Gonadectomy in Raccoons: Anesthetic and Cardiorespiratory Effects of Two Ketamine-Based Pre-Anesthetic Protocols before Sevoflurane-Sufentanil
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Procedures
2.2. Statistical Analysis
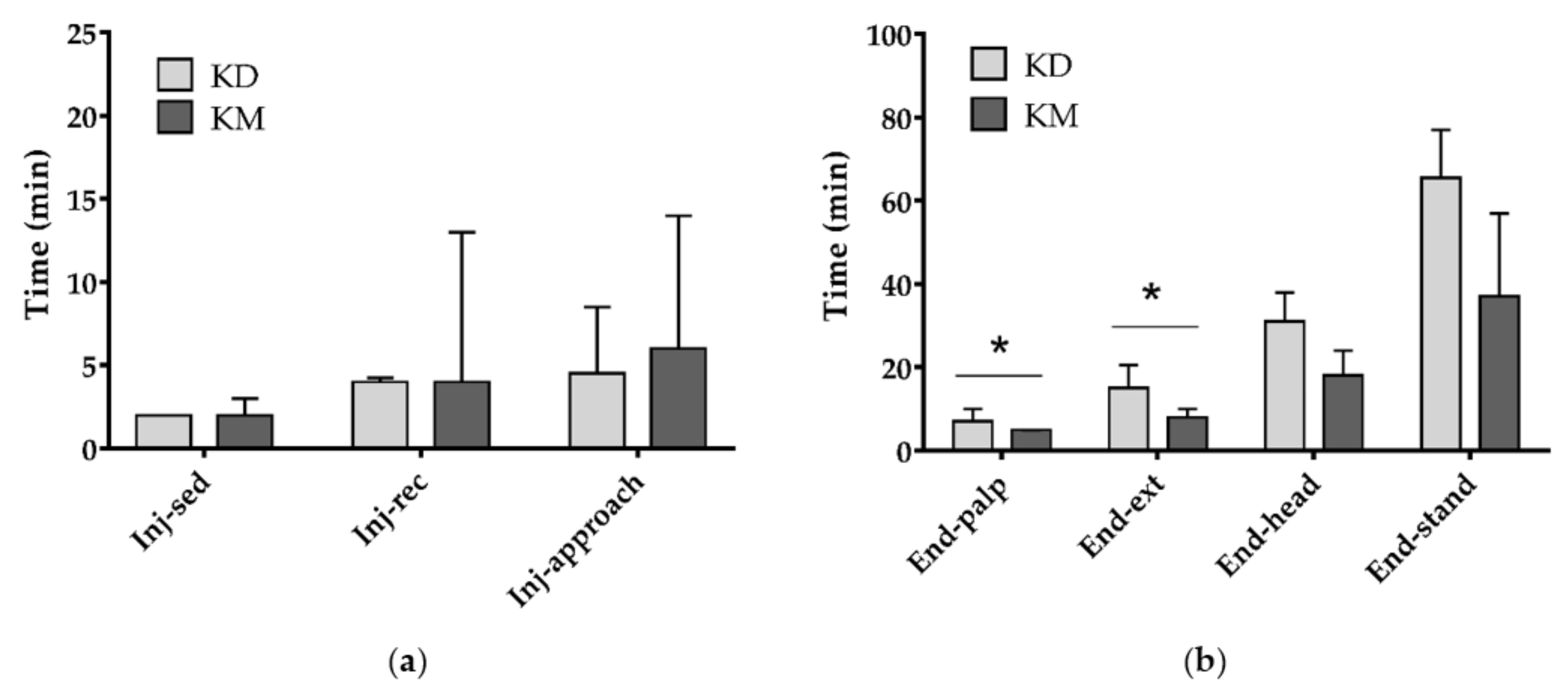
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Decreto del Ministero dell’Ambiente del 19 Aprile 1996. Elenco delle Specie Animali che Possono Costituire Pericolo per la Salute e L’incolumità Pubblica e di cui è Proibita la Detenzione. Available online: https://www.minambiente.it/sites/default/files/archivio/normativa/dim_19_04_1996_aggiornato_2001.pdf (accessed on 22 October 2020).
- Mazzamuto, M.V.; Wauters, L.A.; Bisi, F.; Martinoli, A. Procyon lotor. In Strategia di Azione e Degli Interventi per il Controllo e la Gestione delle Specie Alloctone in Regione Lombardia; Bisi, F., Montagnani, C., Cardarelli, E., Manenti, R., Trasforini, S., Gentili, R., Eds.; 2018; Available online: https://www.naturachevale.it/wp-content/uploads/2019/01/Strategia-per-il-controllo-e-la-gestione-delle-specie-aliene-invasive.pdf (accessed on 13 November 2020).
- Sorvillo, F.; Ash, L.; Berlin, O.G.V.; Morse, S.A. Baylisascaris procyonis: An emerging helminthic zoonosis. Emerg. Infect. Dis. 2002, 8, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Duscher, T.; Hodžić, A.; Glawischnig, W.; Duscher, G.G. The raccoon dog (Nyctereutes procyonoides) and the raccoon (Procyon lotor)—Their role and impact of maintaining and transmitting zoonotic diseases in Austria, Central Europe. Parasitol. Res. 2017, 116, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Mathews, F.; Honess, P.; Wolfensohn, S. Use of inhalation anaesthesia for wild mammals in the field. Vet Rec. 2002, 150, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, S.K.; Strahl-Heldreth, D.; Fiorello, C.V.; Harms, C.A. Best-practice guidelines for field-based surgery and anesthesia of free-ranging wildlife. I. anesthesia and analgesia. J. Wildl. Dis. 2016, 52, S14–S27. [Google Scholar] [CrossRef] [PubMed]
- Seal, U.S.; Kreeger, T.J. Chemical immobilization of furbearers. In Wild Furbearer Management and Conservation in North America; Novak, M.J., Baker, A., Obbard, M.E., Malloch, B., Eds.; Ontario Trappers Association: North Bay, ON, Canada, 1987; pp. 191–215. [Google Scholar]
- Clutton, R.E.; Duggan, L.B. Saffan anesthesia in the Raccoon: A preliminary report. J. Zoo Anim. Med. 1986, 17, 91–99. [Google Scholar] [CrossRef]
- Hoilien, J.; Oates, D. Tranquilizer use in wildlife damage control. In Great Plains Wildlife Damage Control Workshop Proceedings; University of Nebraska: Lincoln, NE, USA, 1981; pp. 71–77. [Google Scholar]
- Belant, J.L. Field Immobilization of Raccoons (Procyon lotor) with Telazol and Xylazine. J. Wildl. Dis. 2004, 40, 787–790. [Google Scholar] [CrossRef]
- Pitt, J.; Larivière, S.; Messier, F. Efficacy of Zoletil® for field immobilization of raccoons. Wildl. Soc. Bull. 2006, 34, 1045–1048. [Google Scholar] [CrossRef]
- Bigler, W.J.; Hoff, G.L. Anesthesia of Raccoons with Ketamine Hydrochloride. J. Wildl. Manag. 1974, 38, 364–366. [Google Scholar] [CrossRef]
- Gregg, D.A.; Olson, L.D. The use of ketamine hydrochloride as an anesthetic for raccoons. J. Wildl. Dis. 1975, 11, 335–337. [Google Scholar] [CrossRef][Green Version]
- Norment, J.L.; Elliott, C.L.; Costello, P.S. Another look at the chemical immobilization of raccoons (Procyon lotor) with ketamine hydrochloride. J. Wildl. Dis. 1994, 30, 541–544. [Google Scholar] [CrossRef][Green Version]
- Baldwin, J.R.; Winstead, J.B.; Hayden-Wing, L.D.; Kreeger, T.J.; Dzialak, M.R. Field sedation of coyotes, red foxes, and raccoons with medetomidine and atipamezole. J. Wildl. Manag. 2008, 72, 1267–1271. [Google Scholar] [CrossRef]
- Allan, M.R. The use of ketamine-xylazine and ketamine-medetomidine with and without their antagonists, yohimbine and atipamezole hydrochloride to immobilize Raccoons (Procyon lotor) in Ontario, Canada. Can. Field Nat. 2015, 129, 84–89. [Google Scholar] [CrossRef][Green Version]
- Reynolds, P.S. When power calculations won’t do: Fermi approximation of animal numbers. Lab. Anim. 2019, 48, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, G.C. Techniques for Determining Age of Raccoons; Biol. Notes; Natural History Survey Division: Urbana, IL, USA, 1961; Volume 45, pp. 1–16. [Google Scholar]
- Nannarone, S.; Moretti, G.; Bellocchi, F.; Menchetti, L.; Bufalari, A. A comparative study of intramuscular alfaxalone- or ketamine-based anesthetic mixtures in gray squirrels undergoing gonadectomy: Clinical and physiologic findings. Animals 2020, 10, 1402. [Google Scholar] [CrossRef]
- Menchetti, L.; Guelfi, G.; Speranza, R.; Carotenuto, P.; Moscati, L.; Diverio, S. Benefits of dietary supplements on the physical fitness of German Shepherd dogs during a drug detection training course. PLoS ONE 2019, 14, e0218275. [Google Scholar] [CrossRef]
- Beck, C. Vetalar® (ketamine hydrochloride), a unique cataleptoid anesthetic agent for multispecies usage. J. Zoo Anim. Med. 1976, 7, 11–38. [Google Scholar] [CrossRef]
- Ramsden, R.O.; Coppin, P.F.; Johnston, D.H. Clinical observations on the use of ketamine hydrochloride in wild carnivores. J. Wildl. Dis. 1976, 2, 221–225. [Google Scholar] [CrossRef]
- Robert, K.; Garant, D.; Pelletier, F. Chemical immobilization of raccoons (Procyon lotor) with ketamine-medetomidine mixture and reversal with atipamezole. J. Wildl. Dis. 2012, 48, 122–130. [Google Scholar] [CrossRef]
- Hime, J.M. Use of ketamine hydrochloride in non-domesticated cats. Vet. Rec. 1974, 31, 193–195. [Google Scholar] [CrossRef]
- Evans, R.H. Raccoons and Relatives (Carnivora, Procyonidae). In Zoological Restraint and Anesthesia; Heard, D., Ed.; International Veterinary Information Service: Ithaca/New York, NY, USA, 2002. [Google Scholar]
- Kollias, G.V.; Abou-Madi, N. Procyonids and Mustelids. In Zoo Animal & Wildlife Immobilization and Anesthesia—Zoo Animal and Wildlife Immobilization and Anesthesia, 1st ed.; West, G., Heard, D., Nigel Caulkett, N., Eds.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 417–427. [Google Scholar]
- Wellington, D.; Mikaelian, I.; Singer, L. Comparison of ketamine-xylazine and ketamine-dexmedetomidine anesthesia and intraperitoneal tolerance in rats. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 481–487. [Google Scholar] [CrossRef]
- Dupras, J.; Vachon, P.; Cuvelliez, S.; Blais, D. Anesthesie du lapin de Nouvelle-Zelande utilisant les combinaisons tiletamine-zolazepam et ketamine-midazolam avec ou sans xylazine. Can. Vet. J. 2001, 42, 455–460. [Google Scholar] [PubMed]
- Valverde, A.; Skelding, A.M. Alternatives to Opioid Analgesia in Small Animal Anesthesia: Alpha-2 Agonists. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Deresienski, D.T.; Rupprecht, C.E. Yohimbine reversal of ketamine-xylazine immobilization of raccoons (Procyon lotor). J. Wildl. Dis. 1989, 25, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Vogler, B.R.; Elias, K.; Steiner-Valentin, K.H.S. Anaesthesia in captive raccoons (Procyon lotor) during seasonal obesity. Proc. Int. Conf. Dis. Zoo Wild Anim. 2010, 6–9. [Google Scholar] [CrossRef]
- Cattet, M.R.L.; Obbard, M.E. Use of hyaluronidase to improve chemical immobilization of free-ranging polar bears (Ursus maritimus). J. Wildl. Dis. 2010, 46, 246–250. [Google Scholar] [CrossRef]
- Kropf, J.; Hughes, J.M.L. Effect of midazolam on the quality and duration of anaesthetic recovery in healthy dogs undergoing elective ovariohysterectomy or castration. Vet. Anaesth. Analg. 2019, 46, 587–596. [Google Scholar] [CrossRef]
- Abdul-Rasool, I.H.; Ward Denham, S. Ventilatory and Cardiovascular Responses to Sufentanil Infusion in Dogs Anesthetized with Isoflurane. Anesth. Analg. 1989, 69, 300–306. [Google Scholar] [CrossRef]
- Polis, I.; Moens, Y.; Hoebenà, D.; Tshamala, M.; Hoybergs, Y.; Gasthuys, F. Cardiopulmonary effects of sufentanil long acting on sevoflurane anaesthesia in dogs. Vet. Anaesth. Analg. 2006, 33, 111–121. [Google Scholar] [CrossRef]
- Reilly, S.; Seddighi, R.; Egger, C.M.; Rohrbach, B.W.; Doherty, T.J.; Qu, W.; Johnson, J.R. The effect of fentanyl on the end-tidal sevoflurane concentration needed to prevent motor movement in dogs. Vet. Anaesth. Analg. 2013, 40, 290–296. [Google Scholar] [CrossRef]
- Chinnadurai, S.K.; Williams, C. The minimum alveolar concentration of sevoflurane in ring-tailed lemurs (Lemur catta) and aye-ayes (Daubentonia madagascariensis). Vet. Anaesth. Analg. 2016, 43, 76–80. [Google Scholar] [CrossRef]

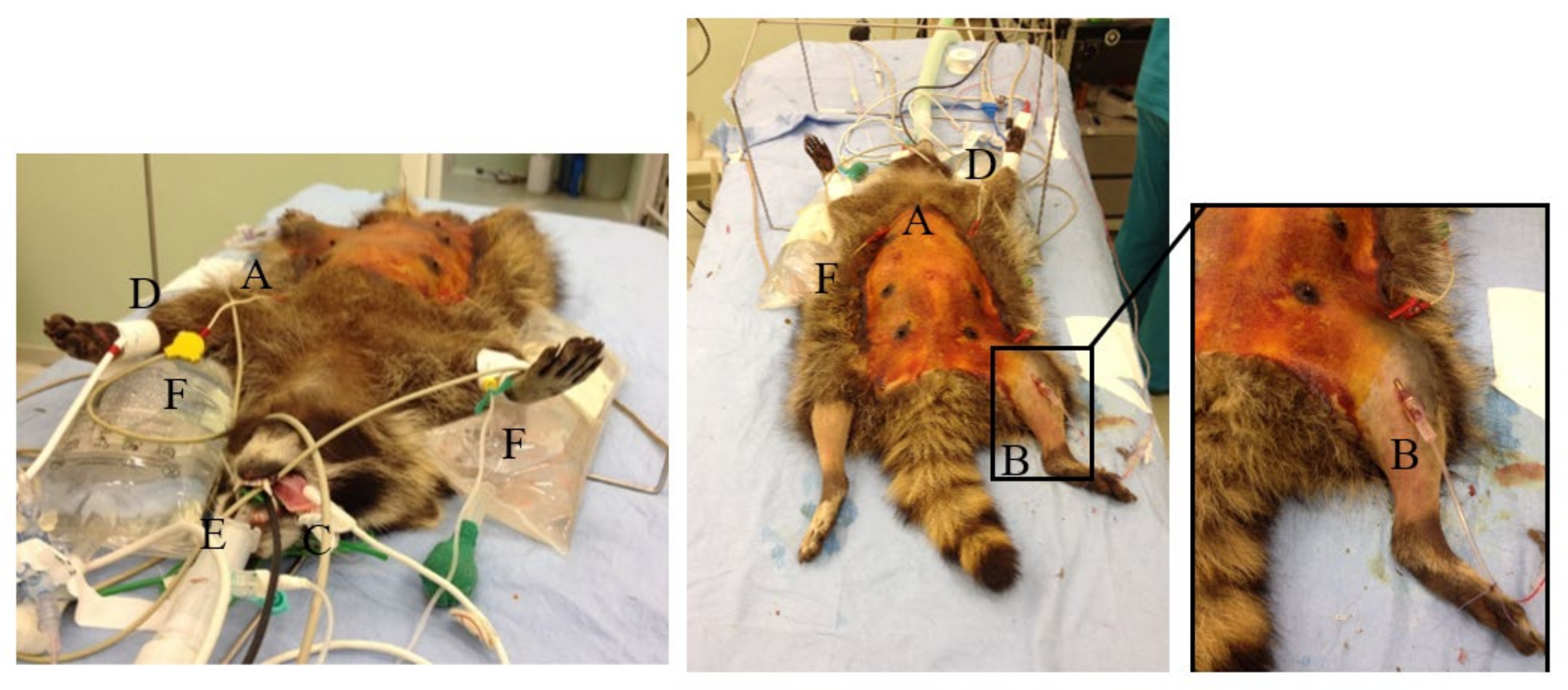
| Score | DEPTH OF ANESTHESIA |
| 1 | Inadequate: Responsive to approach, no safe handling. Additional ½ dose is required |
| 2 | Mild: Purposeful response to stimulation (toe pinch). Additional ½ dose is required |
| 3 | Profound: Relaxed and unresponsive to stimulation (toe pinch) |
| Score | MYORELAXATION |
| 1 | Absent: Muscle tone in the pelvic and thoracic limbs, pedal reflex, persistent jaw tone, no relaxed tongue |
| 2 | Mild: Some muscle tone in thoracic limbs and pedal reflex, no relaxed tongue |
| 3 | Moderate: Some muscle tone in the pelvic or thoracic limbs, no jaw tone |
| 4 | Excellent: No muscle tone in pelvic and thoracic limbs, no jaw tone, relaxed tongue |
| Score | CEPHALIC CATHETERIZATION |
| 1 | Possible, evident limb retraction |
| 2 | Possible, mild limb retraction |
| 3 | Possible, without limb retraction |
| Score | EASE OF INTUBATION |
| 1 | Trachea intubation possible after drug administration (tongue retraction, swallowing reflex) |
| 2 | Trachea intubation possible without drug (no jaw tone, relaxed tongue) |
| Parameter | Group | |
|---|---|---|
| KD | KM | |
| Body weight (kg, mean ± SD) | ||
| 7.00 ± 1.98 | 7.10 ± 0.81 | |
| Gender (n, %) | ||
| Female Male | 5 (50.0%) | 4 (57.1%) |
| 5 (50.0%) | 3 (42.9%) | |
| Pregnant animals (n, % of females) | ||
| No Yes | 2 (40.0%) | 2 (50.0%) |
| 3 (60.0%) | 2 (50.0%) | |
| Age * (n, %) | ||
| YoungAdult | 2 (20.0%) | 1 (14.3%) |
| 8 (80.0%) | 6 (85.7%) | |
| Parameter | Group | Total | p Value | ||
|---|---|---|---|---|---|
| KD | KM | ||||
| Depth of anesthesia | Inadequate | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.593 |
| Mild | 2 (20.0%) | 3 (42.9%) | 5 (29.4%) | ||
| Profound | 8 (80.0%) | 4 (57.1%) | 12 (70.6%) | ||
| Muscle relaxation | Absent | 2 a (20.0%) | 1 a (14.3%) | 3 (17.6%) | 0.003 |
| Mild | 0 a (0.0%) | 3 b (42.9%) | 3 (17.6%) | ||
| Moderate | 1 a (10.0%) | 3 a (42.9%) | 4 (23.5%) | ||
| Excellent | 7 a (70.0%) | 0 b (0.0%) | 7 (41.2%) | ||
| Cephalic catheterization | Evident limb retraction | 0 (0.0%) | 1 (14.3%) | 1 (5.9%) | 0.338 |
| Mild limb retraction | 1 (10.0%) | 2 (28.6%) | 3 (17.6%) | ||
| No limb retraction | 9 (90.0%) | 4 (57.1%) | 13 (76.5%) | ||
| Palpebral reflex | No | 1 (10.0%) | 1 (14.3%) | 2 (11.8%) | 1.000 |
| Yes | 9 (90.0%) | 6 (85.7%) | 15 (88.2%) | ||
| Response to tactile stimulus | No | 8 (80.0%) | 6 (85.7%) | 14 (82.4%) | 1.000 |
| Yes | 2 (20.0%) | 1 (14.3%) | 3 (17.6%) | ||
| Tongue relaxation | No | 3 (30.0%) | 5 (71.4%) | 8 (47.1%) | 0.153 |
| Yes | 7 (70.0%) | 2 (28.6%) | 9 (52.9%) | ||
| Ease of intubation | After propofol | 3 a (30.0%) | 7 b (100.0%) | 10 (58.8%) | 0.010 |
| Without other drugs | 7 a (70.0%) | 0 b (0.0%) | 7 (41.2%) | ||
| Parameter | Baseline Value * | Group | p Value | ||||
|---|---|---|---|---|---|---|---|
| KD | KM | Group | Time | Group × Time | Baseline | ||
| Temp. (°C) | 36.3 | 35.5 ± 0.1 | 35.6 ± 0.1 | 0.376 | 0.007 | 0.406 | <0.001 |
| SAP (mmHg) | 115.4 | 108.2 ± 1.7 | 116.2 ± 1.7 | 0.002 | 0.901 | 0.906 | <0.001 |
| MAP (mmHg) | 90.7 | 86.7 ± 1.5 | 92.4 ± 1.5 | 0.011 | 0.748 | 0.313 | <0.001 |
| DAP (mmHg) | 71.3 | 68.2 ± 1.8 | 75.7 ± 1.8 | 0.006 | 0.621 | 0.332 | <0.001 |
| SpO2 (%) | 97.8 | 98.6 ± 0.2 | 97.7 ± 0.2 | 0.009 | 0.703 | 0.286 | <0.001 |
| RR (breaths/min) | 13.3 | 10.2 ± 0.6 | 10.4 ± 0.6 | 0.788 | 0.098 | 0.106 | <0.001 |
| HR (beats/min) | 114.7 | 105.2 ± 2.5 | 109.3 ± 2.9 | 0.342 | 0.935 | 0.734 | <0.001 |
| etCO2 (mmHg) | 40.6 | 33.1 ± 1.3 | 34.1 ± 1.4 | 0.888 | 0.340 | 0.419 | 0.004 |
| Parameter | Group | p Value | |||
|---|---|---|---|---|---|
| KD | KM | ||||
| Median | IQR | Median | IQR | ||
| pH | 7.26 | (7.19–7.34) | 7.11 | (7.06–7.15) | 0.017 |
| PaCO2 (mmHg) | 49.10 | (40.50–56.50) | 63.10 | (60.40–70.10) | 0.033 |
| PaO2 (mmHg) | 535 | (473–562) | 442 | (423–562) | 0.517 |
| BE (mmol/L) | −4 | (−5–−3) | −7 | (−12–−7) | 0.067 |
| HCO3− (mmol/L) | 22.2 | (20.3–24.6) | 22.3 | (17.7–22.9) | 0.833 |
| TCO2 (mmol/L) | 24 | (22–24) | 24 | (20–25) | 1.000 |
| SaO2 (%) | 100 | (100–100) | 100 | (100–100) | 1.000 |
| Na+ (mmol/L) | 147 | (145–149) | 152 | (150–154) | 0.286 |
| K+ (mmol/L) | 3.8 | (3.6–4.0) | 3.4 | (3.2–3.5) | 0.143 |
| iCa2+ (mmol/L) | 1.26 | (1.22–1.29) | 1.13 | (1.12–1.14) | 0.286 |
| Hct (%) | 29 | (22–38) | 19 | (16–21) | 0.143 |
| Hb (g/dL) | 9.85 | (7.50–12.90) | 6.25 | (5.40–7.10) | 0.143 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nannarone, S.; De Monte, V.; Arcelli, R.; Menchetti, L.; Gialletti, R. Gonadectomy in Raccoons: Anesthetic and Cardiorespiratory Effects of Two Ketamine-Based Pre-Anesthetic Protocols before Sevoflurane-Sufentanil. Animals 2020, 10, 2110. https://doi.org/10.3390/ani10112110
Nannarone S, De Monte V, Arcelli R, Menchetti L, Gialletti R. Gonadectomy in Raccoons: Anesthetic and Cardiorespiratory Effects of Two Ketamine-Based Pre-Anesthetic Protocols before Sevoflurane-Sufentanil. Animals. 2020; 10(11):2110. https://doi.org/10.3390/ani10112110
Chicago/Turabian StyleNannarone, Sara, Valentina De Monte, Rolando Arcelli, Laura Menchetti, and Rodolfo Gialletti. 2020. "Gonadectomy in Raccoons: Anesthetic and Cardiorespiratory Effects of Two Ketamine-Based Pre-Anesthetic Protocols before Sevoflurane-Sufentanil" Animals 10, no. 11: 2110. https://doi.org/10.3390/ani10112110
APA StyleNannarone, S., De Monte, V., Arcelli, R., Menchetti, L., & Gialletti, R. (2020). Gonadectomy in Raccoons: Anesthetic and Cardiorespiratory Effects of Two Ketamine-Based Pre-Anesthetic Protocols before Sevoflurane-Sufentanil. Animals, 10(11), 2110. https://doi.org/10.3390/ani10112110







