVMA-Guidelines-for-the-Depopulation-of-Animals.pdf (accessed on 10 November 2020).
- Department for Environment Food and Rural Affairs. Archive: Foot and Mouth Disease Outbreak Statistics. Available online: https://webarchive.nationalarchives.gov.uk/20130402162506/http://archive.defra.gov.uk/foodfarm/farmanimal/diseases/atoz/fmd/about/stats.htm (accessed on 10 November 2020).
- Meyer, R.E.; Morrow, W.M. Carbon dioxide for emergency on-farm euthanasia of swine. J. Swine Health Prod. 2005, 13, 210–217. [Google Scholar]
- Howden, K.J.; Brockhoff, E.J.; Caya, F.D.; McLeod, L.J.; Lavoie, M.; Ing, J.D.; Bystrom, J.M.; Alexandersen, S.; Pasick, J.M.; Berhane, Y. An investigation into human pandemic influenza virus (H1N1) 2009 on an Alberta swine farm. Can. Vet. J. 2009, 50, 1153. [Google Scholar] [PubMed]
- Mangen, M.-J.; Burrell, A.; Mourits, M. Epidemiological and economic modelling of classical swine fever: Application to the 1997/1998 Dutch epidemic. Agric. Syst. 2004, 81, 37–54. [Google Scholar] [CrossRef]
- American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals: 2020 Edition. Available online: https://www.avma.org/sites/default/files/2020-01/2020-Euthanasia-Final-1-17-20.pdf (accessed on 10 November 2020).
- JAVMA News: Slaughter Delays Lead to Depopulation: Farms Short of Room as Processors Halt or Slow meat Production Because of COVID-19. Available online: https://www.avma.org/javma-news/2020-06-15/slaughter-delays-lead-depopulation (accessed on 10 November 2020).
- Meyer, R.E.; Whitley, J.T.; Morrow, W.E.; Stikeleather, L.F.; Baird, C.L.; Rice, J.M.; Halbert, B.V.; Styles, D.K.; Whisnant, C.S. Effect of physical and inhaled euthanasia methods on hormonal measures of stress in pigs. J. Swine Health Prod. 2013, 21, 261–269. [Google Scholar]
- Vogel, K.; Badtram, G.; Claus, J.; Grandin, T.; Turpin, S.; Weyker, R.; Voogd, E. Head-only followed by cardiac arrest electrical stunning is an effective alternative to head-only electrical stunning in pigs. J. Anim. Sci. 2011, 89, 1412–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijdicks, E.F. The diagnosis of brain death. N. Engl. J. Med. 2001, 344, 1215–1221. [Google Scholar] [CrossRef]
- Terlouw, C.; Bourguet, C.; Deiss, V. Consciousness, unconsciousness and death in the context of slaughter. Part I. Neurobiological mechanisms underlying stunning and killing. Meat Sci. 2016, 118, 133–146. [Google Scholar] [CrossRef]
- Verhoeven, M.T.W.; Gerritzen, M.A.; Hellebrekers, L.J.; Kemp, B. Indicators used in livestock to assess unconsciousness after stunning: A review. Animal 2015, 9, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Verhoeven, M.; Gerritzen, M.; Velarde, A.; Hellebrekers, L.; Kemp, B. Time to loss of consciousness and its relation to Behavior in slaughter Pigs during stunning with 80 or 95% carbon Dioxide. Front. Vet. Sci. 2016, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Terlouw, C.; Bourguet, C.; Deiss, V. Consciousness, unconsciousness and death in the context of slaughter. Part II. Evaluation methods. Meat Sci. 2016, 118, 147–156. [Google Scholar] [CrossRef]
- McKeegan, D. Mass depopulation. In Advances in Poultry Welfare; Elsevier: Cambridge, UK, 2018; pp. 351–372. [Google Scholar]
- Steiner, A.R.; Axiak Flammer, S.; Beausoleil, N.J.; Berg, C.; Bettschart-Wolfensberger, R.; García Pinillos, R.; Golledge, H.D.; Marahrens, M.; Meyer, R.; Schnitzer, T. Humanely Ending the Life of Animals: Research Priorities to Identify Alternatives to Carbon Dioxide. Animals 2019, 9, 911. [Google Scholar] [CrossRef] [Green Version]
- Velarde, A.; Cruz, J.; Gispert, M.; Carrión, D.; de la Torre, R.J.; Diestre, A.; Manteca, X. Aversion to carbon dioxide stunning in pigs: Effect of carbon dioxide concentration and halothane genotype. Anim. Welf. 2007, 16, 513–522. [Google Scholar]
- Raj, A.; Gregory, N. Welfare implications of the gas stunning of pigs 1. Determination of aversion to the initial inhalation of carbon dioxide or argon. Anim. Welf. 1995, 4, 273–280. [Google Scholar]
- Raj, A.; Gregory, N. Welfare implications of the gas stunning of pigs 2. Stress of induction of anaesthesia. Anim. Welf. 1996, 5, 71–78. [Google Scholar]
- Sadler, L.J.; Hagen, C.; Wang, C.; Widowski, T.; Johnson, A.; Millman, S.T. Effects of flow rate and gas mixture on the welfare of weaned and neonate pigs during gas euthanasia. J. Anim. Sci. 2014, 92, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Kells, N.; Beausoleil, N.; Johnson, C.; Sutherland, M. Evaluation of different gases and gas combinations for on-farm euthanasia of pre-weaned pigs. Animals 2018, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.K.; Rault, J.-L.; Gates, R.S.; Lay, D.C. A two-step process of nitrous oxide before carbon dioxide for humanely euthanizing piglets: On-farm trials. Animals 2018, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Becerril-Herrera, M.; Alonso-Spilsbury, M.; Lemus-Flores, C.; Guerrero-Legarreta, I.; Olmos-Hernández, A.; Ramírez-Necoechea, R.; Mota-Rojas, D. CO2 stunning may compromise swine welfare compared with electrical stunning. Meat Sci. 2009, 81, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, J.C.; Foster, J.A.; Reitz, R.L. Development of a self-contained carbon dioxide euthanasia trailer for large-scale euthanasia of feral swine. Wildl. Soc. Bull. 2016, 40, 316–320. [Google Scholar] [CrossRef]
- Stikeleather, L.; Morrow, W.; Meyer, R.; Baird, C.; Halbert, B. Evaluation of CO2 application requirements for on-farm mass depopulation of swine in a disease emergency. Agriculture 2013, 3, 599–612. [Google Scholar] [CrossRef] [Green Version]
- Rice, M.; Baird, C.; Stikeleather, L.; Morrow, W.M.; Meyer, R. Carbon dioxide system for on-farm euthanasia of pigs in small groups. J. Swine Health Prod. 2014, 22, 248–254. [Google Scholar]
- Meyer, R.E.; Morrow, W.M.; Stikeleather, L.F.; Baird, C.L.; Rice, J.M.; Byrne, H.; Halbert, B.V.; Styles, D.K. Evaluation of carbon dioxide administration for on-site mass depopulation of swine in response to animal health emergencies. J. Am. Vet. Med. Assoc. 2014, 244, 924–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, M.; Bryer, P.; Backus, B. The effect of age and method of gas delivery on carbon dioxide euthanasia of pigs. Anim. Welf. 2017, 26, 293–299. [Google Scholar] [CrossRef]
- The EndNote Team. EndNote; Version X9 64 bit; Clarivate: Philadelphia, PA, USA, 2013. [Google Scholar]
- Shea, B.J.; Grimshaw, J.M.; Wells, G.A.; Boers, M.; Andersson, N.; Hamel, C.; Porter, A.C.; Tugwell, P.; Moher, D.; Bouter, L.M. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Llonch, P.; Rodriguez, P.; Jospin, M.; Dalmau, A.; Manteca, X.; Velarde, A. Assessment of unconsciousness in pigs during exposure to nitrogen and carbon dioxide mixtures. Anim. Int. J. Anim. Biosci. 2013, 7, 492. [Google Scholar] [CrossRef]
- Zhang, Q. Euthanasia of Pigs Using Nitrogen Gas and Decompression. In Proceedings of the 2017 International Symposium on Animal Environment and Welfare, Chongqing, China, 23–25 October 2017; Volume 279, p. 286. [Google Scholar]
- Casey-Trott, T.; Millman, S.; Turner, P.; Nykamp, S.; Widowski, T. Effectiveness of a nonpenetrating captive bolt for euthanasia of piglets less than 3 d of age. J. Anim. Sci. 2013, 91, 5477–5484. [Google Scholar] [CrossRef]
- Widowski, T.; Elgie, R.; Lawlis, P. Assessing the effectiveness of a non-penetrating captive bolt for euthanasia of newborn piglets. In Proceedings of the Allen D. Leman Swine Conference, St. Paul, MN, USA, 22 September 2008; Volume 107, p. 111. [Google Scholar]
- Grist, A.; Lines, J.A.; Knowles, T.G.; Mason, C.W.; Wotton, S.B. The use of a non-penetrating captive bolt for the euthanasia of neonate piglets. Animals 2018, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Blackmore, D.; Bowling, M.; Madie, P.; Nutman, A.; Barnes, G.; Davies, A.; Donoghue, M.; Kirk, E. The use of a shotgun for the emergency slaughter or euthanasia of large mature pigs. N. Z. Vet. J. 1995, 43, 134–137. [Google Scholar] [CrossRef]
- Shearer, J.K.; Griffin, D.; Cotton, S.E. Humane Euthanasia and Carcass Disposal. Vet. Clin. Food Anim. Pract. 2018, 34, 355–374. [Google Scholar] [CrossRef]
- Pluimers, F.H.; De Leeuw, P.W.; Smak, J.A.; Elbers, A.R.W.; Stegeman, J.A. Classical swine fever in The Netherlands 1997–1998: A description of organisation and measures to eradicate the disease. Prev. Vet. Med. 1999, 42, 139–155. [Google Scholar] [CrossRef]
- Stegeman, A.; Elbers, A.; de Smit, H.; Moser, H.; Smak, J.; Pluimers, F. The 1997–1998 epidemic of classical swine fever in the Netherlands. Vet. Microbiol. 2000, 73, 183–196. [Google Scholar] [CrossRef]
- Staples, L.D.; Lapidge, S.; Cowled, B.; Humphrys, S. Nitrite Salts as Poisons in Baits for Omnivores. U.S. Patent No. 8,795,649 B2, 5 August 2014. [Google Scholar]
- Snow, N.P.; Foster, J.A.; Kinsey, J.C.; Humphrys, S.T.; Staples, L.D.; Hewitt, D.G.; Vercauteren, K.C. Development of toxic bait to control invasive wild pigs and reduce damage. Wildl. Soc. Bull. 2017, 41, 256–263. [Google Scholar] [CrossRef]
- Lapidge, S.; Wishart, J.; Staples, L.; Fagerstone, K.; Campbell, T.A.; Eisemann, J.D. Development of a feral swine toxic bait (Hog-Gone®) and bait hopper (Hog-HopperTM) in Australia and the USA. In Proceedings of the 14th WDM Conference; Frey, S.N., Ed.; USDA National Wildlife Research Center—Staff Publications: Fort Collins, CO, USA, 2012; Volume 19, p. 24. Available online: https://digitalcommons.unl.edu/icwdm_usdanwrc/1158/ (accessed on 18 November 2020).
- Foster, J.A.; Martin, J.C.; VerCauteren, K.; Phillips, G.E.; Eisemann, J.D. Optimization of Formulations for the Lethal Control of Feral Pigs; University of California: Davis, CA, USA; USDA National Wildlife Research Center—Staff Publications: Fort Collins, CO, USA, 2014; pp. 277–280. Available online: https://core.ac.uk/download/pdf/188125995.pdf (accessed on 18 November 2020).
- Shapiro, L.; Eason, C.; Bunt, C.; Hix, S.; Aylett, P.; MacMorran, D. Efficacy of encapsulated sodium nitrite as a new tool for feral pig management. J. Pest. Sci. 2016, 89, 489–495. [Google Scholar] [CrossRef]
- Ventilation Failure Alarm: 2 Case Studies. Available online: http://www.dicam.co.uk/wp-content/uploads/filebase/research/Case_Study_2_ventilation_failure_incidents.pdf (accessed on 10 November 2020).
- On Farm Euthanasia of Swine: Recommendations for the Producer. Available online: https://www.aasv:documents/2016EuthRec-EN.pdf (accessed on 10 November 2020).
- Zhao, Y.; Xin, H.; Li, L. Modelling and validating the indoor environment and supplemental heat requirement during ventilation shutdown (VSD) for rapid depopulation of hens and turkeys. Biosyst. Eng. 2019, 184, 130–141. [Google Scholar] [CrossRef]
- Killing of Animals for Disease Control Purposes. Available online: https://www.oie.int/fileadmin/home/eng/Health_standards/tahc/current/chapitre_aw_killing.pdf (accessed on 10 November 2020).
- Fiedler, K.; Parsons, R.; Sadler, L.; Millman, S. Effects of stocking rate on measures of efficacy and welfare during argon gas euthanasia of weaned pigs. Anim. Welf. 2016, 25, 83–89. [Google Scholar] [CrossRef]
- Whiting, T.L.; Marion, C.R. Perpetration-induced traumatic stress—A risk for veterinarians involved in the destruction of healthy animals. Can. Vet. J. 2011, 52, 794. [Google Scholar]
- Mort, M.; Convery, I.; Baxter, J.; Bailey, C. Animal disease and human trauma: The psychosocial implications of the 2001 UK foot and mouth disease disaster. J. Appl. Anim. Welf. Sci. 2008, 11, 133–148. [Google Scholar] [CrossRef]
- Galvin, J.; Blokhuis, H.; Chimbombi, M.; Jong, D.; Wotton, S. Killing of animals for disease control purposes. Rev. Sci. Tech. Int. Off. Epizoot. 2005, 24, 711–722. [Google Scholar] [CrossRef]
- United States Hog Inventory Up 5 Percent. Available online: https://www.nass.usda.gov/Newsroom/2020/06-25-2020.php (accessed on 10 November 2020).
- DeNicola, A.J.; Miller, D.S.; DeNicola, V.L.; Meyer, R.E.; Gambino, J.M. Assessment of humaneness using gunshot targeting the brain and cervical spine for cervid depopulation under field conditions. PLoS ONE 2019, 14, e0213200. [Google Scholar] [CrossRef]
- Laureys, S. Death, unconsciousness and the brain. Nat. Rev. Neurosci. 2005, 6, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Forslid, A. Transient neocortical, hippocampal and qmygdaloid EEG silence induced by one minute inhalation of high concentration CO2 in swine. Acta Physiol. Scand. 1987, 130, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Forslid, A.; Augustinsson, O. Acidosis, hypoxia and stress hormone release in response to one-minute inhalation of 80% CO2 in swine. Acta Physiol. Scand. 1988, 132, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Martoft, L.; Stødkilde-Jørgensen, H.; Forslid, A.; Pedersen, H.D.; Jørgensen, P.F. CO2 induced acute respiratory acidosis and brain tissue intracellular pH: A 31P NMR study in swine. Lab. Anim. 2003, 37, 241–248. [Google Scholar] [CrossRef]
- Martoft, L.; Lomholt, L.; Kolthoff, C.; Rodriguez, B.E.; Jensen, E.W.; Jørgensen, P.F.; Pedersen, H.D.; Forslid, A. Effects of CO2 anaesthesia on central nervous system activity in swine. Lab. Anim. 2002, 36, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Anton, F.; Euchner, I.; Handwerker, H.O. Psychophysical examination of pain induced by defined CO2 pulses applied to the nasal mucosa. Pain 1992, 49, 53–60. [Google Scholar] [CrossRef]
- Anil, M. Studies on the return of physical reflexes in pigs following electrical stunning. Meat Sci. 1991, 30, 13–21. [Google Scholar] [CrossRef]
- Jongman, E.; Barnett, J.; Hemsworth, P. The aversiveness of carbon dioxide stunning in pigs and a comparison of the CO2 stunner crate vs. the V-restrainer. Appl. Anim. Behav. Sci. 2000, 67, 67–76. [Google Scholar] [CrossRef]
- Effect of Genetics on Handling and CO2 Stunning of Pigs. Available online: https://www.grandin.com/humane/meatfocus7-92.html (accessed on 10 November 2020).
- Llonch, P.; Dalmau, A.; Rodriguez, P.; Manteca, X.; Velarde, A. Aversion to nitrogen and carbon dioxide mixtures for stunning pigs. Anim. Welf. 2012, 21, 33–39. [Google Scholar] [CrossRef]
- Small, A.; Lea, J.; Niemeyer, D.; Hughes, J.; McLean, D.; McLean, J.; Ralph, J. Development of a microwave stunning system for cattle 2: Preliminary observations on behavioural responses and EEG. Res. Vet. Sci. 2019, 122, 72–80. [Google Scholar] [CrossRef]
- Gurung, S.; Hoffman, J.; Stringfellow, K.; Abi-Ghanem, D.; Zhao, D.; Caldwell, D.; Lee, J.; Styles, D.; Berghman, L.; Byrd, J. Depopulation of caged layer hens with a compressed air foam system. Animals 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, E.; Alphin, R.; Dawson, M.; Malone, G. Use of water-based foam to depopulate ducks and other species. Poult. Sci. 2009, 88, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Bolanos-Lopez, D.; Concepcion-Mendez, M.; Ramirez-Telles, J.; Roldan-Santiago, P.; Flores-Peinado, S.; Mora-Medina, P. Stunning swine with CO2 gas: Controversies related to animal welfare. Int. J. Pharmacol. 2012, 8, 141–151. [Google Scholar] [CrossRef]
- Campler, M.R.; Pairis-Garcia, M.D.; Rault, J.-L.; Coleman, G.; Arruda, A.G. Caretaker attitudes toward swine euthanasia. Transl. Anim. Sci. 2018, 2, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Mullins, C.; Pairis-Garcia, M.; George, K.; Anthony, R.; Johnson, A.; Coleman, G.; Rault, J.; Millman, S. Determination of swine euthanasia criteria and analysis of barriers to euthanasia in the United States using expert opinion. Anim. Welf. 2017, 26, 449–459. [Google Scholar] [CrossRef]
- Pimentel, D.; Lach, L.; Zuniga, R.; Morrison, D. Environmental and economic costs of nonindigenous species in the United States. BioScience 2000, 50, 53–65. [Google Scholar] [CrossRef]
- Jolley, D.B.; Ditchkoff, S.S.; Sparklin, B.D.; Hanson, L.B.; Mitchell, M.S.; Grand, J.B. Estimate of herpetofauna depredation by a population of wild pigs. J. Mammal. 2010, 91, 519–524. [Google Scholar] [CrossRef] [Green Version]
- WAP World Animal Protection (WAP) Urging AVMA to Address Cruel Depopulation Methods. Available online: https://dkt6rvnu67rqj.cloudfront.net/cdn/ff/ed6j2SHzFuIXOPfvvowP9I5LdygW3ZZfwGK_NweWhjU/1588000688/public/media/WAP_Letter_to_AVMA_April_22_2020.pdf (accessed on 10 November 2020).
| Age Category | Method | Preferred | Permitted in Constrained Circumstances |
|---|---|---|---|
| All ages | Injectable | Anesthetic overdose, intravenous barbiturates and barbituric acid derivatives | Expired, compounded, non-pharmaceutical grade injectable euthanasia agents or anesthetics |
| Inhaled/gas | Carbon dioxide | ||
| Physical methods | Gunshot to the head (not appropriate for depopulation of suckling pigs), penetrating captive bolt, electrocution | ||
| Ingested/Oral | Sodium nitrate | ||
| Other | Ventilation shutdown (VSD) or VSD plus | ||
| Only for suckling and young pigs | Physical methods | Manual blunt force trauma, non-penetrating captive bolt |
| National Pork Board (NPB), 2016 [47] | OIE, 2019 [53] | ||||||
|---|---|---|---|---|---|---|---|
| Human Safety Risk | Skill Required | Aesthetics | Limitations | Approved for | Restraint Necessary | Animal Welfare Concerns If Applied Inappropriately | |
| Carbon dioxide (CO2) | moderate | moderate to low, based on equipment design | bloodless, some excitatory movement or vocalization | practical for small pigs only; maintenance of equipment | all ages (may not be practical for pigs over 70 pounds (31.7 kg)) | yes | slow induction of unconsciousness, averseness of induction |
| Gunshot | high | moderate to high | discharge of blood from wound | security of firearms; legal restrictions; maintenance of equipment | nursery pigs or older | no | non-lethal wounding |
| Non-penetrating captive bolt | low | low | minimal to some blood discharge as a 1-step process | may be a 2-step process based on equipment design and size of pig; maintenance of equipment | pigs less than 70 pounds (31.7 kg) | yes | non-lethal wounding |
| Penetrating captive bolt | moderate | moderate | discharge of blood from wound | may be a 2-step process depending on equipment design; maintenance of equipment | pigs greater than 12 pounds (5.4 kg) | yes | ineffective stunning, non-lethal wounding, regaining of consciousness before death |
| Electrocution (head-only and head-to-heart) | low if proper lock out/tag out procedure followed | moderate | muscle contraction; minimal to no blood discharge | adequate amperage and voltage needed; head only is a 2-step process; cleanliness of electrodes | pigs over 3 days of age | yes | pain associated with cardiac arrest after ineffective stunning; design of the stunning tongs not appropriate for the small head or body of neonates |
| Veterinarian administered anesthetic overdose | low | high, veterinary administration only | no blood discharge, limited pig movements | applicable agents available only to licensed veterinarian; carcass disposal | all ages but may not be practical | yes | non-lethal dose, pain associated with injection site |
| Manual blunt force trauma | low | moderate | some blood discharge; objectionable for some | only applicable to small pigs; ability of caretaker to apply sufficient force | pigs up to 12 pounds (5.4 kg) | yes | non-lethal wounding |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arruda, A.G.; Beyene, T.J.; Kieffer, J.; Lorbach, J.N.; Moeller, S.; Bowman, A.S. A Systematic Literature Review on Depopulation Methods for Swine. Animals 2020, 10, 2161. https://doi.org/10.3390/ani10112161
Arruda AG, Beyene TJ, Kieffer J, Lorbach JN, Moeller S, Bowman AS. A Systematic Literature Review on Depopulation Methods for Swine. Animals. 2020; 10(11):2161. https://doi.org/10.3390/ani10112161
Chicago/Turabian StyleArruda, Andréia G., Tariku J. Beyene, Justin Kieffer, Joshua N. Lorbach, Steven Moeller, and Andrew S. Bowman. 2020. "A Systematic Literature Review on Depopulation Methods for Swine" Animals 10, no. 11: 2161. https://doi.org/10.3390/ani10112161






