Detection of Plasmid-Mediated Colistin Resistant mcr-1 Gene in Escherichia coli Isolated from Infected Chicken Livers in Nepal
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Sample Collection, Bacterial Isolation and Identification
2.2. Antimicrobial Susceptibility Testing
2.3. Determination of Minimum Inhibitory Concentration (MIC) of Colistin
2.4. Quality Control
2.5. Extraction of Plasmid DNA and PCR Amplification of Colistin Resistance Gene (mcr-1)
2.6. Statistical Analysis
2.7. Ethics Approval and Consent to Participate
3. Results
3.1. Distribution of E. coli
3.2. Antibiotic Susceptibility Pattern
3.3. Determination of MIC and Colistin-Resistant E. coli Isolates
3.4. Prevalence of mcr-1 among Colistin-Resistant E. coli Isolates from Poultry Infected Livers
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rafique, M.; Potter, R.F.; Ferreiro, A.; Wallace, M.A.; Rahim, A.; Ali Malik, A.; Siddique, N.; Abbas, M.A.; D’Souza, A.W.; Burnham, C.D.; et al. Genomic Characterization of Antibiotic Resistant Escherichia coli Isolated from Domestic Chickens in Pakistan. Front. Microbiol. 2019, 10, 3052. [Google Scholar] [CrossRef] [PubMed]
- De Carli, S.; Ikuta, N.; Lehmann, F.K.; da Silveira, V.P.; de Melo Predebon, G.; Fonseca, A.S.; Lunge, V.R. Virulence gene content in Escherichia coli isolates from poultry flocks with clinical signs of colibacillosis in Brazil. Poult. Sci. 2015, 94, 2635–2640. [Google Scholar] [CrossRef] [PubMed]
- Manges, A.R.; Johnson, J.R. Food-borne origins of Escherichia coli causing extraintestinal infections. Clin. Infect. Dis. 2012, 55, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Kayastha, K.; Dhungel, B.; Karki, S.; Adhikari, B.; Banjara, M.R.; Rijal, K.R.; Ghimire, P. Extended-Spectrum Beta-Lactamase-Producing Escherichia coli and Klebsiella species in Pediatric Patients Visiting International Friendship Children’s Hospital, Kathmandu, Nepal. Infect. Dis. 2020, 13, 1178633720909798. [Google Scholar]
- Dhungana, K.; Awal, B.K.; Dhungel, B.; Sharma, S.; Banjara, M.R.; Rijal, K.R. Detection of Klebsiella pneumoniae carbapenemase (KPC) and metallo betalactamae (MBL) producing Gram negative bacteria isolated from different clinical samples in a Transplant Center, Kathmandu, Nepal. ASMI 2019, 2, 60–69. [Google Scholar]
- Amin, M.B.; Sraboni, A.S.; Hossain, M.I.; Roy, S.; Mozmader, T.; Unicomb, L.; Rousham, E.K.; Islam, M.A. Occurrence and genetic characteristics of mcr-1 positive colistin resistant E. coli from poultry environments in Bangladesh. J. Glob. Antimicrob. Resist. 2020, 22, 546–552. [Google Scholar] [CrossRef]
- Falagas, M.E.; Karageorgopoulos, D.E.; Nordmann, P. Therapeutic options for infections with Enterobacteriaceae producing carbapenem-hydrolyzing enzymes. Future Microbiol. 2011, 6, 653–666. [Google Scholar] [CrossRef]
- MacNair, C.R.; Stokes, J.M.; Carfrae, L.A.; Fiebig-Comyn, A.A.; Coombes, B.K.; Mulvey, M.R.; Brown, E.D. Overcoming mcr-1 mediated colistin resistance with colistin in combination with other antibiotics. Nat. Commun. 2018, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Temkin, E.; Adler, A.; Lerner, A.; Carmeli, Y. Carbapenem-resistant Enterobacteriaceae: Biology, epidemiology, and management. Ann. N. Y. Acad. Sci. 2014, 1323, 22–42. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Poirel, L.; Kieffer, N.; Liassine, N.; Thanh, D.; Nordmann, P. Plasmid-mediated carbapenem and colistin resistance in a clinical isolate of Escherichia coli. Lancet Infect. Dis. 2016, 16, 281. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Wang, J.; Butaye, P.; Huang, K.; Qiu, H.; Zhang, X.; Gong, W.; Wang, C. Molecular detection of colistin resistance genes (mcr-1 to mcr-5) in human vaginal swabs. BMC Res. Notes 2018, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Mello, M.M.; Vieira, J.; Simoes, F.V.; Freire, S.V.; Moraes, R.; Santos, R.P. Microbial resistance to colistin in consequence of mutations in the mcr-1 gene of Escherichia coli. MOJ Public Health 2018, 7, 59–63. [Google Scholar]
- Van Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T.; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing antimicrobial use in food animals. Science 2017, 357, 1350–1352. [Google Scholar] [CrossRef]
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef]
- Chowdhury, R.; Haque, M.; Islam, K.M.S.; Khaleduzzaman, A.B.M. A review on Antibiotics in an Animal Feed. Bangladesh J. Anim. Sci. 2009, 38, 22–32. [Google Scholar] [CrossRef]
- Rokka, M.; Jestoi, M.; Peltonen, K. Trace level determination of polyether ionophores in feed. BioMed Res. Int. 2013, 2013, 151363. [Google Scholar] [CrossRef]
- Kabir, J.; Umoh, V.J.; Audu-okoh, E.; Umoh, J.U.; Kwaga, J.K.P. Veterinary Drug Use in Poultry Farms and Determination of Antimicrobial Drug Residues in Commercial Eggs and Slaughtered Chicken in Kaduna State, Nigeria. Food Control 2004, 15, 99–105. [Google Scholar] [CrossRef]
- Seri, H.I. Introduction to Veterinary Drug Residues: Hazards and Risks. In Proceedings of the Workshop: Veterinary Drug Residues in Food Derived from Animals (Our Goal of Protecting Consumers), The National Medicinal and Poisons Board, Khartoum, Sudan, 26–27 May 2013; Available online: http://www.sustech.edu/staff_publications/2013070315212363.pdf (accessed on 22 December 2019).
- Dominguez, J.E.; Redondo, L.M.; Figueroa Espinosa, R.A.; Cejas, D.; Gutkind, G.O.; Chacana, P.A.; Di Conza, J.A.; Fernandez Miyakawa, M.E. Simultaneous Carriage of mcr-1 and Other Antimicrobial Resistance Determinants in Escherichia coli from Poultry. Front. Microbiol. 2018, 9, 1679. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Gautam, G.; Devkota, B.; Thapaliya, S. Recent case flow pattern in veterinary teaching Hospital of Agriculture and Forestry University, Chitwan, Nepal. J. Agric. For. Univ. 2017, 1, 119–128. [Google Scholar]
- Shrestha, E.K.; Dhakal, I.P.; Sapkota, M.; Manandhar, P.; Rijal, T.B. Antimicrobial resistance pattern of Eshcerichia coli isolates from chicken and human samples in Chitwan. Nepal. Vet. J. 2011, 30, 38–44. [Google Scholar]
- Subedi, M.; Luitel, H.; Devkota, B.; Bhattarai, R.K.; Phuyal, S.; Panthi, P.; Shrestha, A.; Chaudhary, D.K. Antibiotic resistance pattern and virulence genes content in avian pathogenic Escherichia coli (APEC) from broiler chickens in Chitwan, Nepal. BMC Vet. Res. 2018, 14, 113. [Google Scholar] [CrossRef]
- Overdevest, I.; Willemsen, I.; Rijnsburger, M.; Eustace, A.; Xu, L.; Hawkey, P.; Heck, M.; Savelkoul, P.; Vandenbroucke-Grauls, C.; van der Zwaluw, K.; et al. Extended-spectrum beta-lactamase genes of Escherichia coli in chicken meat and humans, The Netherlands. Emerg. Infect. Dis. 2011, 17, 1216–1222. [Google Scholar] [CrossRef]
- Guragin, N.; Pradhan, A.; Dhungel, B.; Banjara, M.R.; Rijal, K.R.; Ghimire, P. Extended spectrum B-lactamase producing Gram Negative bacterial isolates from urine of patients visiting Everest Hospital, Kathmandu, Nepal. TUJM 2019, 6, 26–31. [Google Scholar] [CrossRef]
- Clinical Laboratory Standards Institute (CLSI). M100 Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; An Informational Supplement; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. [Google Scholar] [CrossRef]
- Gurung, S.; Kafle, S.; Dhungel, B.; Adhikari, N.; Shrestha, U.T.; Adhikari, B.; Banjara, M.R.; Rijal, K.R.; Ghimire, P. Detection of OXA-48 Gene in Carbapenem-Resistant Escherichia coli and Klebsiella pneumoniae from Urine Samples. Infect. Drug Resist. 2020, 13, 2311–2321. [Google Scholar] [CrossRef]
- Birnboim, H.C.; Doly, J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979, 7, 1513–1523. [Google Scholar] [CrossRef]
- Newton-Foot, M.; Snyman, Y.; Maloba, M.R.B.; Whitelaw, A.C. Plasmid-mediated mcr-1 colistin resistance in Escherichia coli and Klebsiella spp. clinical isolates from the Western Cape region of South Africa. Antimicrob. Resist. Infect. Control 2017, 6, 78. [Google Scholar] [CrossRef]
- Mellata, M. Human and avian extraintestinal pathogenic Escherichia coli: Infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog. Dis. 2013, 10, 916–932. [Google Scholar] [CrossRef]
- Adeyanju, G.T.; Ishola, O. Salmonella and Escherichia coli contamination of poultry meat from a processing plant and retail markets in Ibadan, Oyo State, Nigeria. Springerplus 2014, 3, 139. [Google Scholar] [CrossRef]
- Lee, G.Y.; Jang, H.I.; Hwang, I.G.; Rhee, M.S. Prevalence and classification of pathogenic Escherichia coli isolated from fresh beef, poultry, and pork in Korea. Int. J. Food Microbiol. 2009, 134, 196–200. [Google Scholar] [CrossRef]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef]
- Joshi, P.R.; Thummeepak, R.; Leungtongkam, U.; Pooarlai, R.; Paudel, S.; Acharya, M.; Dhital, S.; Sitthisak, S. The emergence of colistin-resistant Escherichia coli in chicken meats in Nepal. FEMS Microbiol. Lett. 2019, 366. [Google Scholar] [CrossRef]
- Sobur, M.A.; Ievy, S.; Haque, Z.F.; Nahar, A.; Zaman, S.B.; Rahman, M.T. Emergence of colistin-resistant Escherichia coli in poultry, house flies, and pond water in Mymensingh, Bangladesh. J. Adv. Vet. Anim. Res. 2019, 6, 50–53. [Google Scholar]
- Omer, M.M.; Abusalab, S.M.; Gumaa, M.M.; Mulla, S.A.; Omer, E.A.; Jeddah, I.E.; Al-Hassan, A.M.; Hussein, M.A.; Ahmed, A.M. Outbreak of colibacillosis among broiler and layer flocks in Intensive and semi Intensive poultry farms in Kassala State, Eastern Sudan. Asian J. Poult. Sci. 2010, 4, 173–181. [Google Scholar] [CrossRef][Green Version]
- Ferens, W.A.; Hovde, C.J. Escherichia coli O157:H7: Animal reservoir and sources of human infection. Foodborne Pathog. Dis. 2011, 8, 465–487. [Google Scholar] [CrossRef]
- Matin, M.A.; Islam, M.A.; Khatun, M.M. Prevalence of colibacillosis in chickens in greater Mymensingh district of Bangladesh. Vet. World 2017, 10, 29–33. [Google Scholar] [CrossRef]
- Tonu, N.S.; Sufian, M.A.; Sarker, S.; Kamal, M.M.; Rahman, M.H.; Hossain, M.M. Pathological study of colibacillosis in chicken and detection of E. coli by PCR, Bangladesh. J. Vet. Med. 2011, 9, 17–25. [Google Scholar]
- Landman, W.J.; van Eck, J.H. The incidence and economic impact of the Escherichia coli peritonitis syndrome in Dutch poultry farming. Avian Pathol. 2015, 44, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Omer, M.M.; Khaldga, A.; Abusalab, S.M.; Gumma Mulla, S.A.; Ahmed, A.M. An outbreak of gumboro disease associated with Colibacillosis among broiler and layer chicks in Kassala state, Eastern Sudan. Res. J. Poult. Sci. 2008, 2, 27–28. [Google Scholar]
- Koenen, M.E.; Boonstra-Blom, A.G.; Jeurissen, S.H.M. Immunological differences between layer- and broiler-type chickens. Vet. Immunol. Immunopathol. 2002, 89, 47–56. [Google Scholar] [CrossRef]
- Annual Technical Report 2074/075 (2017/018): Government of Nepal MoAaLD, Department of Livestock Services, Central Veterinary Laboratory, Tripureshwor, Kathmandu, Nepal. Available online: www.cvl.gov.np (accessed on 20 January 2020).
- Sarker, S.; Mannan, S.; Ali, Y.; Bayzid Ahad, A.; Bupasha, Z.B. Antibiotic resistance of E. coli isolated from broilers sold at live bird markets in Chattogramm Bangladesh. J. Adv. Vet. Anim. Res. 2019, 3, 272–277. [Google Scholar] [CrossRef]
- van den Bogaard, A.E.; Stobberingh, E.E. Epidemiology of resistance to antibiotics. Links between animals and humans. Int. J. Antimicrob. Agents 2000, 14, 327–335. [Google Scholar] [CrossRef]
- Khanal, T.; Raut, S.B.; Paneru, U. Study of antibiotic resistance in E. coli in commercial poultry of Nepal. Nepal. Vet. J. 2017, 34, 6–17. [Google Scholar] [CrossRef]
- van den Bogaard, A.E.; London, N.; Driessen, C.; Stobberingh, E.E. Antibiotic resistance of faecal Escherichia coli in poultry, poultry farmers and poultry slaughterers. J. Antimicrob. Chemother. 2001, 47, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Muktan, B.; Thapa Shrestha, U.; Dhungel, B.; Mishra, B.C.; Shrestha, N.; Adhikari, N.; Banjara, M.R.; Adhikari, B.; Rijal, K.R.; Ghimire, P. Plasmid mediated colistin resistant mcr-1 and co-existence of OXA-48 among Escherichia coli from clinical and poultry isolates: First report from Nepal. Gut Pathog. 2020, 12, 4. [Google Scholar] [CrossRef]
- Pokharel, S.; Raut, S.; Adhikari, B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob. Health 2019, 4, e002104. [Google Scholar] [CrossRef]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Wang, J.; Yassin, A.K.; Butayae, P.; Kelly, P.; Gong, J.; Guo, W.; Li, J.; Li, M.; et al. Molecular detection of colistin resistance genes (mcr-1, mcr-2 and mcr-3) in nasal/oropharyngeal and anal/cloacal swabs from pigs and poultry. Sci. Rep. 2018, 8, 3705. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Wang, J.; Butayae, P.; Kelly, P.; Li, M.; Yang, F.; Gong, J.; Yassin, A.K.; Guo, W.; et al. Newly identified colistin resistance genes, mcr-4 and mcr-5, from upper and lower alimentary tract of pigs and poultry in China. PLoS ONE 2018, 13, e0193957. [Google Scholar] [CrossRef]
- Morales, A.S.; Fragoso de Araujo, J.; de Moura Gomes, V.T.; Reis Costa, A.T.; dos Prazeres Rodrigues, D.; Porfida Ferreira, T.S.; de Lima Filsner, P.H.; Felizardo, M.R.; Micke Moreno, A. Colistin resistance in Escherichia coli and Salmonella enterica strains isolated from swine in Brazil. Sci. World J. 2012, 2012, 109795. [Google Scholar] [CrossRef]
- Barlaam, A.; Parisi, A.; Spinelli, E.; Caruso, M.; Taranto, P.D.; Normanno, G. Global Emergence of Colistin-Resistant Escherichia coli in Food Chains and Associated Food Safety Implications: A Review. J. Food Prot. 2019, 82, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Shen, Z.; Zhang, C.; Song, L.; Wang, B.; Shang, J.; Yue, X.; Qu, Z.; Li, X.; Wu, L.; et al. Surveillance of antimicrobial resistance among Escherichia coli from chicken and swine, China, 2008–2015. Vet. Microbiol. 2017, 203, 49–55. [Google Scholar] [CrossRef]
- Azam, M.; Ehsan, I.; Sajjad Ur, R.; Saleemi, M.K.; Javed, M.R.; Mohsin, M. Detection of the colistin resistance gene mcr-1 in avian pathogenic Escherichia coli in Pakistan. J. Glob. Antimicrob. Resist. 2017, 11, 152–153. [Google Scholar] [CrossRef]
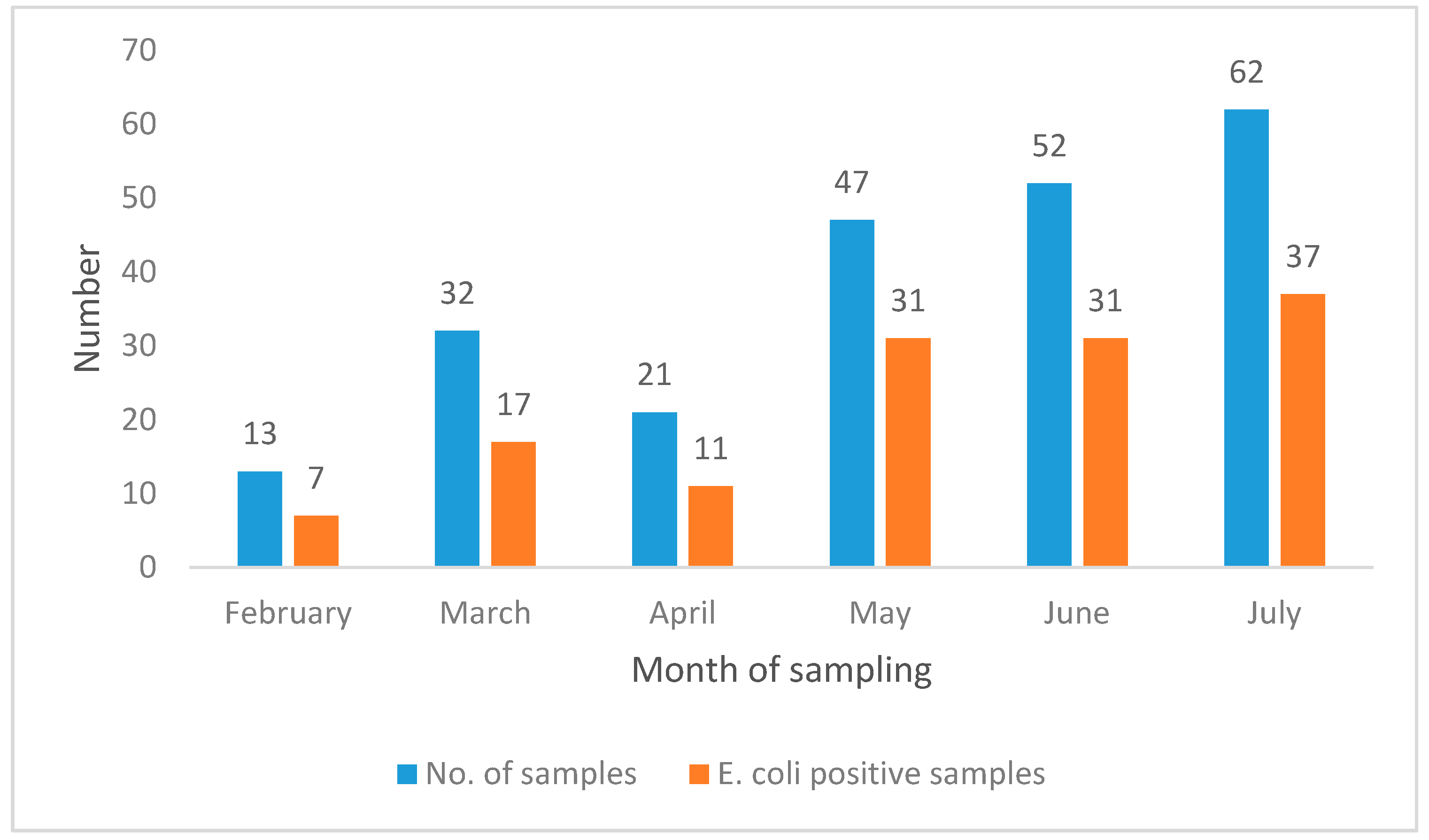

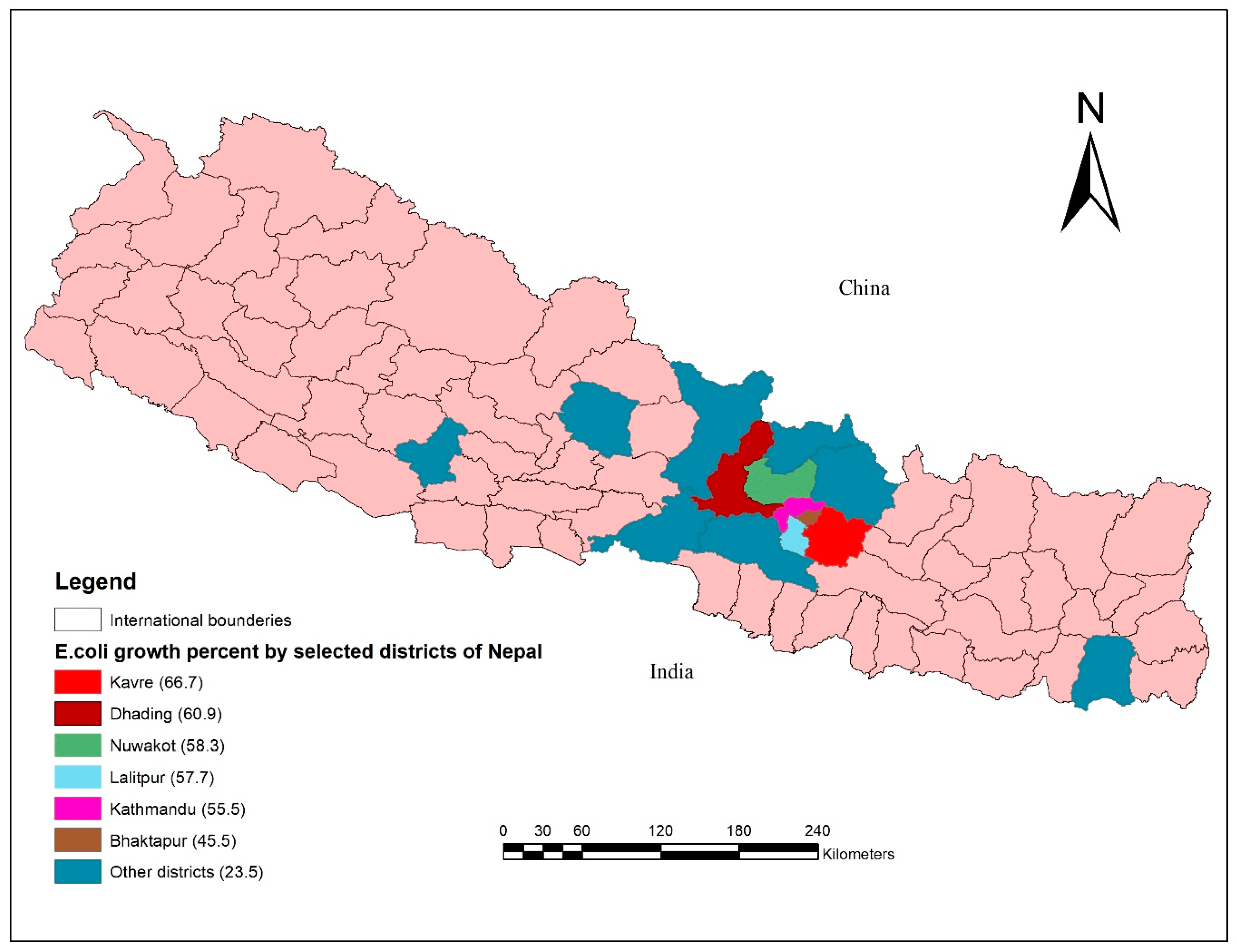
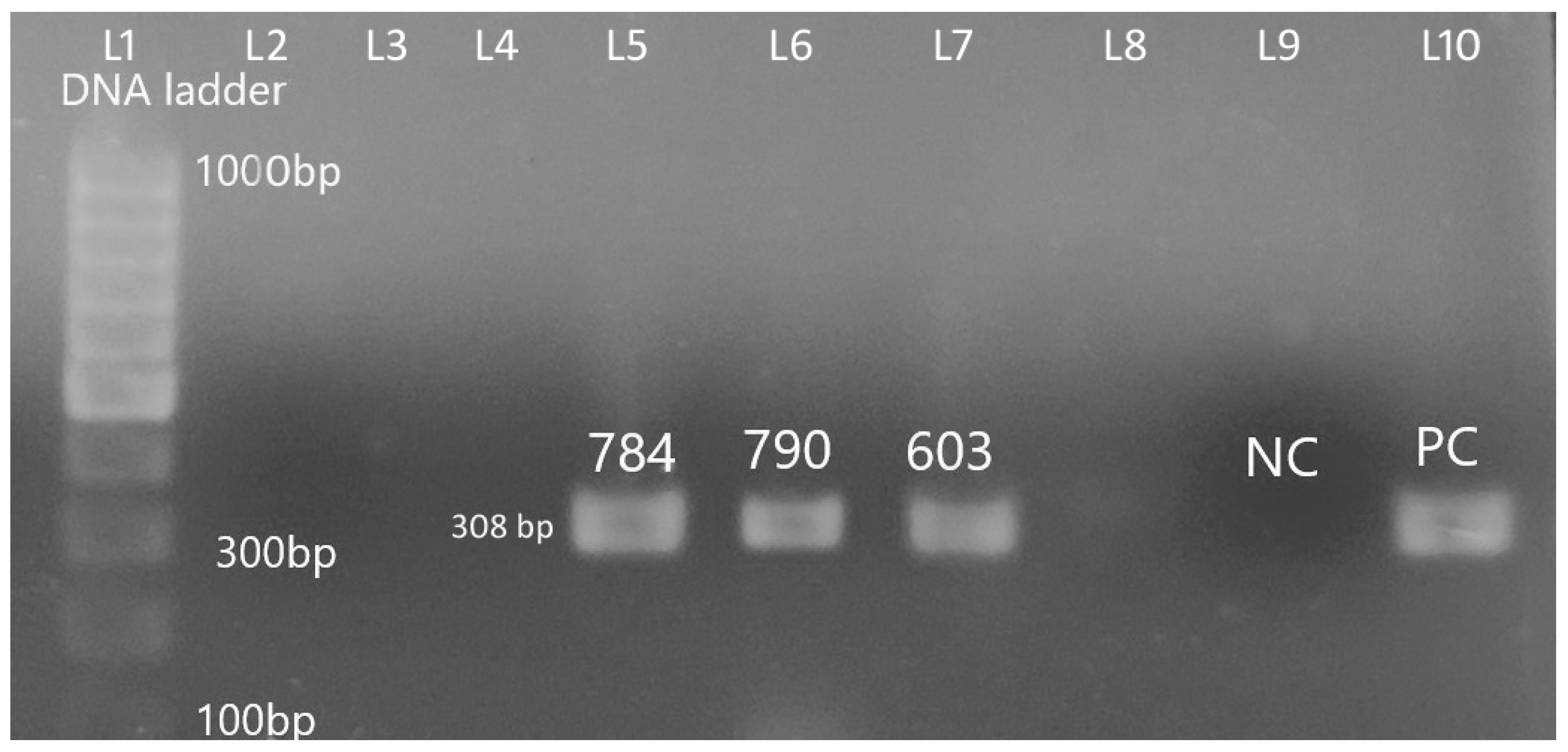
| Age (Days) | E. coli | No Growth | Total | p-Value |
| ≤40 | 109(54%) | 93(46%) | 202(74.8) | 0.72 |
| >40 | 35(51.5%) | 33(48.5%) | 68(25.2%) | |
| Total | 144 | 126 | 270(100%) | |
| Flock Size | E. coli | No Growth | Total | p-Value |
| >1500 | 67(74.4%) | 23(25.6%) | 90(33.3%) | 0.0001 |
| ≤1500 | 77(42.8%) | 103(57.2) | 180(66.7% | |
| Total | 144 | 126 | 270(100%) | |
| Type of Poultry | E. coli | No Growth | Total | p-Value |
| Broiler | 133(58.6%) | 94(41.4%) | 227(84.1%) | 0.0001 |
| Layer | 11(25.6%) | 32(74.4%) | 43(15.9%) | |
| Total | 144 | 126 | 270 (100%) |
| Antibiotic Category | Antibiotics | Susceptibility Pattern | |||
|---|---|---|---|---|---|
| Resistant | Sensitive | ||||
| N. | % | N. | % | ||
| Aminoglycosides | Amikacin | 103 | 71.5 | 41 | 28.5 |
| Gentamicin | 110 | 76.4 | 34 | 23.6 | |
| Penicillins | Ampicillin | 88 | 61.1 | 56 | 38.9 |
| Cephamycins | Cefoxitin | 19 | 13.2 | 125 | 86.8 |
| 3rd generation cephalosporins | Ceftriaxone | 46 | 31.9 | 98 | 68.1 |
| Phenicols | Chloramphenicol | 84 | 58.3 | 60 | 41.7 |
| Fluoroquinolones | Ciprofloxacin | 119 | 82.6 | 25 | 17.4 |
| Levofloxacin | 110 | 76.4 | 34 | 23.6 | |
| Folate pathway inhibitors | Cotrimoxazole | 98 | 68.1 | 46 | 31.9 |
| Carbapenems | Imipenem | 37 | 25.7 | 107 | 74.3 |
| Tetracyclines | Tetracycline | 137 | 95.1 | 7 | 4.9 |
| Organism | Number | Concentration of Colistin | Total | |||
|---|---|---|---|---|---|---|
| 4 mg/L | 8 mg/L | 16 mg/L | 32 mg/L | |||
| E. coli | 144 | 14 (34.1%) | 10 (24.4%) | 8 (19.5%) | 9 (22%) | 41 (28.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bista, S.; Thapa Shrestha, U.; Dhungel, B.; Koirala, P.; Gompo, T.R.; Shrestha, N.; Adhikari, N.; Joshi, D.R.; Banjara, M.R.; Adhikari, B.; et al. Detection of Plasmid-Mediated Colistin Resistant mcr-1 Gene in Escherichia coli Isolated from Infected Chicken Livers in Nepal. Animals 2020, 10, 2060. https://doi.org/10.3390/ani10112060
Bista S, Thapa Shrestha U, Dhungel B, Koirala P, Gompo TR, Shrestha N, Adhikari N, Joshi DR, Banjara MR, Adhikari B, et al. Detection of Plasmid-Mediated Colistin Resistant mcr-1 Gene in Escherichia coli Isolated from Infected Chicken Livers in Nepal. Animals. 2020; 10(11):2060. https://doi.org/10.3390/ani10112060
Chicago/Turabian StyleBista, Sayara, Upendra Thapa Shrestha, Binod Dhungel, Pragya Koirala, Tulsi Ram Gompo, Nabaraj Shrestha, Nabaraj Adhikari, Dev Raj Joshi, Megha Raj Banjara, Bipin Adhikari, and et al. 2020. "Detection of Plasmid-Mediated Colistin Resistant mcr-1 Gene in Escherichia coli Isolated from Infected Chicken Livers in Nepal" Animals 10, no. 11: 2060. https://doi.org/10.3390/ani10112060
APA StyleBista, S., Thapa Shrestha, U., Dhungel, B., Koirala, P., Gompo, T. R., Shrestha, N., Adhikari, N., Joshi, D. R., Banjara, M. R., Adhikari, B., Rijal, K. R., & Ghimire, P. (2020). Detection of Plasmid-Mediated Colistin Resistant mcr-1 Gene in Escherichia coli Isolated from Infected Chicken Livers in Nepal. Animals, 10(11), 2060. https://doi.org/10.3390/ani10112060







