The Effect of Dietary Rye Inclusion and Xylanase Supplementation on Structural Organization of Bone Constitutive Phases in Laying Hens Fed a Wheat-Corn Diet
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Birds and Experimental Diets
2.3. Bone Measurements and Bone Sample Preparation
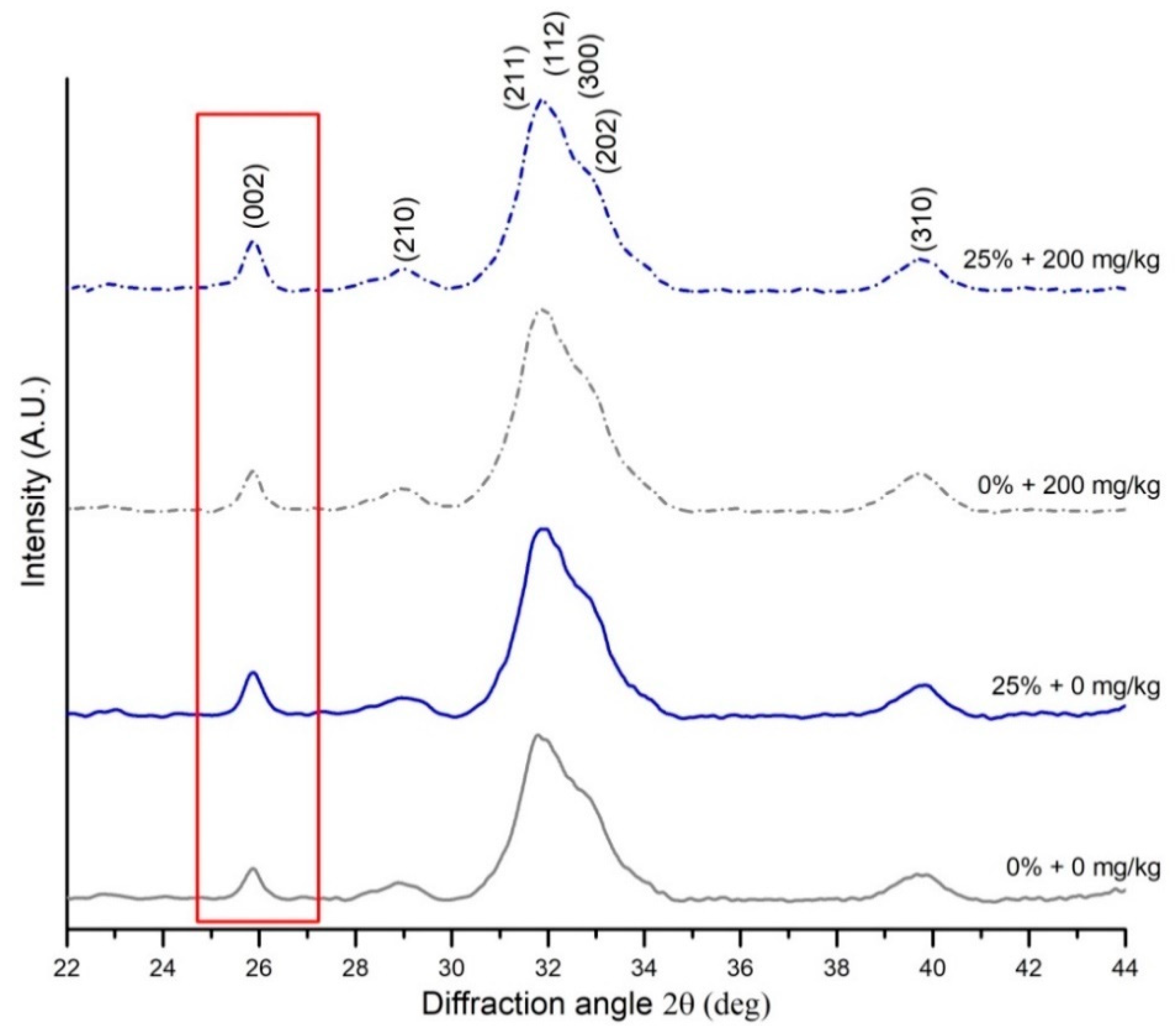
2.4. X-Ray Diffraction (XRD) Measurements
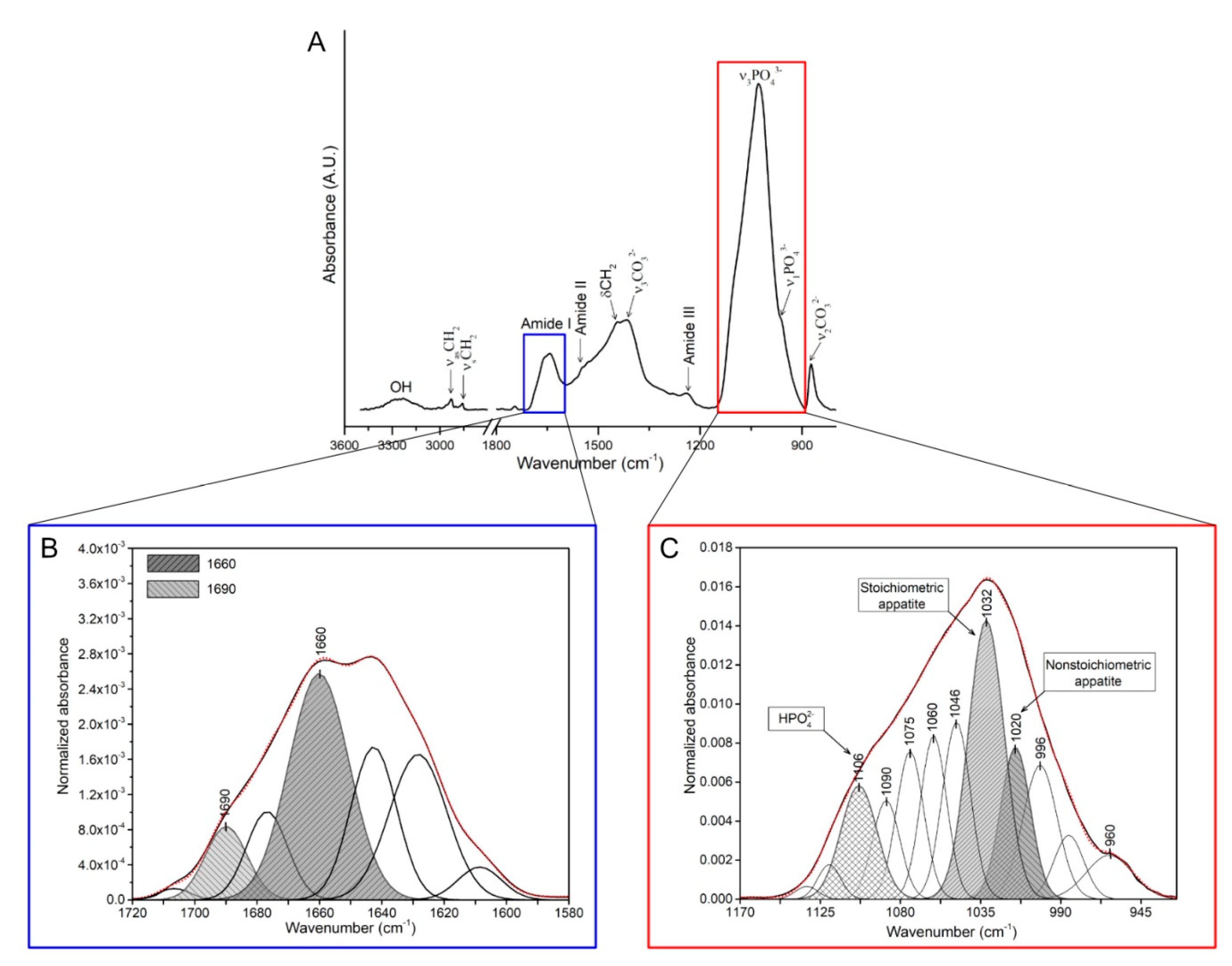
2.5. FTIR Measurements
| Parameter | Formula | Description | Reference |
|---|---|---|---|
| Size of the hydroxyapatite crystals in the c-axis | K—a constant related to the crystallite shape (0.94), λ—the wavelength of X-ray radiation (1.54178 Å), β—the full width at half-maximum intensity (FWHM) of (002) reflection peak, counting the apparatus broadening of 0.08°, θ—the (002) peak position | [47,48] | |
| Mineral crystallinity index | KA—a constant set to 0.24, FWHM002—the full width at half-maximum intensity of (002) reflection peak | [49] |
| Parameter | Formula | Description | Reference |
|---|---|---|---|
| Mineral-to-matrix ratio | The ratio of the main phosphate (ν1, ν3, PO43−; 900–1200 cm−1) to amide I (1600–1700 cm−1) integrated band area (Figure 1A). | [50] | |
| Collagen maturity | The ratio of the area ratio between amide I sub-bands at mature cross-links (1660 cm−1) and immature cross-links (1690 cm−1) (Figure 1B). | [51,52] | |
| Carbonate-to-phosphate ratio | The ratio of the integrated area of the carbonate spectral region (840–890 cm−1) to integrated area of the ν1, ν3, PO43− spectral region (900–1200 cm−1). | [46,52,53] | |
| Crystallinity index | The ratio between phosphate sub-band areas of stoichiometric (at 1030 cm−1) to nonstoichiometric (1020 cm−1) hydroxyapatites (Figure 1C). | [54] |
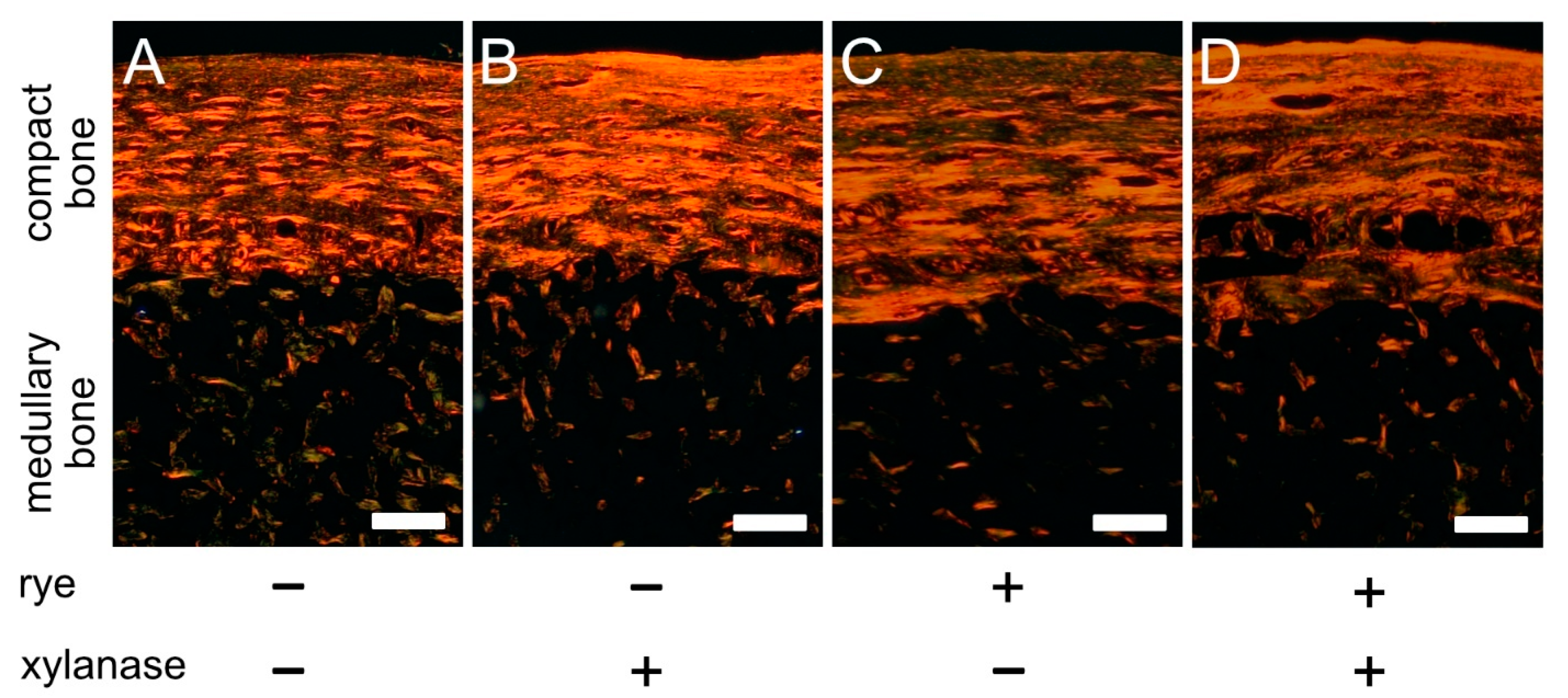
2.6. PSR Staining
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rath, N.C.; Huff, G.R.; Huff, W.E.; Balog, J.M. Factors Regulating Bone Maturity and Strength in Poultry. Poult. Sci. 2000, 79, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Dacke, C.G.; Arkle, S.; Cook, D.J.; Wormstone, I.M.; Jones, S.; Zaidi, M.; Bascal., Z.A. Medullary bone and avian calcium regulation. J. Exp. Biol. 1993, 184, 63–88. [Google Scholar]
- Van De Velde, J.; Vermeiden, J.; Bloot, A. Medullary bone matrix formation, mineralization, and remodeling related to the daily egg-laying cycle of Japanese quail: A histological and radiological study. Bone 1985, 6, 321–327. [Google Scholar] [CrossRef]
- Bai, S.; Jin, G.; Li, D.; Ding, X.; Wang, J.; Zhang, K.; Zeng, Q.; Ji, F.; Zhao, J. Dietary organic trace minerals level influences eggshell quality and minerals retention in hens. Ann. Anim. Sci. 2017, 17, 503–515. [Google Scholar] [CrossRef]
- Świątkiewicz, S.; Arczewska-Włosek, A.; Krawczyk, J.; Szczurek, W.; Puchała, M.; Józefiak, D. Effect of selected feed additives on egg performance and eggshell quality in laying hens fed a diet with standard or decreased calcium content. Ann. Anim. Sci. 2018, 18, 167–183. [Google Scholar] [CrossRef]
- Whitehead, C.C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004, 83, 193–199. [Google Scholar] [CrossRef]
- Gregory, N.G.; Wilkins, L.J. Broken bones in domestic fowl: Handling and processing damage in end-of-lay battery hens. Br. Poult. Sci. 1989, 30, 555–562. [Google Scholar] [CrossRef]
- Bishop, S.C.; Fleming, R.H.; McCormack, H.A.; Flock, D.K.; Whitehead, C.C. Inheritance of bone characteristics affecting osteoporosis in laying hens. Br. Poult. Sci. 2000, 41, 33–40. [Google Scholar] [CrossRef]
- Whitehead, C.; Fleming, R.H. Osteoporosis in Cage Layers. Poult. Sci. 2000, 79, 1033–1041. [Google Scholar] [CrossRef]
- Jansen, S.; Baulain, U.; Habig, C.; Weigend, A.; Halle, I.; Scholz, A.M.; Simianer, H.; Sharifi, A.R.; Weigend, S. Relationship between Bone Stability and Egg Production in Genetically Divergent Chicken Layer Lines. Animals 2020, 10, 850. [Google Scholar] [CrossRef]
- Jansen, S.; Bues, M.; Baulain, U.; Habig, C.; Halle, I.; Petow, S.; Sharifi, A.R.; Weigend, A.; Wilkens, M.R.; Weigend, S. Bone Health or Performance? Adaptation Response of Genetically Divergent Chicken Layer Lines to a Nutritive Calcium Depletion. Animals 2020, 10, 1645. [Google Scholar] [CrossRef] [PubMed]
- Kierończyk, B.; Rawski, M.; Józefiak, D.; Świątkiewicz, S. Infectious and non-infectious factors associated with leg disorders in poultry—A review. Ann. Anim. Sci. 2017, 17, 645–669. [Google Scholar] [CrossRef]
- Olgun, O.; Yildiz, A.Ö. Effects of dietary supplementation of inorganic, organic or nano zinc forms on performance, eggshell quality, and bone characteristics in laying hens. Ann. Anim. Sci. 2017, 17, 463–476. [Google Scholar] [CrossRef]
- Świątkiewicz, S.; Arczewska-Włosek, A.; Szczurek, W.; Calik, J.; Krawczyk, J.; Józefiak, D. The influence of selected feed additives on mineral utilisation and bone characteristics in laying hens. Ann. Anim. Sci. 2018, 18, 781–793. [Google Scholar] [CrossRef]
- Nolte, T.; Jansen, S.; Halle, I.; Scholz, A.M.; Simianer, H.; Sharifi, A.R.; Weigend, S. Egg Production and Bone Stability of Local Chicken Breeds and Their Crosses Fed with Faba Beans. Animals 2020, 10, 1480. [Google Scholar] [CrossRef]
- Baird, H.T.; Eggett, D.L.; Fullmer, S. Varying Ratios of Omega-6: Omega-3 Fatty Acids on the Pre-and Postmortem Bone Mineral Density, Bone Ash, and Bone Breaking Strength of Laying Chickens. Poult. Sci. 2008, 87, 323–328. [Google Scholar] [CrossRef]
- Adhikari, R.; White, D.; House, J.; Kim, W. Effects of additional dosage of vitamin D3, vitamin D2, and 25-hydroxyvitamin D3 on calcium and phosphorus utilization, egg quality and bone mineralization in laying hens. Poult. Sci. 2020, 99, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Donalson, L.M.; Mitchell, A.D.; Kubena, L.F.; Nisbet, D.J.; Ricke, S.C. Effects of Alfalfa and Fructooligosaccharide on Molting Parameters and Bone Qualities Using Dual Energy X-Ray Absorptiometry and Conventional Bone Assays. Poult. Sci. 2006, 85, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Olgun, O.; Altay, Y.; Yildiz, A.O. Effects of carbohydrase enzyme supplementation on performance, eggshell quality, and bone parameters of laying hens fed on maize- and wheat-based diets. Br. Poult. Sci. 2018, 59, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Świątkiewicz, S.; Arczewska-Włosek, A.; Józefiak, D. Bones quality indices in laying hens fed diets with a high level of DDGS and supplemented with selected feed additives. Czech J. Anim. Sci. 2014, 59, 61–68. [Google Scholar] [CrossRef]
- Bederska-Lojewska, D.; Świątkiewicz, S.; Arczewska-Włosek, A.; Schwarz, T. Rye non-starch polysaccharides: Their impact on poultry intestinal physiology, nutrients digestibility and performance indices—A review. Ann. Anim. Sci. 2017, 17, 351–369. [Google Scholar] [CrossRef]
- Boros, D.; Fraś, A. Nutritional value and prohealthy properties of cereal and rapeseed varieties approved for cultivation in Poland. In Monographs and Dissertations; 49/2015; Plant Breeding and Acclimatization Institute—National Research Institute: Radzików, Poland, 2015. [Google Scholar]
- Mourão, J.; Ponte, P.I.P.; Prates, J.A.; Centeno, M.S.J.; Ferreira, L.M.A.; Soares, M.A.C.; Fontes, C.M.G.A. Use of β-Glucanases and β-1,4-Xylanases to Supplement Diets Containing Alfalfa and Rye for Laying Hens: Effects on Bird Performance and Egg Quality. J. Appl. Poult. Res. 2006, 15, 256–265. [Google Scholar] [CrossRef]
- Rajtar, P.; Górka, P.; Schwarz, T.; Micek, P. Effect of Hybrid Rye and Maize Grain Processing on Ruminal and Postruminal Digestibility Parameters. Ann. Anim. Sci. 2020, 20, 1065–1083. [Google Scholar] [CrossRef]
- Choct, M.; Hughes, R.J.; Wang, J.; Bedford, M.R.; Morgan, A.J.; Annison, G. Increased small intestinal fermentation is partly responsible for the anti-nutritive activity of non-starch polysaccharides in chickens. Br. Poult. Sci. 1996, 37, 609–621. [Google Scholar] [CrossRef]
- Bedford, M.R.; Schulze, H. Exogenous enzymes for pigs and poultry. Nutr. Res. Rev. 1998, 11, 91–114. [Google Scholar] [CrossRef]
- Adeola, O.; Cowieson, A.J. Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 2011, 89, 3189–3218. [Google Scholar] [CrossRef]
- Bautil, A.; Verspreet, J.; Buyse, J.; Goos, P.; Bedford, M.; Courtin, C.M. Age-related arabinoxylan hydrolysis and fermentation in the gastrointestinal tract of broilers fed wheat-based diets. Poult. Sci. 2019, 98, 4606–4621. [Google Scholar] [CrossRef] [PubMed]
- Smulikowska, S.; Rutkowski, A. Recommended Allowances and Nutritive Value of Feedstuffs. In Poultry Feeding Standards, 5th ed.; The Kielanowski Institute of Animal Physiology and Nutrition PAS: Jabłonna, Poland, 2018; pp. 66–74. [Google Scholar]
- Costa, F.G.P.; Goulart, C.D.C.; Oliveira, C.; Figueiredo, D.; Neto, R. Use of Exogenous Enzymes on Laying Hens Feeding During the Second Production Cycle. Int. J. Poult. Sci. 2008, 7, 333–338. [Google Scholar] [CrossRef]
- Mirzaie, S.; Zaghari, M.; Aminzadeh, S.; Shivazad, M.; Mateos, G.G. Effects of wheat inclusion and xylanase supplementation of the diet on productive performance, nutrient retention, and endogenous intestinal enzyme activity of laying hens. Poult. Sci. 2012, 91, 413–425. [Google Scholar] [CrossRef]
- Silversides, F.G.; Scott, T.A.; Korver, D.R.; Afsharmanesh, M.; Hruby, M. A Study on the Interaction of Xylanase and Phytase Enzymes in Wheat-Based Diets Fed to Commercial White and Brown Egg Laying Hens. Poult. Sci. 2006, 85, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Węsierska, E.; Niemczyńska, K.; Pasternak, M.; Arczewska-Włosek, A. Selected Physical and Chemical Characteristics of Eggs Laid by Hens Fed Diets with Different Levels of Hybrid Rye. Ann. Anim. Sci. 2019, 19, 1009–1020. [Google Scholar] [CrossRef]
- Lazaro, R.; Garcia, M.; Aranibar, M.J.; Mateos, G.G. Effect of enzyme addition to wheat-, barley- and rye-based diets on nutrient digestibility and performance of laying hens. Br. Poult. Sci. 2003, 44, 256–265. [Google Scholar] [CrossRef]
- Brufau, J.; Cos, R.; Pérez-Vendrell, A.; Esteve-Garcia, E. Performance of laying hens as affected by the supplementation of a barley-based diet with a crude enzyme preparation from Trichoderma viride. Can. J. Anim. Sci. 1994, 74, 129–133. [Google Scholar] [CrossRef]
- Pan, C.F.; Igbasan, F.A.; Guenter, W.; Marquardt, R.R. The effects of enzyme and inorganic phosphorus supplements in wheat- and rye-based diets on laying hen performance, energy, and phosphorus availability. Poult. Sci. 1998, 77, 83–89. [Google Scholar] [CrossRef]
- Fengler, A.I.; Marquardt, R.R. Water-soluble pentosans from rye II. Effects on the rate of dialysis and the retention of nutrients by the chicks. Cereal Chem. 1988, 65, 298–302. [Google Scholar]
- Latorre, J.D.; Hernandez-Velasco, X.; Bielke, L.R.; Vicente, J.L.; Wolfenden, R.; Menconi, A.; Hargis, B.M.; Tellez, G. Evaluation of a Bacillus direct-fed microbial candidate on digesta viscosity, bacterial translocation, microbiota composition and bone mineralization in broiler chickens fed on a rye-based diet. Br. Poult. Sci. 2015, 56, 723–732. [Google Scholar] [CrossRef]
- Langhout, D.J.; Schutte, J.B.; Geerse, C.; Kies, A.K.; De Jong, J.; Verstegen, M.W.A. Effects on chick performance and nutrient digestibility of an endo-xylanase added to a wheat- and rye-based diet in relation to fat source. Br. Poult. Sci. 1997, 38, 557–563. [Google Scholar] [CrossRef]
- Jürgens, H.-U.; Jansen, G.; Wegener, C.B. Characterisation of Several Rye Cultivars with Respect to Arabinoxylans and Extract Viscosity. J. Agric. Sci. 2012, 4, p1. [Google Scholar] [CrossRef]
- Arczewska-Wlosek, A.; Swiatkiewicz, S.; Bederska-Lojewska, D.; Orczewska-Dudek, S.; Szczurek, W.; Boros, D.; Fras, A.; Tomaszewska, E.; Dobrowolski, P.; Muszynski, S.; et al. The Efficiency of Xylanase in Broiler Chickens Fed with Increasing Dietary Levels of Rye. Animals 2019, 9, 46. [Google Scholar] [CrossRef]
- Janssen, W.M.M.A. European Table of Energy Values for Poultry Feedstuffs, 3rd ed.; Subcommittee Energy of the Working Group nr. 2 Nutrition of the European Federation of Branches of the World’s Poultry Science Association; WPSA: Beekbergen, The Netherlands, 1989; ISBN 90-71463-00-0. [Google Scholar]
- Bederska-Łojewska, D.; Arczewska-Włosek, A.; Świątkiewicz, S.; Orczewska-Dudek, S.; Schwarz, T.; Puchała, M.; Krawczyk, J.; Boros, D.; Fraś, A.; Micek, P.; et al. The effect of different dietary levels of hybrid rye and xylanase addition on the performance and egg quality in laying hens. Br. Poult. Sci. 2019, 60, 423–430. [Google Scholar] [CrossRef]
- Jing, M.; Zhao, S.; Rogiewicz, A.; Slominski, B.A.; House, J.D. Assessment of the minimal available phosphorus needs of laying hens: Implications for phosphorus management strategies. Poult. Sci. 2018, 97, 2400–2410. [Google Scholar] [CrossRef]
- Świetlicka, I.; Kuc, D.; Świetlicki, M.; Arczewska, M.; Muszyński, S.; Tomaszewska, E.; Prószyński, A.; Gołacki, K.; Błaszczak, J.; Cieślak, K.; et al. Near-Surface Studies of the Changes to the Structure and Mechanical Properties of Human Enamel under the Action of Fluoride Varnish Containing CPP–ACP Compound. Biomolecules 2020, 10, 765. [Google Scholar] [CrossRef]
- Świetlicka, I.; Arczewska, M.; Muszyński, S.; Tomaszewska, E.; Świetlicki, M.; Kuc, D.; Mielnik-Błaszczak, M.; Gołacki, K.; Cieślak, K. Surface analysis of etched enamel modified during the prenatal period. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117271. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. Kolloidchem. Lehrb. 1912, 2, 387–409. [Google Scholar] [CrossRef]
- Rajesh, R.; Hariharasubramanian, A.; Ravichandran, Y.D. Chicken Bone as a Bioresource for the Bioceramic (Hydroxyapatite). Phosphorus Sulfur Silicon Relat. Elem. 2012, 187, 914–925. [Google Scholar] [CrossRef]
- Reyes-Gasga, J.; Martínez-Piñeiro, E.L.; Rodríguez-Álvarez, G.; Tiznado-Orozco, G.E.; García-García, R.; Brès, É.F. XRD and FTIR crystallinity indices in sound human tooth enamel and synthetic hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 4568–4574. [Google Scholar] [CrossRef]
- Boskey, A.L. Assessment of bone mineral and matrix using backscatter electron imaging and FTIR imaging. Curr. Osteoporos. Rep. 2006, 4, 71–75. [Google Scholar] [CrossRef]
- Donnelly, E.; Boskey, A.L.; Baker, S.P.; Van Der Meulen, M.C.H. Effects of tissue age on bone tissue material composition and nanomechanical properties in the rat cortex. J. Biomed. Mater. Res. Part A 2009, 92, 1048–1056. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Mendelsohn, R.; Boskey, A.L. Infrared Assessment of Bone Quality: A Review. Clin. Orthop. Relat. Res. 2011, 469, 2170–2178. [Google Scholar] [CrossRef]
- Ou-Yang, H.; Paschalis, E.P.; Mayo, W.E.; Boskey, A.L.; Mendelsohn, R. Infrared Microscopic Imaging of Bone: Spatial Distribution of CO32−. J. Bone Miner. Res. 2001, 16, 893–900. [Google Scholar] [CrossRef]
- Miller, L.M.; Vairavamurthy, V.; Chance, M.R.; Mendelsohn, R.; PaschaIis, E.P.; Betts, F.; Boskey, A.L. In situ analysis of mineral content and crystallinity in bone using infrared micro-spectroscopy of the ν4 PO43− vibration. Biochim. Biophys. Acta 2001, 1527, 11–19. [Google Scholar] [CrossRef]
- Junqueira, L.C.U.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. J. Mol. Histol. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Kamiński, D.M.; Czech, A.; Grela, E.; Wiącek, D.; Tomczyk-Warunek, A. Dried fermented post-extraction rapeseed meal given to sows as an alternative protein source for soybean meal during pregnancy improves bone development of their offspring. Livest. Sci. 2019, 224, 60–68. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, P.; Kong, X.; Xie, S.; Li, Q.; Li, Z.; Zhou, Z. Delicate changes of bioapatite mineral in pig femur with addition of dietary xylooligosaccharide: Evidences from Raman spectroscopy and ICP. Anim. Sci. J. 2017, 88, 1820–1826. [Google Scholar] [CrossRef]
- Rudyk, H.; Tomaszewska, E.; Kotsyumbas, I.; Muszyński, S.; Tomczyk-Warunek, A.; Szymańczyk, S.; Dobrowolski, P.; Wiącek, D.; Kamiński, D.; Brezvyn, O. Bone Homeostasis in Experimental Fumonisins Intoxication of Rats. Ann. Anim. Sci. 2019, 19, 403–419. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.; Wang, S.-J.; Zhang, L.; Qiu, J.-Y.; Wu, Y.; Zhou, Z.-L. Rapid increase of carbonate in cortical bones of hens during laying period. Poult. Sci. 2016, 95, 2889–2894. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, A.B.; Gaines, K.F.; Romanek, C.S.; Masson, G.R. Mineralization of Clapper Rail Eggshell from a Contaminated Salt Marsh System. Arch. Environ. Contam. Toxicol. 2002, 43, 449–460. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, A.; McCormack, H.; Fleming, R.; Alvarez-Lloret, P.; Romero-Pastor, J.; Dominguez-Gasca, N.; Prozorov, T.; Dunn, I.C. Influence of physical activity on tibial bone material properties in laying hens. J. Struct. Biol. 2018, 201, 36–45. [Google Scholar] [CrossRef]
- Muszyński, S.; Tomaszewska, E.; Dobrowolski, P.; Kwiecień, M.; Wiącek, D.; Świetlicka, I.; Skibińska, M.; Szymańska-Chargot, M.; Orzeł, J.; Świetlicki, M.; et al. Analysis of bone osteometry, mineralization, mechanical and histomorphometrical properties of tibiotarsus in broiler chickens demonstrates a influence of dietary chickpea seeds (Cicer arietinum L.) inclusion as a primary protein source. PLoS ONE 2018, 13, e0208921. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Kwiecień, M.; Muszyński, S.; Dobrowolski, P.; Kasperek, K.; Blicharski, T.; Jeżewska-Witkowska, G.; Grela, E.R. Long-bone properties and development are affected by caponisation and breed in Polish fowls. Br. Poult. Sci. 2017, 58, 312–318. [Google Scholar] [CrossRef]
- Téllez, G.; Latorre, J.D.; Kuttappan, V.A.; Kogut, M.H.; Ewolfenden, A.; Ehernandez-Velasco, X.; Hargis, B.M.; Bottje, W.G.; Bielke, L.R.; Faulkner, O.B. Utilization of rye as energy source affects bacterial translocation, intestinal viscosity, microbiota composition, and bone mineralization in broiler chickens. Front. Genet. 2014, 5, 339. [Google Scholar] [CrossRef]
- Téllez, G.; Latorre, J.D.; Kuttappan, V.A.; Hargis, B.M.; Hernandez-Velasco, X. Rye Affects Bacterial Translocation, Intestinal Viscosity, Microbiota Composition and Bone Mineralization in Turkey Poults. PLoS ONE 2015, 10, e0122390. [Google Scholar] [CrossRef]
- Muszyński, S.; Świątkiewicz, S.; Arczewska-Włosek, A.; Dobrowolski, P.; Piedra, J.L.V.; Arciszewski, M.B.; Szymańczyk, S.; Zacharko-Siembida, A.; Kowalik, S.; Hułas-Stasiak, M.; et al. Analysis of mechanical properties of bones and tendons shows that modern hybrid rye can be introduced to corn-wheat based diet in broiler chickens as an alternative energy source irrespective of xylanase supplementation. Poult. Sci. 2019, 98, 5613–5621. [Google Scholar] [CrossRef]
- Jäger, I.; Fratzl, P. Mineralized Collagen Fibrils: A Mechanical Model with a Staggered Arrangement of Mineral Particles. Biophys. J. 2000, 79, 1737–1746. [Google Scholar] [CrossRef]
- Ruppel, M.E.; Miller, L.M.; Burr, D.B. The effect of the microscopic and nanoscale structure on bone fragility. Osteoporos. Int. 2008, 19, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Sparke, A.; Sims, T.; Avery, N.; Bailey, A.J.; Fleming, R.H.; Whitehead, C.C. Differences in composition of avian bone collagen following genetic selection for resistance to osteoporosis. Br. Poult. Sci. 2002, 43, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Gasca, N.; Benavides-Reyes, C.; Sánchez-Rodríguez, E.; Rodríguez-Navarro, A.B. Changes in avian cortical and medullary bone mineral composition and organization during acid-induced demineralization. Eur. J. Miner. 2019, 31, 209–216. [Google Scholar] [CrossRef]
- Farlay, D.; Panczer, G.; Rey, C.; Delmas, P.D.; Boivin, G. Mineral maturity and crystallinity index are distinct characteristics of bone mineral. J. Bone Miner. Metab. 2010, 28, 433–445. [Google Scholar] [CrossRef]
- Boskey, A.L.; Imbert, L. Bone quality changes associated with aging and disease: A review. Ann. N. Y. Acad. Sci. 2017, 1410, 93–106. [Google Scholar] [CrossRef]
- Nishimura, K.; Miyamoto, S.; Takeda, T.; Ayukawa, E.; Sugiyama, T.; Kusuhara, S. Changes in the Outer Shape, Inner Structure and Bone Mineral Density of the Tibia in Growing Japanese Quail, Coturnix japonica. J. Poult. Sci. 2007, 44, 426–432. [Google Scholar] [CrossRef]
- Burnell, J.; Baylink, D.; Chestnut, C.H.; Mathews, M.; Teubner, E. Bone matrix and mineral abnormalities in postmenopausal osteoporosis. Metabolism 1982, 31, 1113–1120. [Google Scholar] [CrossRef]
- Fujisaki, K.; Todoh, M.; Niida, A.; Shibuya, R.; Kitami, S.; Tadano, S. Orientation and deformation of mineral crystals in tooth surfaces. J. Mech. Behav. Biomed. Mater. 2012, 10, 176–182. [Google Scholar] [CrossRef]
- Gamal, G.A.; Al-Mufadi, F.A.; Said, A.H. Effect of iron additives on the microstructure of hydroxyapatite. ETASR Eng. Technol. Appl. Sci. Res. 2013, 3, 532–539. [Google Scholar]
- Rey, C.; Renugopalakrishman, V.; Collins, B.; Glimcher, M.J. Fourier transform infrared spectroscopic study of the carbonate ions in bone mineral during aging. Calcif. Tissue Int. 1991, 49, 251–258. [Google Scholar] [CrossRef]
- Hester, P.Y.; Schreiweis, M.A.; Orban, J.I.; Mazzuco, H.; Kopka, M.N.; Ledur, M.C.; Moody, D.E. Assessing bone mineral density in vivo: Dual energy X-ray absorptiometry. Poult. Sci. 2004, 83, 215–221. [Google Scholar] [CrossRef]
- Schreiweis, M.A.; Orban, J.I.; Ledur, M.C.; Moody, D.E.; Hester, P.Y. Validation of dual-energy X-ray absorptiometry in live White Leghorns. Poult. Sci. 2005, 84, 91–99. [Google Scholar] [CrossRef]
- Knott, L.; Bailey, A.J. Collagen cross-links in mineralising tissue: A review of their chemistry, function and clinical relevance. Bone 1998, 22, 181–187. [Google Scholar] [CrossRef]
- Knott, L.; Whitehead, C.C.; Fleming, R.H.; Bailey, A.J. Biochemical changes in the collagenous matrix of osteoporotic avian bone. Biochem. J. 1995, 310, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
| Item | Diet | |
|---|---|---|
| Wheat–Corn | Rye–Wheat–Corn | |
| Ingredients (g/kg) | ||
| Rye | 0.00 | 250.00 |
| Wheat | 382.30 | 250.80 |
| Corn | 290.00 | 150.00 |
| Soybean meal | 200.00 | 210.00 |
| Rapeseed oil | 15.00 | 27.00 |
| Limestone | 91.00 | 90.00 |
| Monocalcium phosphate | 12.00 | 12.00 |
| NaCl | 3.00 | 3.00 |
| DL-Methionine | 1.10 | 1.10 |
| L-Lysine hydrochloride | 0.60 | 0.10 |
| Vitamin–mineral premix 1 | 5.00 | 5.00 |
| Metabolizable energy, MJ/kg 3 | 11.55 | |
| Nutrient composition (g/kg DM) 2 | ||
| Crude protein | 170.00 | |
| Lys | 7.20 | |
| Met | 3.40 | |
| Ca | 36.00 | |
| Available P | 3.75 | |
| Na | 1.50 | |
| Cl | 2.19 | |
| Factors 1 | DXA Measurement | Ash | |||
|---|---|---|---|---|---|
| Rye | Xylanase | BMD, g/cm2 | Area, cm2 | BMC, g | Ash, % |
| Treatment 2 | |||||
| 0% | 0 mg/kg | 0.275 b | 4.14 | 1.151 b | 29.9 a |
| 200 mg/kg | 0.238 ab | 3.62 | 0.902 a | 34.2 b | |
| 25% | 0 mg/kg | 0.198 a | 4.57 | 0.899 a | 33.0 b |
| 200 mg/kg | 0.246 b | 4.12 | 1.012 ab | 31.7 ab | |
| SEM 3 | 0.018 | 0.09 | 0.081 | 0.6 | |
| Main factors | |||||
| 0% | 0.257 | 3.88 a | 1.027 | 32.4 | |
| 25% | 0.222 | 4.33 b | 0.956 | 32.1 | |
| 0 mg/kg | 0.236 | 4.34 a | 1.025 | 31.5 | |
| 200 mg/kg | 0.243 | 3.87 b | 0.957 | 33.0 | |
| p-value | |||||
| Rye | 0.067 | <0.001 | 0.392 | 0.603 | |
| Xylanase | 0.722 | <0.001 | 0.409 | 0.020 | |
| Rye x xylanase | 0.027 | 0.592 | 0.034 | <0.001 | |
| Factors 1 | XRD Diffraction | FTIR Spectroscopy | |||||
|---|---|---|---|---|---|---|---|
| Rye | Xylanase | HA c-Axis Size, nm | Crystallinity Index | Mineral-to-Matrix Ratio | Carbonate-to-Phosphate Ratio | Crystallinity | Collagen Maturity |
| Treatment 2 | |||||||
| 0% | 0 mg/kg | 19.28 | 0.161 | 10.57 a | 0.0263 | 1.19 | 5.25 b |
| 200 mg/kg | 19.44 | 0.165 | 12.85 b | 0.0239 | 1.15 | 5.31 b | |
| 25% | 0 mg/kg | 19.47 | 0.165 | 12.75 b | 0.0276 | 1.25 | 4.06 a |
| 200 mg/kg | 19.72 | 0.172 | 11.62 ab | 0.0250 | 1.17 | 5.36 b | |
| SEM 3 | 0.28 | 0.070 | 0.55 | 0.0007 | 0.03 | 0.18 | |
| Main factors | |||||||
| 0% | 19.36 | 0.163 | 11.71 | 0.0251 | 1.17 | 5.28 | |
| 25% | 19.60 | 0.169 | 12.19 | 0.0263 | 1.21 | 4.71 | |
| 0 mg/kg | 19.37 | 0.163 | 11.66 | 0.0270 b | 1.22 b | 4.65 | |
| 200 mg/kg | 19.58 | 0.168 | 12.23 | 0.0245 a | 1.16 a | 5.34 | |
| p-value | |||||||
| Rye | 0.406 | 0.398 | 0.396 | 0.118 | 0.114 | 0.005 | |
| Xylanase | 0.466 | 0.465 | 0.310 | 0.002 | 0.033 | 0.001 | |
| Rye x xylanase | 0.871 | 0.842 | 0.005 | 0.876 | 0.523 | 0.002 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muszyński, S.; Arczewska, M.; Świątkiewicz, S.; Arczewska-Włosek, A.; Dobrowolski, P.; Świetlicka, I.; Hułas-Stasiak, M.; Blicharski, T.; Donaldson, J.; Schwarz, T.; et al. The Effect of Dietary Rye Inclusion and Xylanase Supplementation on Structural Organization of Bone Constitutive Phases in Laying Hens Fed a Wheat-Corn Diet. Animals 2020, 10, 2010. https://doi.org/10.3390/ani10112010
Muszyński S, Arczewska M, Świątkiewicz S, Arczewska-Włosek A, Dobrowolski P, Świetlicka I, Hułas-Stasiak M, Blicharski T, Donaldson J, Schwarz T, et al. The Effect of Dietary Rye Inclusion and Xylanase Supplementation on Structural Organization of Bone Constitutive Phases in Laying Hens Fed a Wheat-Corn Diet. Animals. 2020; 10(11):2010. https://doi.org/10.3390/ani10112010
Chicago/Turabian StyleMuszyński, Siemowit, Marta Arczewska, Sylwester Świątkiewicz, Anna Arczewska-Włosek, Piotr Dobrowolski, Izabela Świetlicka, Monika Hułas-Stasiak, Tomasz Blicharski, Janine Donaldson, Tomasz Schwarz, and et al. 2020. "The Effect of Dietary Rye Inclusion and Xylanase Supplementation on Structural Organization of Bone Constitutive Phases in Laying Hens Fed a Wheat-Corn Diet" Animals 10, no. 11: 2010. https://doi.org/10.3390/ani10112010
APA StyleMuszyński, S., Arczewska, M., Świątkiewicz, S., Arczewska-Włosek, A., Dobrowolski, P., Świetlicka, I., Hułas-Stasiak, M., Blicharski, T., Donaldson, J., Schwarz, T., & Tomaszewska, E. (2020). The Effect of Dietary Rye Inclusion and Xylanase Supplementation on Structural Organization of Bone Constitutive Phases in Laying Hens Fed a Wheat-Corn Diet. Animals, 10(11), 2010. https://doi.org/10.3390/ani10112010







