An Unusual Case of Testicular Disorder in Sex Development of Arabian Mare (64,XX SRY-Negative)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
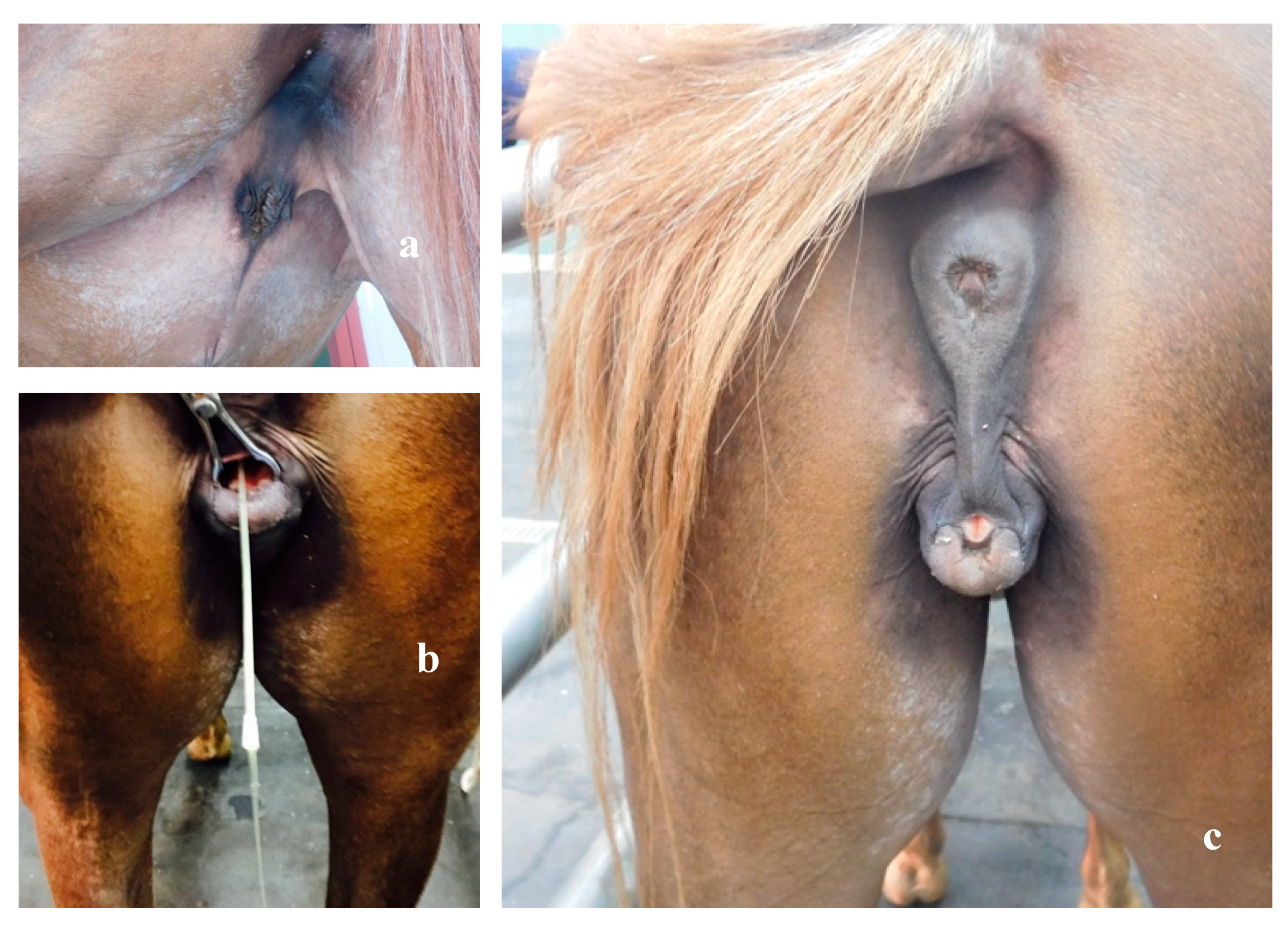
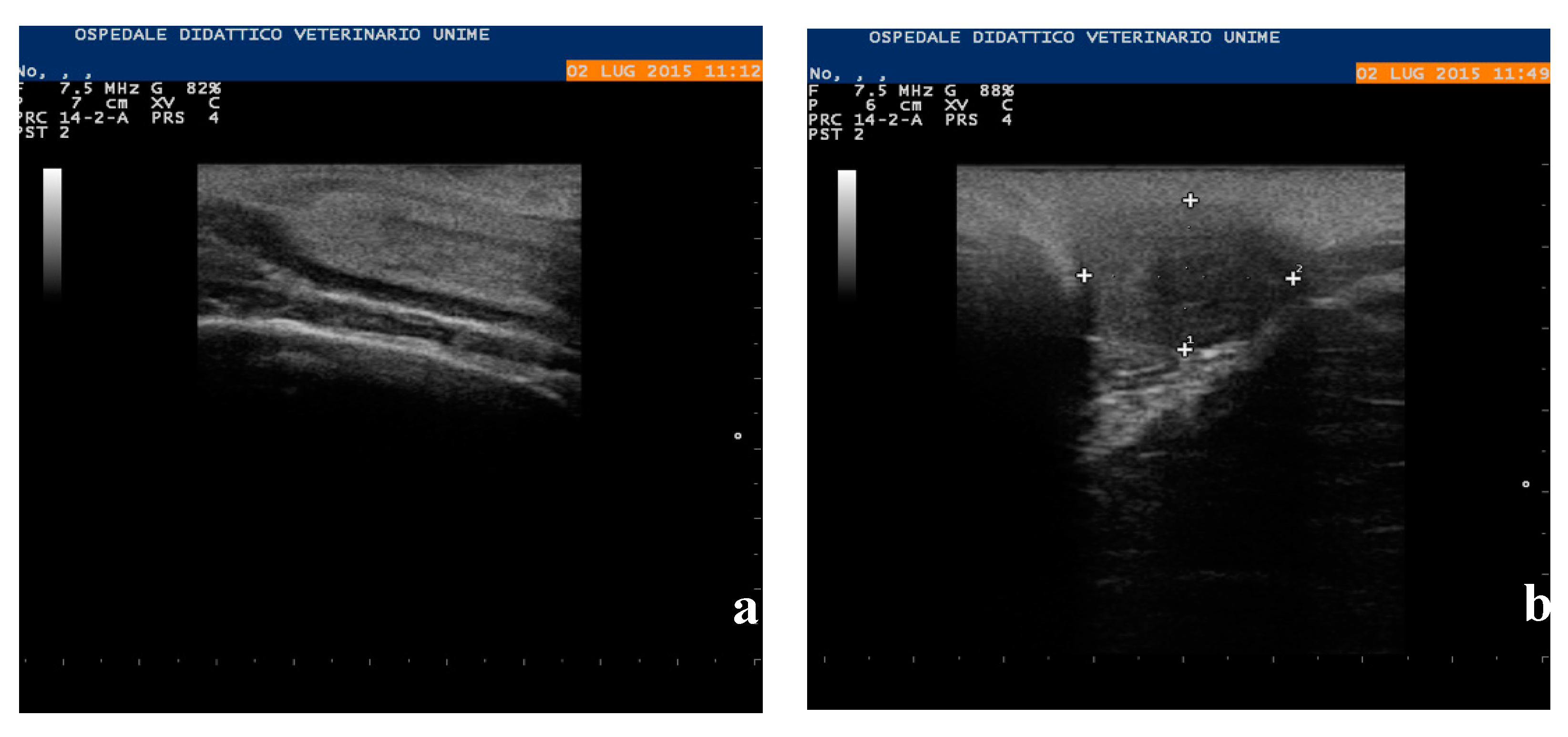
2.2. Clinical Examination
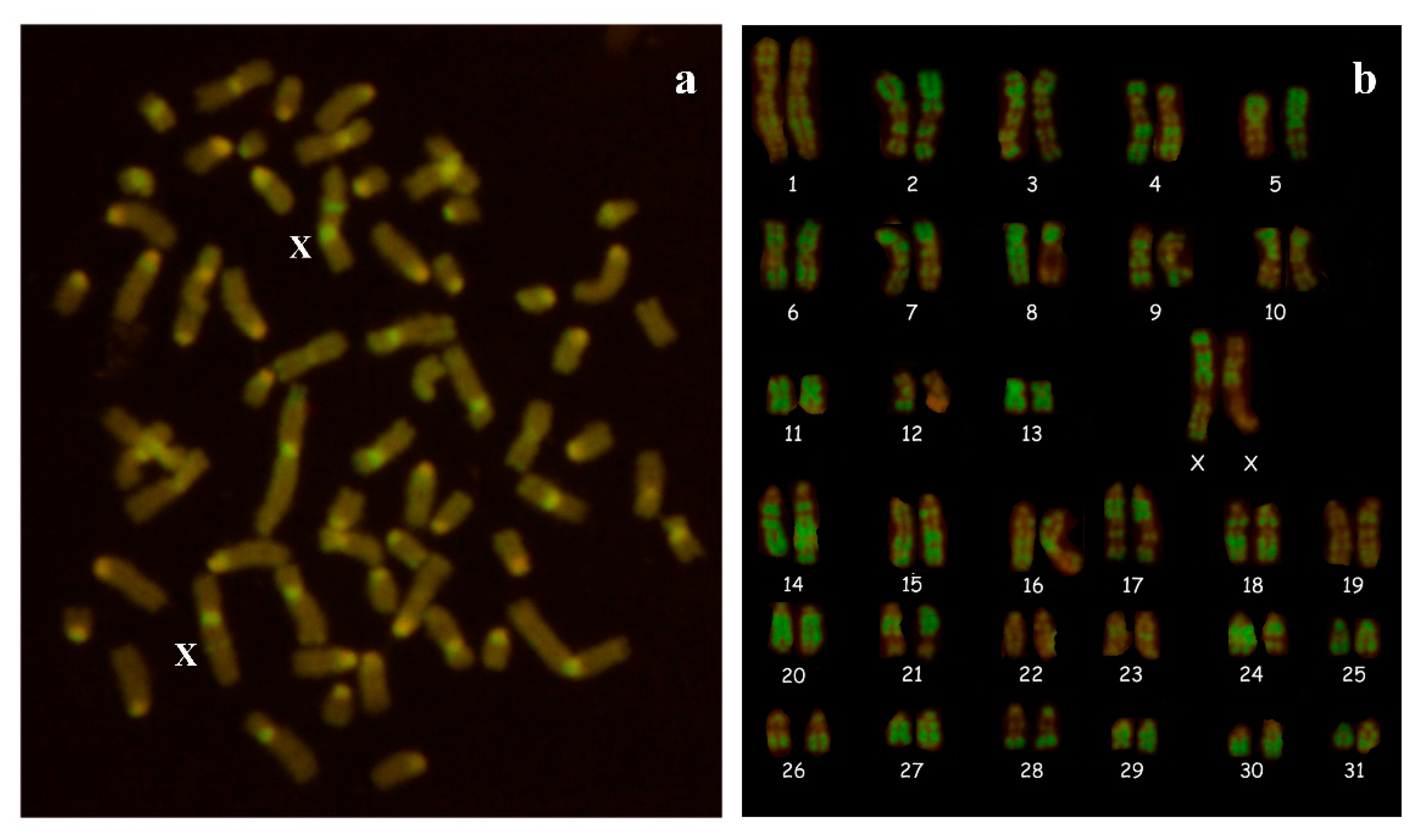
2.3. Cytogenetic Analysis
2.4. Hormonal Analysis
2.5. Molecular Analysis
2.6. Histopathological Methods
3. Results
3.1. Cytogenetic and Molecular Analyses
3.2. Testosterone Concentrations
3.3. Necropsy and Anatomopathological Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ali, A.; Derar, D.R.; AlShahed, M. Management strategies, reproductive performance and causes of infertility in sheep flocks in the central region of Saudi Arabia. Trop. Anim. Health Prod. 2019, 52, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Chakravarty, A.K.; Singh, A.; Upadhyay, A.; Singh, M.; Yousuf, S. Effect of heat stress on reproductive performances of dairy cattle and buffaloes: A review. Veter. World 2016, 9, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, C.; Ciani, F.; Tafuri, S.; Pasolini, M.P.; Della Valle, G.; Palumbo, V.; Abbondante, L.; Calamo, A.; Barbato, V.; Gualtieri, R.; et al. Effect of superoxide dismutase, catalase, and glutathione peroxidase supplementation in the extender on chilled semen of fertile and hypofertile dogs. J. Veter. Sci. 2018, 19, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Tafuri, S.; Cocchia, N.; Vassetti, A.; Carotenuto, D.; Esposito, L.; Maruccio, L.; Avallone, L.; Ciani, F. Lepidium meyenii (Maca) in male reproduction. Nat. Prod. Res. 2019, 5, 1–10. [Google Scholar] [CrossRef]
- Tafuri, S.; Cocchia, N.; Carotenuto, D.; Vassetti, A.; Staropoli, A.; Mastellone, V.; Peretti, V.; Ciotola, F.; Albarella, S.; Del Prete, C.; et al. Chemical Analysis of Lepidium meyenii (Maca) and Its Effects on Redox Status and on Reproductive Biology in Stallions †. Molecules 2019, 24, 1981. [Google Scholar] [CrossRef]
- Bugno, M.; Klukowska, J.; Słota, E.; Tischner, M.; Świtoński, M. A sporadic case of the sex-reversed mare (64,XY; SRY-negative): Molecular and cytogenetic studies of the Y chromosome. Theriogenology 2003, 59, 1597–1603. [Google Scholar] [CrossRef]
- Lear, T.; McGee, R. Disorders of Sexual Development in the Domestic Horse, Equus caballus. Sex. Dev. 2012, 6, 61–71. [Google Scholar] [CrossRef]
- Albarella, S.; Ciotola, F.; D’Anza, E.; Coletta, A.; Zicarelli, L.; Peretti, V. Congenital Malformations in River Buffalo (Bubalus bubalis). Animals 2017, 7, 9. [Google Scholar] [CrossRef]
- De Lorenzi, L.; Arrighi, S.; Rossi, E.; Grignani, P.; Previderè, C.; Bonacina, S.; Cremonesi, F.; Parma, P. XY (SRY-positive) Ovarian Disorder of Sex Development in Cattle. Sex. Dev. 2018, 12, 196–203. [Google Scholar] [CrossRef]
- Albarella, S.; D’Anza, E.; Galdiero, G.; Esposito, L.; De Biase, D.; Paciello, O.; Ciotola, F.; Peretti, V. Cytogenetic Analyses in Ewes with Congenital Abnormalities of the Genital Apparatus. Animals 2019, 9, 776. [Google Scholar] [CrossRef]
- Vaiman, D.; Koutita, O.; Oustry, A.; Elsen, J.-M.; Manfredi, E.; Fellous, M.; Cribiu, E.P. Genetic mapping of the autosomal region involved in XX sex-reversal and horn development in goats. Mamm. Genome 1996, 7, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Pailhoux, E.; Cotinot, C.; Popescu, P.C.; Boscher, J.; Legault, C.; Fellous, M.; Parma, P.; Molteni, L. Genetic analysis of 38XX males with genital ambiguities and true hermaphrodites in pigs. Anim. Genet. 2009, 25, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Krzemińska, P.; D’Anza, E.; Ciotola, F.; Paciello, O.; Restucci, B.; Peretti, V.; Albarella, S.; Switonski, M. Polymorphisms of MAMLD1, SRD5A2, and AR Candidate Genes in Seven Dogs (78,XY; SRY-Positive) Affected by Hypospadias or Cryptorchidism. Sex. Dev. 2019, 13, 92–98. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzi, L.; Banco, B.; Previderè, C.; Bonacina, S.; Romagnoli, S.; Grieco, V.; Parma, P. Testicular XX (SRY-Negative) Disorder of Sex Development in Cat. Sex. Dev. 2017, 11, 210–216. [Google Scholar] [CrossRef]
- D’Ovidio, D.; Melidone, R.; Rossi, G.; Albarella, S.; Noviello, E.; Fioretti, A.; Meomartino, L. Multiple Congenital Malformations in a Ferret (Mustela putorius furo). J. Exot. Pet. Med. 2015, 24, 92–97. [Google Scholar] [CrossRef]
- Villagómez, D.; Lear, T.; Chenier, T.; Lee, S.; McGee, R.; Cahill, J.; Foster, R.; Reyes, E.; John, E.S.; King, W. Equine Disorders of Sexual Development in 17 Mares Including XX, SRY-Negative, XY, SRY-Negative and XY, SRY-Positive Genotypes. Sex. Dev. 2011, 5, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Albarella, S.; De Lorenzi, L.; Catone, G.; Magi, G.E.; Petrucci, L.; Vullo, C.; D’Anza, E.; Parma, P.; Raudsepp, T.; Ciotola, F.; et al. Diagnosis of XX/XY Blood Cell Chimerism at a Low Percentage in Horses. J. Equine Veter. Sci. 2018, 69, 129–135. [Google Scholar] [CrossRef]
- Blaschko, S.D.; Cunha, G.R.; Baskin, L.S. Molecular mechanisms of external genitalia development. Differentiation 2012, 84, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Pujar, S.; Meyers-Wallen, V. Sequence Variations in Equine Candidate Genes For XX and XY Inherited Disorders of Sexual Development. Reprod. Domest. Anim. 2012, 47, 827–834. [Google Scholar] [CrossRef]
- Mäkinen, A.; Hasegawa, T.; Mäkilä, M.; Katila, T. Infertility in two mares with XY and XXX sex chromosomes. Equine Veter. J. 1999, 31, 346–349. [Google Scholar] [CrossRef]
- Kuiper, H.; Distl, O. Intersexuality in horses. DTW. Dtsch. Tierarztl. Wochenschr. 2007, 114, 50–56. [Google Scholar] [PubMed]
- Peer, M.; Neuhauser, S.; Klaus, C.; Kuiper, H.; Gruber, A.D.; Distl, O.; Lischer, C.; Handler, J. Laparoscopic Gonadectomy in Two Intersex Warmblood Horses. J. Equine Veter. Sci. 2012, 32, 117–122. [Google Scholar] [CrossRef]
- Pienkowska-Schelling, A.; Becker, D.; Bracher, V.; Pineroli, B.; Schelling, C. Zytogenetische und molekulargenetische Abklärungen bei einem Pferd mit SRY-negativer Sex-Umkehr. Schweiz Arch. Tierheilkd 2014, 156, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Raudsepp, T.; Durkin, K.; Lear, T.L.; Das, P.J.; Avila, F.; Kachroo, P.; Chowdhary, B.P. Molecular heterogeneity of XY sex reversal in horses. Anim. Genet. 2010, 41, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Switonski, M.; Chmurzyńska, A.; Szczerbal, I.; Lipczynski, A.; Yang, F.; Nowicka-Posluszna, A. Sex reversal syndrome (64,XY; SRY-positive) in a mare demonstrating masculine behaviour. J. Anim. Breed. Genet. 2005, 122, 60–63. [Google Scholar] [CrossRef]
- Eggers, S.; Ohnesorg, T.; Sinclair, A.H. Genetic regulation of mammalian gonad development. Nat. Rev. Endocrinol. 2014, 10, 673–683. [Google Scholar] [CrossRef]
- Parma, P.; Radi, O.; Vidal, V.; Chaboissier, M.C.; Dellambra, E.; Valentini, S.; Guerra, L.; Schedl, A.; Camerino, G. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat. Genet. 2006, 38, 1304–1309. [Google Scholar] [CrossRef]
- Bodvarsdottir, S.K.; Imsland, F.; Thorisson, B.; Steinarsdottir, M.; Eyfjord, J.E. 64,XX, SRY-, and ZFY-Negative Icelandic Horse Likely to Be True Hermaphrodite. J. Equine Veter. Sci. 2009, 29, 734–738. [Google Scholar] [CrossRef]
- Jaszczak, K.; Sysa, P.; Sacharczuk, M.; Parada, R.; Romanowicz, K.; Kawka, M.; Jarmuż, W. SRY-negative, 64, XX sex reversal in a Konik Polski horse: A case report. Anim. Sci. Papers Rep. 2010, 28, 381–388. [Google Scholar]
- Vaughan, L.; Schofield, W.; Ennis, S. SRY-negative XX sex reversal in a pony: A case report. Theriogenology 2001, 55, 1051–1057. [Google Scholar] [CrossRef]
- Moreno-Millán, M.; Demyda, S.E.; Saleno, D.R. Sex Chromosomes Abnormalities in Purebred Spanish Horses with Reproductive Problems: Cases Report. Bullet. UASMV Vet. Med. 2012, 69, 1–7. [Google Scholar]
- Buoen, L.C.; Zhang, T.Q.; Weber, A.F.; Ruth, G.R. SRY-negative, XX intersex horses: The need for pedigree studies to examine the mode of inheritance of the condition. Equine Veter. J. 2000, 32, 78–81. [Google Scholar] [CrossRef]
- Ciotola, F.; Albarella, S.; Pasolini, M.; Auletta, L.; Esposito, L.; Iannuzzi, L.; Peretti, V. Molecular and Cytogenetic Studies in a Case of XX SRY-Negative Sex Reversal in an Arabian Horse. Sex. Dev. 2012, 6, 104–107. [Google Scholar] [CrossRef]
- Torres, A.; Silva, J.F.; Bernardes, N.; Luis, J.P.S.; Da Costa, L.L. 64, XX, SRY-negative, testicular DSD syndrome in a Lusitano horse. Reprod. Domest. Anim. 2012, 48, e33–e37. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, L.; Di Meo, G.; Perucatti, A.; Incarnato, D.; Peretti, V.; Ciotola, F.; Barbieri, V. An improved characterization of horse (Equus caballus, 2n = 64) chromosomes by using replicating G and R banding patterns. Caryologia 2003, 56, 205–211. [Google Scholar] [CrossRef][Green Version]
- Vilar, J.M.; Batista, M.; Carrillo, J.M.; Rubio, M.; Sopena, J.; Álamo, D. Histological, cytogenetic and endocrine evaluation in twenty-five unilateral cryptorchid horses. J. Appl. Anim. Res. 2017, 46, 441–444. [Google Scholar] [CrossRef]
- Han, S.-H.; Yang, B.-C.; Ko, M.-S.; Oh, H.-S.; Lee, S.-S. Length difference between equine ZFX and ZFY genes and its application for molecular sex determination. J. Assist. Reprod. Genet. 2010, 27, 725–728. [Google Scholar] [CrossRef][Green Version]
- Kent, M.; Shoffner, R.; Hunter, A.; Elliston, K.O.; Schroder, W.; Tolley, E.; Wachtel, S. XY sex reversal syndrome in the mare: Clinical and behavioral studies, H-Y phenotype. Qual. Life Res. 1988, 79, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Meyers-Wallen, V.N.; Hurtgen, J.; Schlafer, D.; Tulleners, E.; Cleland, W.R.; Ruth, G.R.; Acland, G.M. Sry-negative XX true hermaphroditism in a Pasa Fino horse. Equine Veter. J. 1997, 29, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Bannasch, D.L.; Rinaldo, C.; Millon, L.; Latson, K.; Spangler, T.; Hubberty, S.; Galuppo, L.; Lowenstine, L. SRY negative 64,XX intersex phenotype in an American saddlebred horse. Veter. J. 2007, 173, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-F.; Wu, Q.-Y.; Zhang, C.; Li, W.-W.; Zhou, Q.; Jiang, W.-J.; Cui, Y.-X.; Xia, X.-Y.; Shi, Y.-C. 46,XX testicular disorder of sexual development with SRY-negative caused by some unidentified mechanisms: A case report and review of the literature. BMC Urol. 2014, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Radi, O.; De Lorenzi, L.; Vetro, A.; Groppetti, D.; Bigliardi, E.; Luvoni, G.C.; Rota, A.; Camerino, G.; Zuffardi, O.; et al. Sox9 Duplications Are a Relevant Cause of Sry-Negative XX Sex Reversal Dogs. PLoS ONE 2014, 9, e101244. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Ji, X.; Xing, Y.; Chen, Y.W.; Tao, J. A rare case of 46, XX SRYnegative male with approximately 74-kb duplication in a region upstream of SOX9. Eur. J. Med. Genet. 2013, 56, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Benko, S.; Gordon, C.T.; Mallet, D.; Sreenivasan, R.; Thauvin-Robinet, C.; Brendehaug, A.; Thomas, S.; Bruland, O.; David, M.; Nicolino, M.; et al. Disruption of a long distance regulatory region upstream of SOX9 in isolated disorders of sex development. J. Med. Genet. 2011, 48, 825–830. [Google Scholar] [CrossRef]
- Albarella, S.; De Lorenzi, L.; Rossi, E.; Prisco, F.; Riccardi, M.G.; Restucci, B.; Ciotola, F.; Parma, P. Analysis of XX SRY-Negative Sex Reversal Dogs. Animals 2020, 10, 1667. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, A.; Desmangles, J.-C.; Gartner, L.A.; Buchlis, J. Duplication of dosage sensitive sex reversal area in a 46, XY patient with normal sex determining region of Y causing complete sex reversal. J. Pediatr. Endocrinol. Metab. 2013, 26, 775–779. [Google Scholar] [CrossRef]
- Mizuno, K.; Kojima, Y.; Kamisawa, H.; Moritoki, Y.; Nishio, H.; Kohri, K.; Hayashi, Y. Gene Expression Profile During Testicular Development in Patients With SRY-negative 46,XX Testicular Disorder of Sex Development. Urology 2013, 82, 1453.e1–1453.e7. [Google Scholar] [CrossRef]
- Moalem, S.; Babul-Hirji, R.; Stavropolous, D.J.; Wherrett, D.; Bägli, D.J.; Thomas, P.; Chitayat, D. XX male sex reversal with genital abnormalities associated with a de novoSOX3gene duplication. Am. J. Med. Genet. Part. A 2012, 1759–1764. [Google Scholar] [CrossRef]
- Polanco, J.C.; Wilhelm, D.; Davidson, T.-L.; Knight, D.; Koopman, P. Sox10 gain-of-function causes XX sex reversal in mice: Implications for human 22q-linked disorders of sex development. Hum. Mol. Genet. 2009, 19, 506–516. [Google Scholar] [CrossRef]
- Jeays-Ward, K.; Dandonneau, M.; Swain, A. Wnt4 is required for proper male as well as female sexual development. Dev. Biol. 2004, 276, 431–440. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peretti, V.; Satué, K.; Ciotola, F.; Cristarella, S.; De Majo, M.; Biondi, V.; D’Anza, E.; Albarella, S.; Quartuccio, M. An Unusual Case of Testicular Disorder in Sex Development of Arabian Mare (64,XX SRY-Negative). Animals 2020, 10, 1963. https://doi.org/10.3390/ani10111963
Peretti V, Satué K, Ciotola F, Cristarella S, De Majo M, Biondi V, D’Anza E, Albarella S, Quartuccio M. An Unusual Case of Testicular Disorder in Sex Development of Arabian Mare (64,XX SRY-Negative). Animals. 2020; 10(11):1963. https://doi.org/10.3390/ani10111963
Chicago/Turabian StylePeretti, Vincenzo, Katiuska Satué, Francesca Ciotola, Santo Cristarella, Massimo De Majo, Vito Biondi, Emanuele D’Anza, Sara Albarella, and Marco Quartuccio. 2020. "An Unusual Case of Testicular Disorder in Sex Development of Arabian Mare (64,XX SRY-Negative)" Animals 10, no. 11: 1963. https://doi.org/10.3390/ani10111963
APA StylePeretti, V., Satué, K., Ciotola, F., Cristarella, S., De Majo, M., Biondi, V., D’Anza, E., Albarella, S., & Quartuccio, M. (2020). An Unusual Case of Testicular Disorder in Sex Development of Arabian Mare (64,XX SRY-Negative). Animals, 10(11), 1963. https://doi.org/10.3390/ani10111963






