Validation of an Alternative Feather Sampling Method to Measure Corticosterone
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Husbandry
2.3. Sampling Protocol
2.4. Analysis of the Corticosterone Level in Feathers
2.5. Statistical Analysis
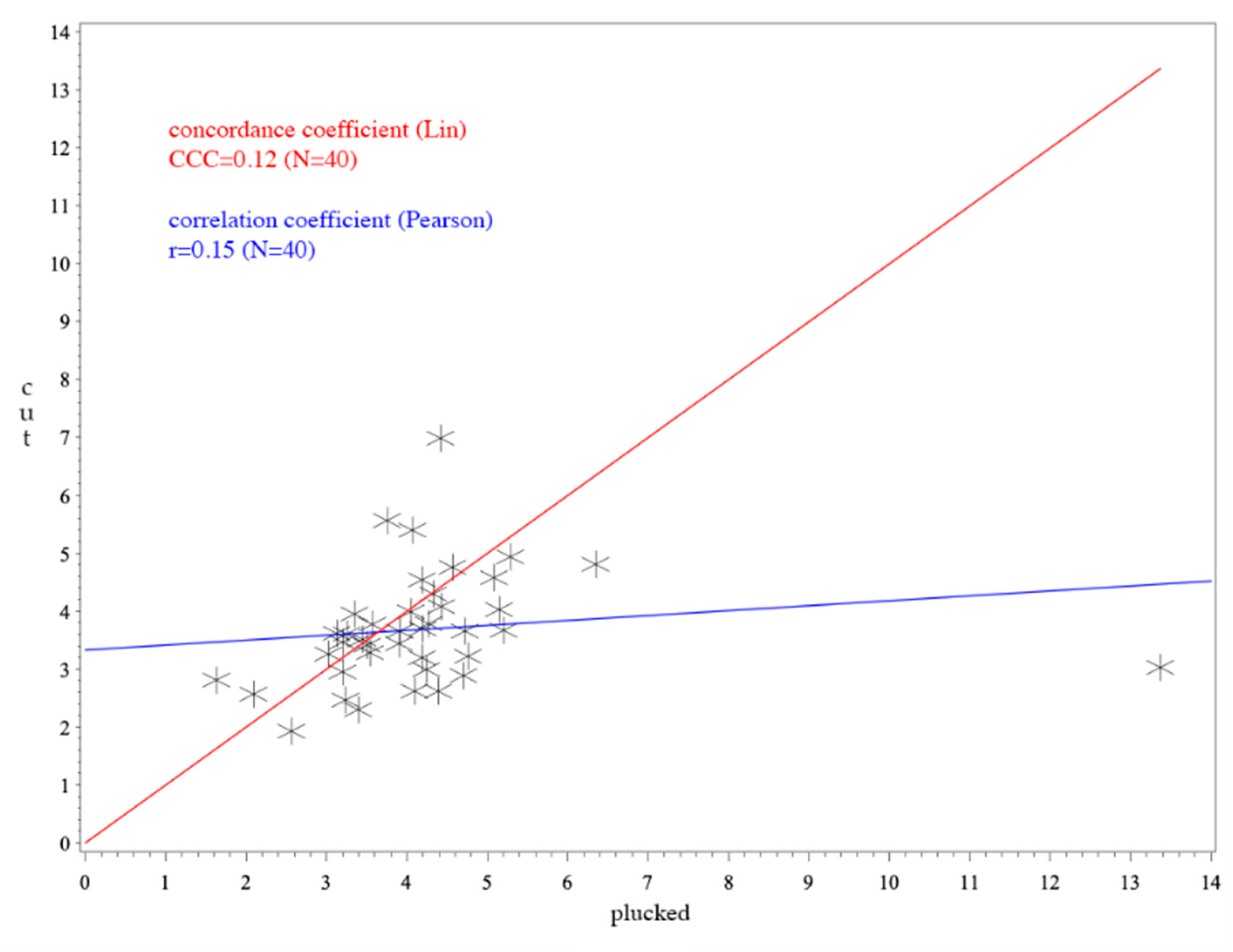
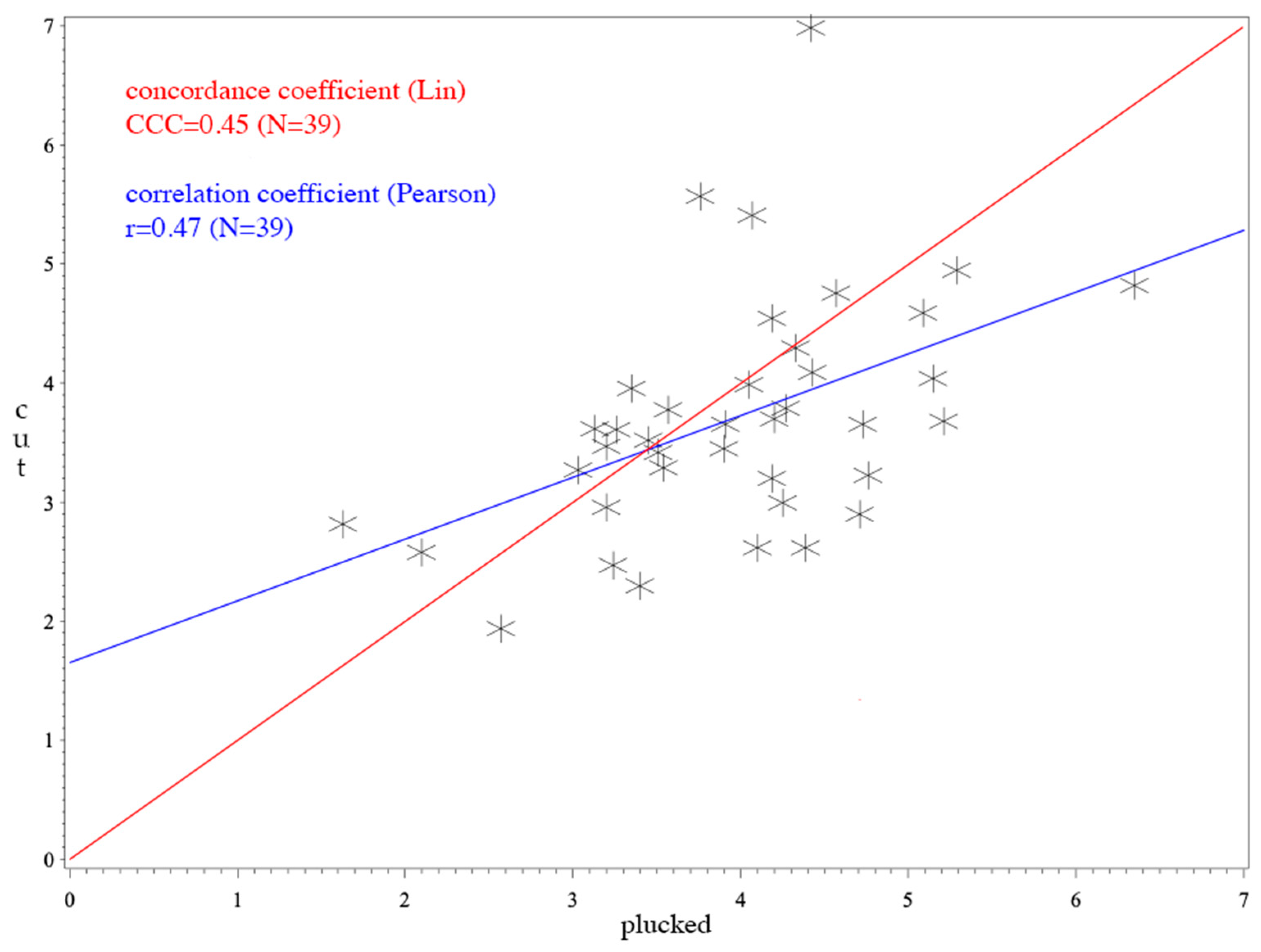
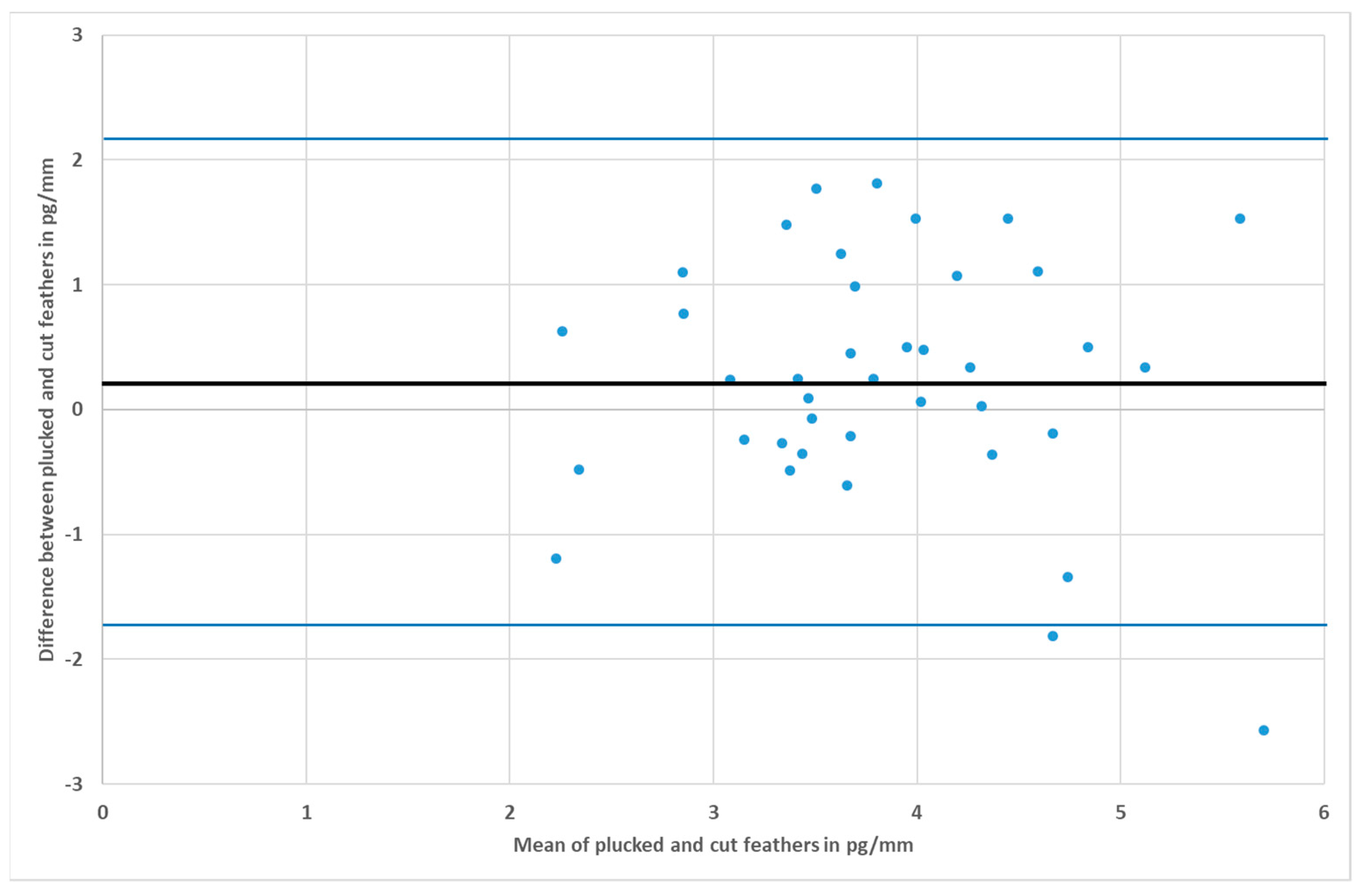
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Romero, L.M.; Fairhurst, G.D. Measuring corticosterone in feathers: Strengths, limitations, and suggestions for the future. Comp. Biochem. Phys. A 2016, 202, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [PubMed]
- Hau, M.; Ricklefs, R.E.; Wikelski, M.; Lee, K.A.; Brawn, J.D. Corticosterone, testosterone and life-history strategies of birds. Proc. Royal Soc. B 2010, 277, 3203–3212. [Google Scholar] [CrossRef] [PubMed]
- Kitaysky, A.S.; Kitaiskaia, E.V.; Wingfield, J.C.; Piatt, J.F. Dietary restriction causes chronic elevation of corticosterone and enhances stress response in red-legged kittiwake chicks. J. Comp. Physiol. B 2001, 171, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Kitaysky, A.; Kitaiskaia, E.; Piatt, J.F.; Wingfield, J. Benefits and costs of increased levels of corticosterone in seabird chicks. Horm. Behav. 2003, 43, 140–149. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Stress, the Aging Brain, and the Mechanisms of Neuron Death; MIT Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Harvey, S.; Merry, B.J.; Phillips, J.G. Influence of stress on the secretion of corticosterone in the duck (Anas platyrhynchos). J. Endocrinol. 1980, 87, 161–171. [Google Scholar] [CrossRef]
- Dufty, A.M., Jr. Stress responsiveness in nestlings: A comparison of two sampling techniques. Auk 2008, 125, 225–229. [Google Scholar] [CrossRef][Green Version]
- Bortolotti, G.R.; Marchant, T.A.; Blas, J.; German, T. Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Funct. Ecol. 2008, 22, 494–500. [Google Scholar] [CrossRef]
- Dehnhard, M.; Schreer, A.; Krone, O.; Jewgenow, K.; Krause, M.; Grossmann, R. Measurement of plasma corticosterone and fecal glucocorticoid metabolites in the chicken (Gallus domesticus), the great cormorant (Phalacrocorax carbo), and the goshawk (Accipiter gentilis). Gen. Comp. Endocrinol. 2003, 131, 345–352. [Google Scholar] [CrossRef]
- Möstl, E.; Rettenbacher, S.; Palme, R. Measurement of corticosterone metabolites in birds’ droppings: An analytical approach. Ann. N. Y. Acad. Sci. 2005, 1046, 17–34. [Google Scholar] [CrossRef]
- Kotrschal, K.; Dittami, J.; Hirschenhauser, K.; Möstl, E.; Peczely, P. Effects of physiological and social challenges in different seasons on fecal testosterone and corticosterone in male domestic geese (Anser domesticus). Acta Ethol. 2000, 2, 115–122. [Google Scholar] [CrossRef]
- Royo, F.; Mayo, S.; Carlsson, H.-E.; Hau, J. Egg corticosterone: A noninvasive measure of stress in egg-laying birds. J. Avian Med. Surg. 2008, 22, 310–314. [Google Scholar] [CrossRef]
- Downing, J.; Bryden, W. Determination of corticosterone concentrations in egg albumen: A non-invasive indicator of stress in laying hens. Physiol. Behav. 2008, 95, 381–387. [Google Scholar] [CrossRef]
- Hahn, A.; Reitemeier, S.; Gottschalk, J.; Haense, M.; Schmidt, V.; Steinbach-Sobiraj, K.; Krautwald-Junghanns, M.; Einspanier, A. Endocrinologic studies of male psittacine birds for the evaluation of their reproductive status. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2011, 39, 249. [Google Scholar] [PubMed]
- Gentle, M.J.; Waddington, D.; Hunter, L.N.; Jones, R.B. Behavioural evidence for persistent pain following partial beak amputation in chickens. Appl. Anim. Behav. Sci. 1990, 27, 149–157. [Google Scholar] [CrossRef]
- Gentle, M.; Hunter, L. Physiological and behavioural responses associated with feather removal in Gallus gallus var domesticus. Res. Vet. Sci. 1991, 50, 95–101. [Google Scholar] [CrossRef]
- Gentle, M.J. Pain in birds. Anim. Welf. 1992, 1, 235–247. [Google Scholar]
- Malik, A.; Valentine, A. Pain in birds: A review for veterinary nurses. Vet. Nurs. J. 2018, 33, 11–25. [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 18 May 2020).
- Russell, W.M.S.; Burch, R.L.; Hume, C.W. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959; Volume 238.
- Dufty, A.M., Jr.; Belthoff, J.R. Corticosterone and the stress response in young western screech-owls: Effects of captivity, gender, and activity period. Physiol. Zool. 1997, 70, 143–149. [Google Scholar] [CrossRef]
- Sims, C.G.; Holberton, R.L. Development of the corticosterone stress response in young northern mockingbirds (Mimus polyglottos). Gen. Comp. Endocrinol. 2000, 119, 193–201. [Google Scholar] [CrossRef]
- Fairhurst, G.D.; Dawson, R.D.; van Oort, H.; Bortolotti, G.R. Synchronizing feather-based measures of corticosterone and carotenoid-dependent signals: What relationships do we expect? Oecologia 2014, 174, 689–698. [Google Scholar] [CrossRef]
- Angelier, F.; Moe, B.; Blanc, S.; Chastel, O. What factors drive prolactin and corticosterone responses to stress in a long-lived bird species (snow petrel Pagodroma nivea)? Physiol. Biochem. Zool. 2009, 82, 590–602. [Google Scholar] [CrossRef]
- Bortolotti, G.R.; Marchant, T.; Blas, J.; Cabezas, S. Tracking stress: Localisation, deposition and stability of corticosterone in feathers. J. Exp. Biol. 2009, 212, 1477–1482. [Google Scholar] [CrossRef]
- Harris, C.M.; Madliger, C.L.; Love, O.P. An evaluation of feather corticosterone as a biomarker of fitness and an ecologically relevant stressor during breeding in the wild. Oecologia 2017, 183, 987–996. [Google Scholar] [CrossRef]
- Astheimer, L.; Buttemer, W.; Wingfield, J. Seasonal and acute changes in adrenocortical responsiveness in an arctic-breeding bird. Horm. Behav. 1995, 29, 442–457. [Google Scholar] [CrossRef]
- Legagneux, P.; Harms, N.J.; Gauthier, G.; Chastel, O.; Gilchrist, H.G.; Bortolotti, G.; Bêty, J.; Soos, C. Does feather corticosterone reflect individual quality or external stress in arctic-nesting migratory birds? PLoS ONE 2013, 8, e82644. [Google Scholar] [CrossRef]
- Marra, P.P.; Holberton, R.L. Corticosterone levels as indicators of habitat quality: Effects of habitat segregation in a migratory bird during the non-breeding season. Oecologia 1998, 116, 284–292. [Google Scholar] [CrossRef]
- Romero, L.M.; Soma, K.K.; Wingfield, J.C. Hypothalamic-pituitary-adrenal axis changes allow seasonal modulation of corticosterone in a bird. Am. J. Physiol. 1998, 274, R1338–R1344. [Google Scholar] [CrossRef]
- Dollinger, P.; Pagel, T.; Baumgartner, K.; Encke, D.; Engel, H.; Filz, A. Flugunfähigmachen von Vögeln–Für und Wider. Zool. Gart. 2013, 82, 293–339. [Google Scholar] [CrossRef]
- TVT. TVT-Stellungn._Flugunfähigmachen_von_Vögeln__Mai_2015. Available online: https://www.tierschutz-tvt.de/alle-merkblaetter-und-stellungnahmen/?no_cache=1&download=TVT-Stellungn._Flugunf%C3%A4higmachen_von_V%C3%B6geln__Mai_2015_.pdf&did=175 (accessed on 30 March 2020).
- Bennett, R.A.; Baumgartner, K. Avian deflighting techniques. In Fowler’s Zoo and Wild Animal Medicine; Elsevier: Amsterdam, The Netherlands, 2015; Volume 8, pp. 650–660. [Google Scholar]
- Hesterman, H.; Gregory, N.; Boardman, W. Deflighting procedures and their welfare implications in captive birds. Anim. Welf. 2001, 10, 405–419. [Google Scholar]
- Reese, L.; Ladwig-Wiegard, M.; von Fersen, L.; Haase, G.; Will, H.; Merle, R.; Encke, D.; Maegdefrau, H.; Baumgartner, K.; Thöne-Reineke, C. Deflighting zoo birds and its welfare considerations. Anim. Welf. 2020, 29, 69–80. [Google Scholar] [CrossRef]
- Tierschutzgesetz. Tierschutzgesetz in der Fassung der Bekanntmachung vom 18. Mai 2006 (BGBl. I S. 1206, 1313), das Zuletzt Durch Artikel 1 des Gesetzes vom 17. Dezember 2018 (BGBl. I S. 2586) Geändert Worden Ist. Verbraucherschutz, B.d.J.u.f., Ed.; Available online: https://www.gesetze-im-internet.de/tierschg/BJNR012770972.html (accessed on 15 April 2020).
- Bračko, A.; King, C. Advantages of aviaries and the Aviary Database Project: A new approach to an old housing option for birds. Int. Zoo Yearb. 2014, 48, 166–183. [Google Scholar] [CrossRef]
- Captive Animals’ Protection Society (CAPS). Mutilated for Your Viewing Pleasure: Pinioning Birds in English Zoos. Available online: https://forms.freedomforanimals.org.uk/wp-content/uploads/2013/03/CAPS_Birds_in_Zoos_Summary_0313_FINAL_v2.pdf (accessed on 13 May 2020).
- PeTA. Systematische Verstümmelung von Vögeln—PETA erstattet Strafanzeige gegen Tierpark Cottbus sowie 19 weitere Zoos und Tierparks (Press Release 2017, November 8). Available online: https://www.peta.de/systematische-verstuemmelung-von-voegeln-peta-erstattet-strafanzeige-gegen-zoo (accessed on 12 May 2020).
- Tyson, E. For an end to pinioning: The case against the legal mutilation of birds in captivity. J. Anim. Ethics 2014, 4, 1–4. [Google Scholar] [CrossRef]
- Baumgartner, K. Flugunfähigmachen von Vögeln in zoologischen Einrichtungen. Dtsch. Tierärztebl. 2015, 2, 172–178. [Google Scholar]
- Council Directive 1999/22/EC. Council Directive of the European Union Relating to the Keeping of Wild Animals in Zoos. Official Journal L 094, 9 April 1999, pp. 24–26. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=uriserv:OJ.L_.1999.094.01.0024.01.ENG (accessed on 18 May 2020).
- WAZA. WAZA Code of Ethics and Animal Welfare. 2003. Available online: https://www.waza.org/wp-content/uploads/2019/05/WAZA-Code-of-Ethics.pdf (accessed on 30 March 2020).
- EAZA. Standards for the Accommodation and Care of Animals in Zoos and Aquaria. 2014. Available online: https://www.eaza.net/assets/Uploads/Standards-and-policies/Standards-for-the-Accommodation-and-Care-of-Animals-2014.pdf (accessed on 30 March 2020).
- Rodenburg, T.B.; Bracke, M.; Berk, J.; Cooper, J.; Faure, J.; Guémené, D.; Guy, G.; Harlander, A.; Jones, T.; Knierim, U. Welfare of ducks in European duck husbandry systems. World’s Poult. Sci. J. 2005, 61, 633–646. [Google Scholar] [CrossRef]
- Europarat. Ständiger Ausschuss des Europäischen Übereinkommens zum Schutz von Tieren in Landwirtschaftlichen Tierhaltungen BMEL. 1999. Available online: https://www.bmel.de/SharedDocs/Downloads/DE/_Tiere/Tierschutz/Gutachten-Leitlinien/eu-haltung-gaense.pdf?__blob=publicationFile&v=2 (accessed on 14 February 2020).
- Reese, L.; Baumgartner, K.; Fersen, L.v.; Merle, R.; Ladwig-Wiegard, M.; Will, H.; Haase, G.; Tallo-Parra, O.; Carbajal, A.; Lopez-Bejar, M. Feather Corticosterone Measurements of Greater Flamingos Living under Different Forms of Flight Restraint. Animals 2020, 10, 605. [Google Scholar] [CrossRef]
- Grindstaff, J.L.; Lovern, M.B.; Burtka, J.L.; Hallmark-Sharber, A. Structural coloration signals condition, parental investment, and circulating hormone levels in Eastern bluebirds (Sialia sialis). J. Comp. Physiol. A 2012, 198, 625–637. [Google Scholar] [CrossRef]
- Kennedy, E.A.; Lattin, C.R.; Romero, L.M.; Dearborn, D.C. Feather coloration in museum specimens is related to feather corticosterone. Behav. Ecol. Sociobiol. 2013, 67, 341–348. [Google Scholar] [CrossRef]
- Lendvai, Á.; Giraudeau, M.; Németh, J.; Bakó, V.; McGraw, K. Carotenoid-based plumage coloration reflects feather corticosterone levels in male house finches (Haemorhous mexicanus). Behav. Ecol. Sociobiol. 2013, 67, 1817–1824. [Google Scholar] [CrossRef]
- Monclús, L.; Carbajal, A.; Tallo-Parra, O.; Sabés-Alsina, M.; Darwich, L.; Molina-López, R.; Lopez-Bejar, M. Relationship between feather corticosterone and subsequent health status and survival in wild Eurasian Sparrowhawk. J. Ornithol. 2017, 158, 773–783. [Google Scholar] [CrossRef]
- Buchanan, K.L.; Goldsmith, A.R. Noninvasive endocrine data for behavioural studies: The importance of validation. Anim. Behav. 2004, 67, 183–185. [Google Scholar] [CrossRef]
- Rohwer, S.; Ricklefs, R.E.; Rohwer, V.G.; Copple, M.M. Allometry of the duration of flight feather molt in birds. PLoS Biol. 2009, 7, e1000132. [Google Scholar] [CrossRef]
- Bortolotti, G.R. Flaws and pitfalls in the chemical analysis of feathers: Bad news–good news for avian chemoecology and toxicology. Ecol. Appl. 2010, 20, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- McBride, G. A proposal for strength-of-agreement criteria for Lin’s concordance correlation coefficient. In NIWA Client Report: HAM2005-062; National Institute of Water & Atmospheric Research Ltd: Hamilton, New Zealand, 2005. [Google Scholar]
- Lawrence, I.; Lin, K. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Carlitz, E.H.; Kirschbaum, C.; Miller, R.; Rukundo, J.; van Schaik, C.P. Effects of body region and time on hair cortisol concentrations in chimpanzees (Pan troglodytes). Gen. Comp. Endocrinol. 2015, 223, 9–15. [Google Scholar] [CrossRef]
- Terwissen, C.; Mastromonaco, G.; Murray, D. Influence of adrenocorticotrophin hormone challenge and external factors (age, sex, and body region) on hair cortisol concentration in Canada lynx (Lynx canadensis). Gen. Comp. Endocrinol. 2013, 194, 162–167. [Google Scholar] [CrossRef]
- Tallo-Parra, O.; Manteca, X.; Sabes-Alsina, M.; Carbajal, A.; Lopez-Bejar, M. Hair cortisol detection in dairy cattle by using EIA: Protocol validation and correlation with faecal cortisol metabolites. Animal 2015, 9, 1059–1064. [Google Scholar] [CrossRef]
- Andreasson, U.; Perret-Liaudet, A.; van Waalwijk van Doorn, L.J.C.; Blennow, K.; Chiasserini, D.; Engelborghs, S.; Fladby, T.; Genc, S.; Kruse, N.; Kuiperij, H.B.; et al. A Practical Guide to Immunoassay Method Validation. Front. Neurol. 2015, 6, 179. [Google Scholar] [CrossRef]
- Hanneman, S.K.; Cox, C.D.; Green, K.E.; Kang, D.-H. Estimating Intra- and Inter-Assay Variability in Salivary Cortisol. Biol. Res. Nurs. 2011, 13, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, S.M.; Ghassabian, S. Linearity of Calibration Curves for Analytical Methods: A Review of Criteria for Assessment of Method Reliability. In Calibration and Validation of Analytical Methods: A Sampling of Current Approaches; IntechOpen: London, UK, 2018. [Google Scholar]
- Carbajal, A.; Tallo-Parra, O.; Sabes-Alsina, M.; Mular, I.; Lopez-Bejar, M. Feather corticosterone evaluated by ELISA in broilers: A potential tool to evaluate broiler welfare. Poultr. Sci. 2014, 93, 2884–2886. [Google Scholar] [CrossRef]


| Fattening Period | Geese | Ducks | Additional Information |
|---|---|---|---|
| 1. About two weeks | Crushed oats, pressed feed (Gallugold® Enten-/Gänsekorn), fattening feed for geese (Gallugold® Enten-/Gänsekorn) | Crushed wheat, pressed feed (Gallugold® Enten-/Gänsekorn) | Periods 1 to 4: Water and pasture ad libitum |
| 2. About two weeks | Oats, pressed feed (Gallugold® Enten-/Gänsekorn) | Wheat, pressed feed (Gallugold® Enten-/Gänsekorn) | Periods 1 to 3: Geese and ducks separated |
| 3. One to two months | Oats | Wheat | |
| 4. Final weeks before slaughter | Oats, wheat | Oats, wheat | Period 4: Geese and ducks together |
| Length in mm | Weight in mg | Corticosterone in pg/mg | Corticosterone in pg/mm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anser | Anas | Anser | Anas | Anser | Anas | Anser | Anas | |||||||||
| Plucked | Cut | Plucked | Cut | Plucked | Cut | Plucked | Cut | Plucked | Cut | Plucked | Cut | Plucked | Cut | Plucked | Cut | |
| Mean | 222 | 217 | 224 | 225 | 110.3 | 106.0 | 82.0 | 78.7 | 8.01 (8.42) | 7.59 | 4.78 | 5.16 | 3.96 (4.20) | 3.69 | 1.75 | 1.77 |
| Max | 249 | 242 | 251 | 248 | 135.2 | 134.0 | 109.0 | 106.0 | 12.90 (24.49) | 11.54 | 10.88 | 12.24 | 6.35 (13.37) | 6.99 | 4.28 | 3.71 |
| Min | 202 | 201 | 201 | 202 | 74.0 | 56.6 | 53.8 | 52.4 | 3.85 | 4.73 | 1.76 | 1.38 | 1.63 | 1.94 | 0.55 | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voit, M.; Merle, R.; Baumgartner, K.; von Fersen, L.; Reese, L.; Ladwig-Wiegard, M.; Will, H.; Tallo-Parra, O.; Carbajal, A.; Lopez-Bejar, M.; et al. Validation of an Alternative Feather Sampling Method to Measure Corticosterone. Animals 2020, 10, 2054. https://doi.org/10.3390/ani10112054
Voit M, Merle R, Baumgartner K, von Fersen L, Reese L, Ladwig-Wiegard M, Will H, Tallo-Parra O, Carbajal A, Lopez-Bejar M, et al. Validation of an Alternative Feather Sampling Method to Measure Corticosterone. Animals. 2020; 10(11):2054. https://doi.org/10.3390/ani10112054
Chicago/Turabian StyleVoit, Marielu, Roswitha Merle, Katrin Baumgartner, Lorenzo von Fersen, Lukas Reese, Mechthild Ladwig-Wiegard, Hermann Will, Oriol Tallo-Parra, Annaïs Carbajal, Manel Lopez-Bejar, and et al. 2020. "Validation of an Alternative Feather Sampling Method to Measure Corticosterone" Animals 10, no. 11: 2054. https://doi.org/10.3390/ani10112054
APA StyleVoit, M., Merle, R., Baumgartner, K., von Fersen, L., Reese, L., Ladwig-Wiegard, M., Will, H., Tallo-Parra, O., Carbajal, A., Lopez-Bejar, M., & Thöne-Reineke, C. (2020). Validation of an Alternative Feather Sampling Method to Measure Corticosterone. Animals, 10(11), 2054. https://doi.org/10.3390/ani10112054






