A Few TH-Immunoreactive Neurons Closely Appose DMX-Located Neuronal Somata Projecting to the Stomach Prepyloric Region in the Pig
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
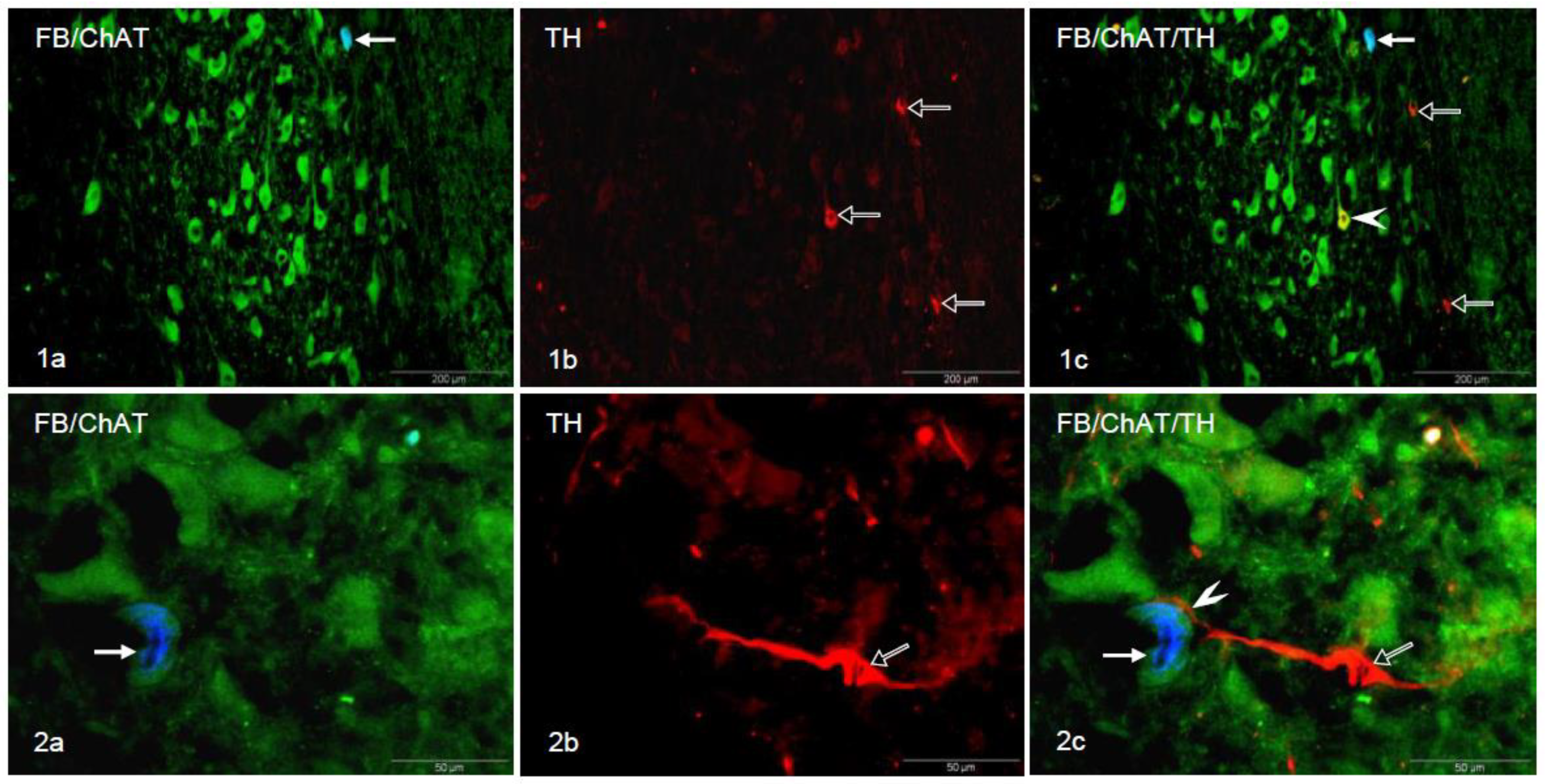
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalia, M.; Mesulam, M.M. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J. Comp. Neurol. 1980, 193, 435–465. [Google Scholar] [CrossRef] [PubMed]
- Chernicky, C.L.; Barnes, K.L.; Ferrario, C.M.; Conomy, J.P. Brainstem distribution of neurons with efferent projections in the cervical vagus of the dog. Brain Res. Bull. 1983, 10, 345–351. [Google Scholar] [CrossRef]
- Scharoun, S.L.; Barone, F.C.; Wayner, M.J.; Jones, S.M. Vagal and gastric connections to the central nervous system determined by the transport of horseradish peroxidase. Brain Res. Bull. 1984, 13, 573–583. [Google Scholar] [CrossRef]
- Kitchell, R.L.; Stromberg, M.W.; Davis, L.H. Comparative study of the dorsal motor nucleus of the vagus nerve. Am. J. Vet. Res. 1977, 38, 37–49. [Google Scholar] [PubMed]
- Hopkins, D.A.; Gootman, P.M.; Gootman, N.; Di Russo, S.M.; Zeballos, M.E. Brainstem cells of origin of the cervical vagus and cardiopulmonary nerves in the neonatal pig (Sus scrofa). Brain Res. 1984, 306, 63–72. [Google Scholar] [CrossRef]
- Gańko, M.; Całka, J. Localization and chemical coding of the dorsal motor vagal nucleus (DMX) neurons projecting to the porcine stomach prepyloric area in the physiological state and after stomach partial resection. J. Mol. Neurosci. 2014, 52, 90–100. [Google Scholar] [CrossRef]
- Wu, M.; Majewski, M.; Wojtkiewicz, J.; Vanderwinden, J.M.; Adriaensen, D.; Timmermans, J.P. Anatomical and neurochemical features of the extrinsic and intrinsic innervation of the striated muscle in the porcine esophagus: Evidence for regional and species differences. Cell Tiss. Res. 2000, 311, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Gańko, M.; Rychlik, A.; Całka, J. Immunohistochemical characterization of neurons and neuronal processes in the dorsal vagal nucleus of the pig. Pol. J. Vet. Sci. 2013, 16, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.M.; Ross, C.A.; Pickel, V.M.; Joh, T.H.; Reis, D.J. Distribution of dopamine-, noradrenaline-, and adrenaline-containing cell bodies in the rat medulla oblongata: Demonstrated by the immunocytochemical localization of catecholamine biosynthetic enzymes. J. Comp. Neurol. 1982, 212, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Dormer, K.J.; Anwar, M.; Ashlock, S.R.; Ruggiero, D.A. Organization of presumptive catecholamine-synthesizing neurons in the canine medulla oblongata. Brain Res. 1993, 601, 41–64. [Google Scholar] [CrossRef]
- Tillet, Y.; Thibault, J. Catecholamine-containing neurons in the sheep brainstem and diencephalon: Immunohistochemical study with tyrosine hydroxylase (TH) and dopamine-beta-hydroxylase (DBH) antibodies. J. Comp. Neurol. 1989, 290, 69–104. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.M.; Li, Y.W.; Joh, T.H.; Cotton, R.G.; Howe, P.R.; Geffen, L.B.; Blessing, W.W. Distribution of monoamine-synthesizing neurons in the human medulla oblongata. J. Comp. Neurol. 1988, 273, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Chaillou, E.; Tillet, Y.; Malbert, C.H. Organisation of the catecholaminergic system in the vagal motor nuclei of pigs: A retrograde fluorogold tract tracing study combined with immunohistochemistry of catecholaminergic synthesizing enzymes. J. Chem. Neuroanat. 2009, 38, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Gyires, K.; Toth, V.E.; Zadori, Z.S. Gastric mucosal protection: From the periphery to the central nervous system. J. Physiol. Pharmacol. 2015, 66, 319–329. [Google Scholar] [PubMed]
- Tsukamoto, K.; Hayakawa, T.; Maeda, S.; Tanaka, K.; Seki, M.; Yamamura, T. Projections to the alimentary canal from the dopaminergic neurons in the dorsal motor nucleus of the vagus of the rat. Auton. Neurosci. 2005, 123, 12–18. [Google Scholar] [CrossRef]
- Guo, J.J.; Browning, K.N.; Rogers, R.C.; Travagli, R.A. Catecholaminergic neurons in rat dorsal motor nucleus of vagus project selectively to gastric corpus. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G361–G367. [Google Scholar]
- Rosin, D.L.; Talley, E.M.; Lee, A.; Stornetta, R.L.; Gaylinn, B.D.; Guyenet, P.G.; Lynch, K.R. Distribution of alpha-2C –Adrenergic Receptor-Like Immunoreactivity in the Rat Central Nervous System. J. Comp. Neurol. 1996, 372, 135–165. [Google Scholar] [CrossRef]
- Tavares, A.; Handy, D.E.; Bogdanova, N.N.; Rosene, D.L.; Gavras, H. Localization of α2A and α2B-Adrenergic Receptor Subtypes in Brain. Hypertension 1996, 27, 449–455. [Google Scholar] [CrossRef] [PubMed]
| Antigen | Species | Dilution | Code | Manufacturer/Supplier |
|---|---|---|---|---|
| Primary Antibodies | ||||
| TH | Mouse | 1:200 | MAB318 | Millipore, Temecula, CA, USA |
| ChAT | Goat | 1:50 | AB144P-1ML | Millipore, Temecula, CA, USA |
| Secondary Antibodies | ||||
| Alexa Fluor 488 nm | anti-goat | 1:1000 | A11055 | Thermo Fisher Scientific, Waltham, MA, USA |
| Alexa Fluor 546 nm | anti-mouse | 1:1000 | A10036 | Thermo Fisher Scientific, Waltham, MA, USA |
| Pig | I | II | III | IV | V | Mean ± Standard Error of Mean (SEM) |
|---|---|---|---|---|---|---|
| Number of appositions between CHAT+/FB+ and TH+ neurons | 1 | 2 | 0 | 2 | 3 | 1.6±0.5 |
| Number of FB+ cells | 508 | 633 | 375 | 463 | 447 | 485.2± 42.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calka, J.; Ganko, M.; Rychlik, A. A Few TH-Immunoreactive Neurons Closely Appose DMX-Located Neuronal Somata Projecting to the Stomach Prepyloric Region in the Pig. Animals 2020, 10, 2008. https://doi.org/10.3390/ani10112008
Calka J, Ganko M, Rychlik A. A Few TH-Immunoreactive Neurons Closely Appose DMX-Located Neuronal Somata Projecting to the Stomach Prepyloric Region in the Pig. Animals. 2020; 10(11):2008. https://doi.org/10.3390/ani10112008
Chicago/Turabian StyleCalka, Jaroslaw, Marta Ganko, and Andrzej Rychlik. 2020. "A Few TH-Immunoreactive Neurons Closely Appose DMX-Located Neuronal Somata Projecting to the Stomach Prepyloric Region in the Pig" Animals 10, no. 11: 2008. https://doi.org/10.3390/ani10112008
APA StyleCalka, J., Ganko, M., & Rychlik, A. (2020). A Few TH-Immunoreactive Neurons Closely Appose DMX-Located Neuronal Somata Projecting to the Stomach Prepyloric Region in the Pig. Animals, 10(11), 2008. https://doi.org/10.3390/ani10112008




