Analysis of XX SRY-Negative Sex Reversal Dogs
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Statement
2.2. Cases
2.3. Morphological Analyses
2.4. Cytogenetic Analyses
2.5. Molecular Analyses
2.6. qPCR Analyses
2.7. Array-CGH Analyses
3. Results
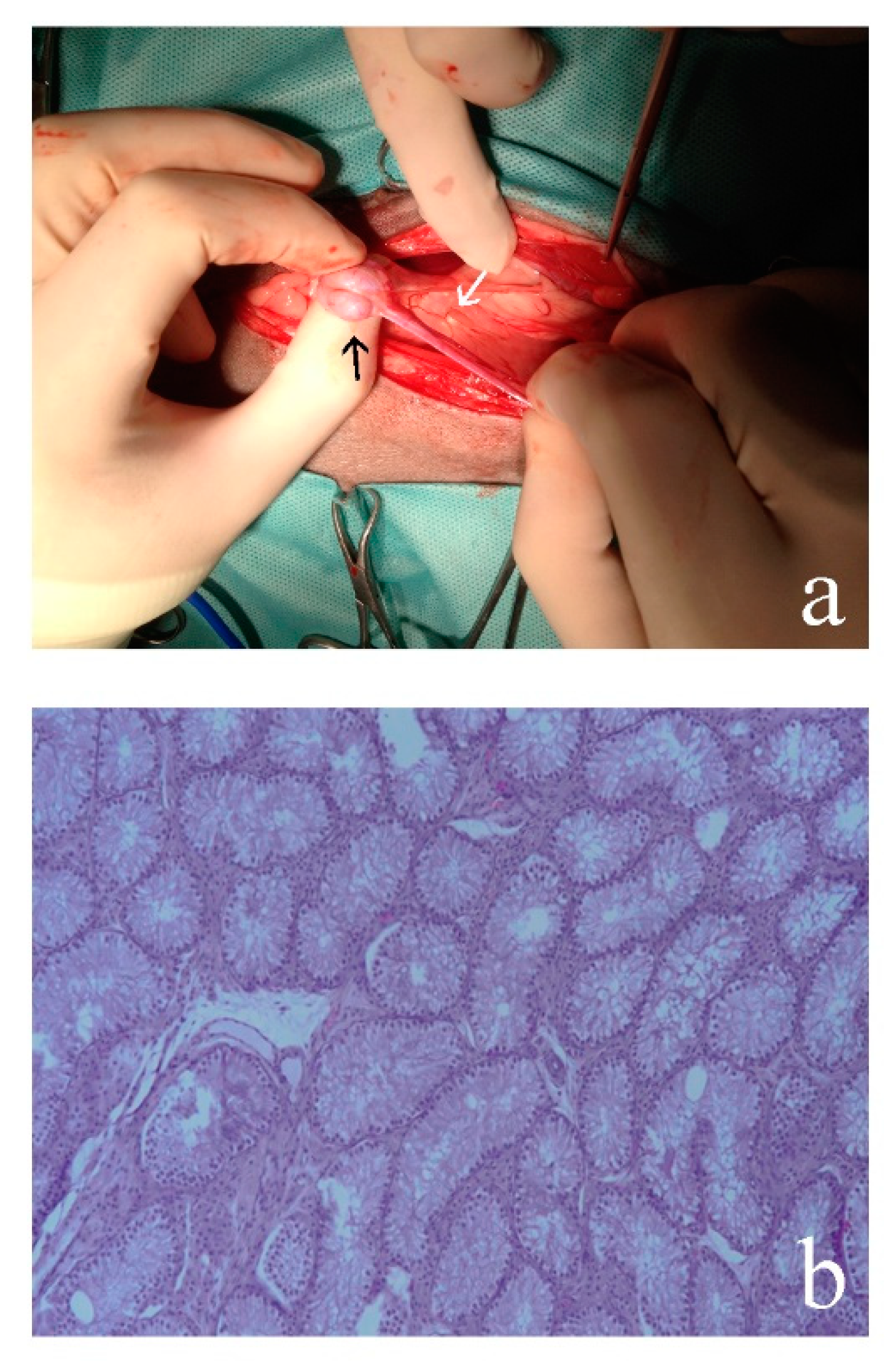
3.1. Clinical Findings
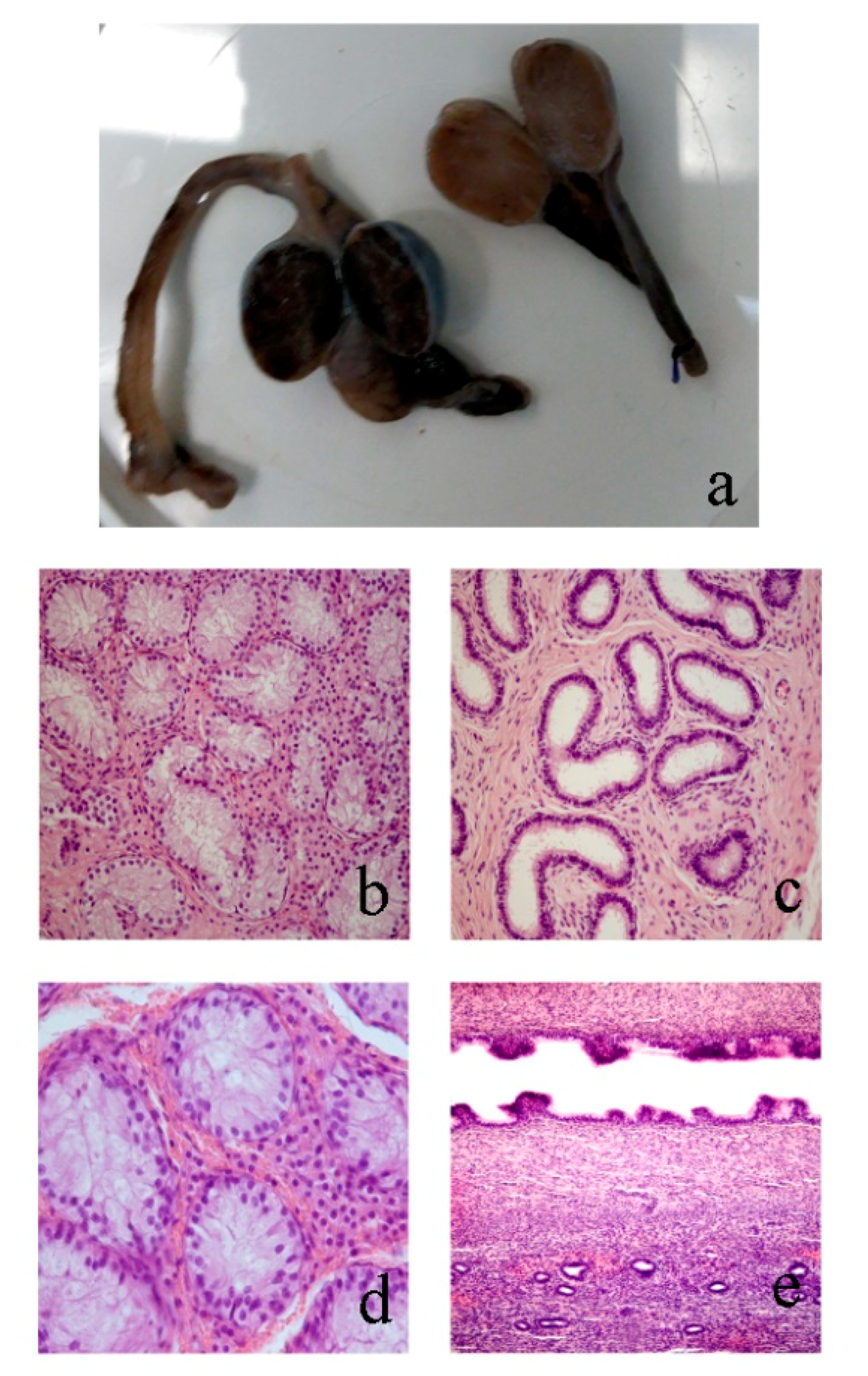
3.2. Morphological Analyses
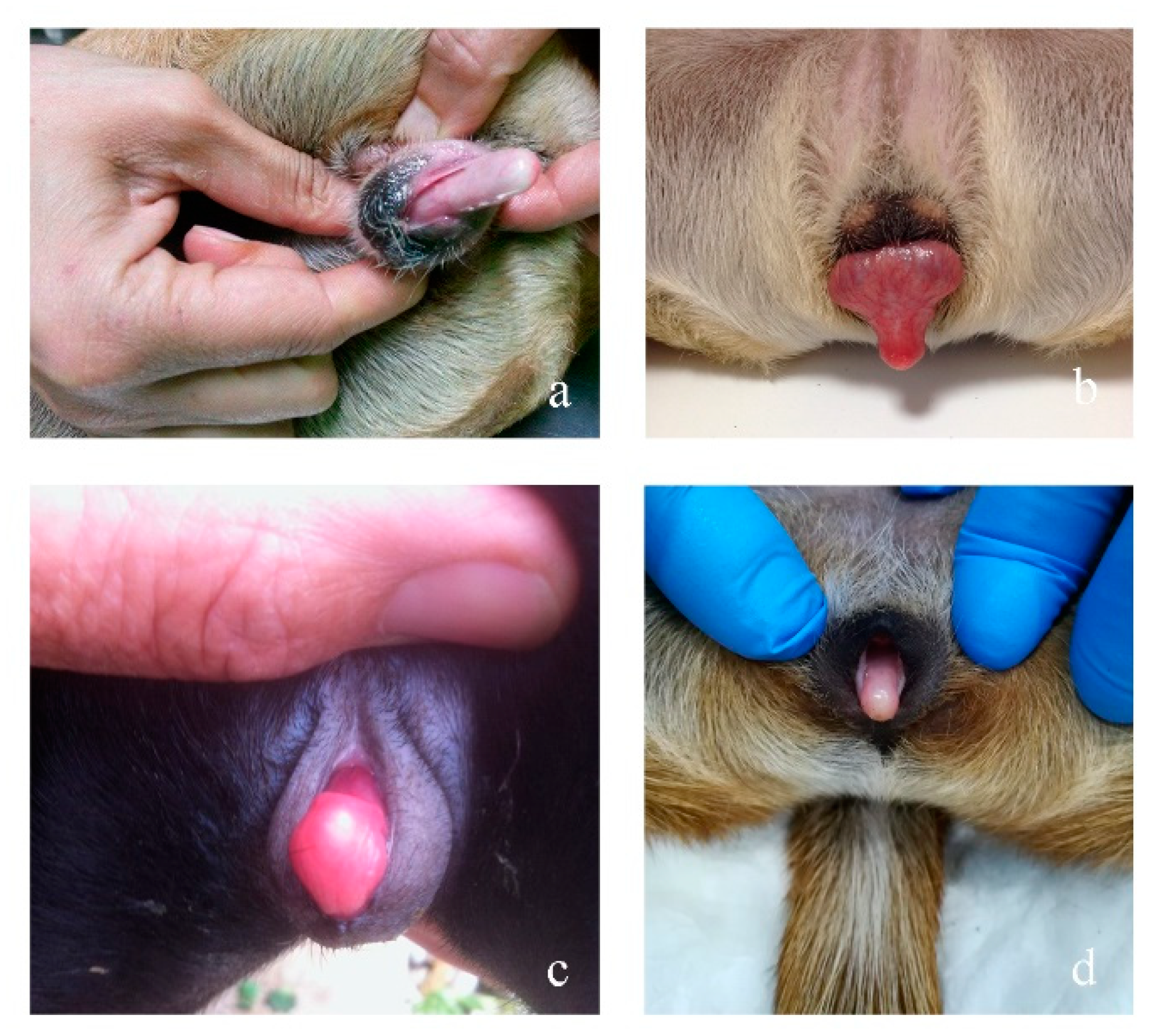
3.2.1. Case 1
3.2.2. Case 2
3.2.3. Case 3
3.2.4. Case 4
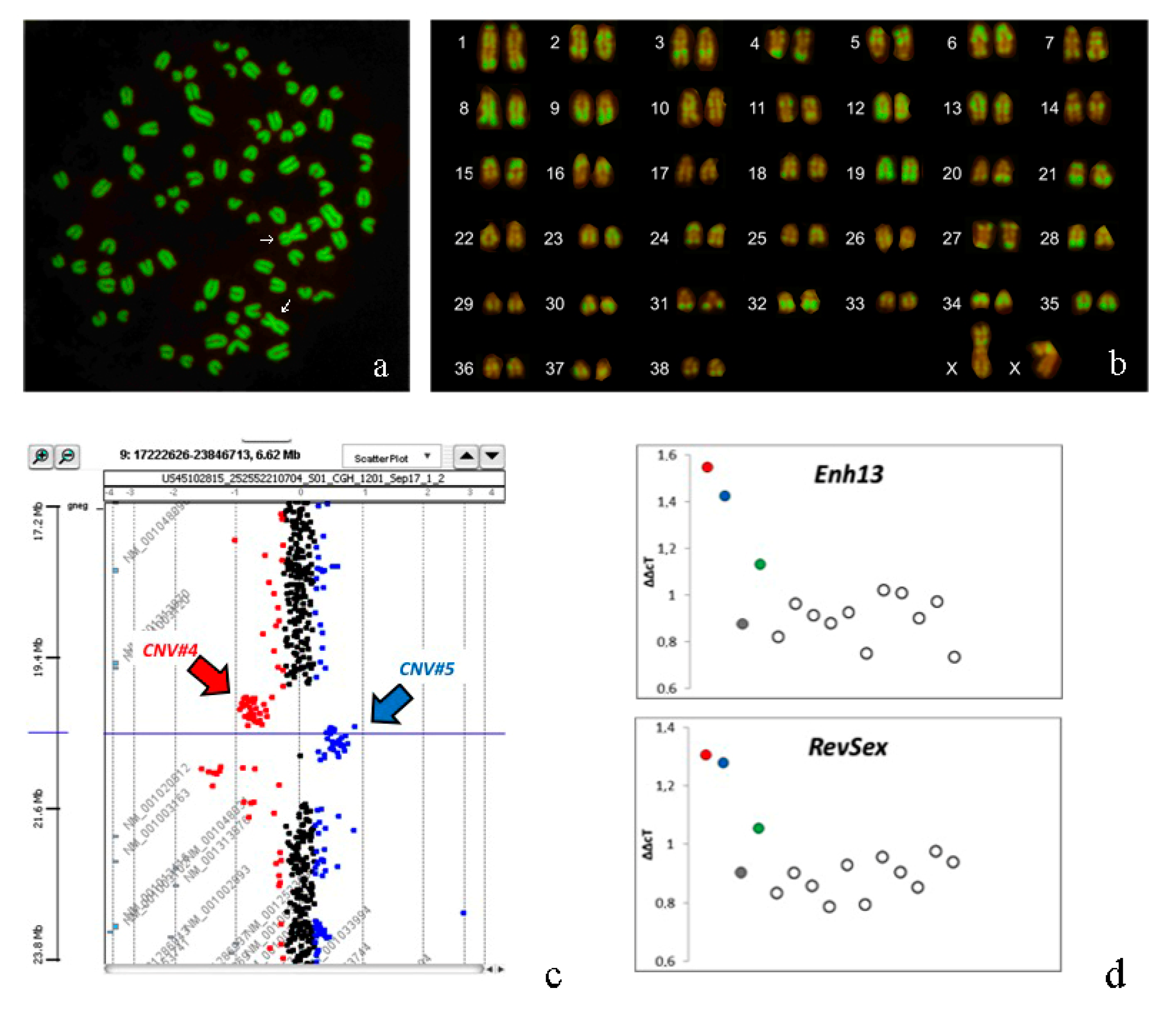
3.3. Cytogenetic Analyses
3.4. Molecular Analyses
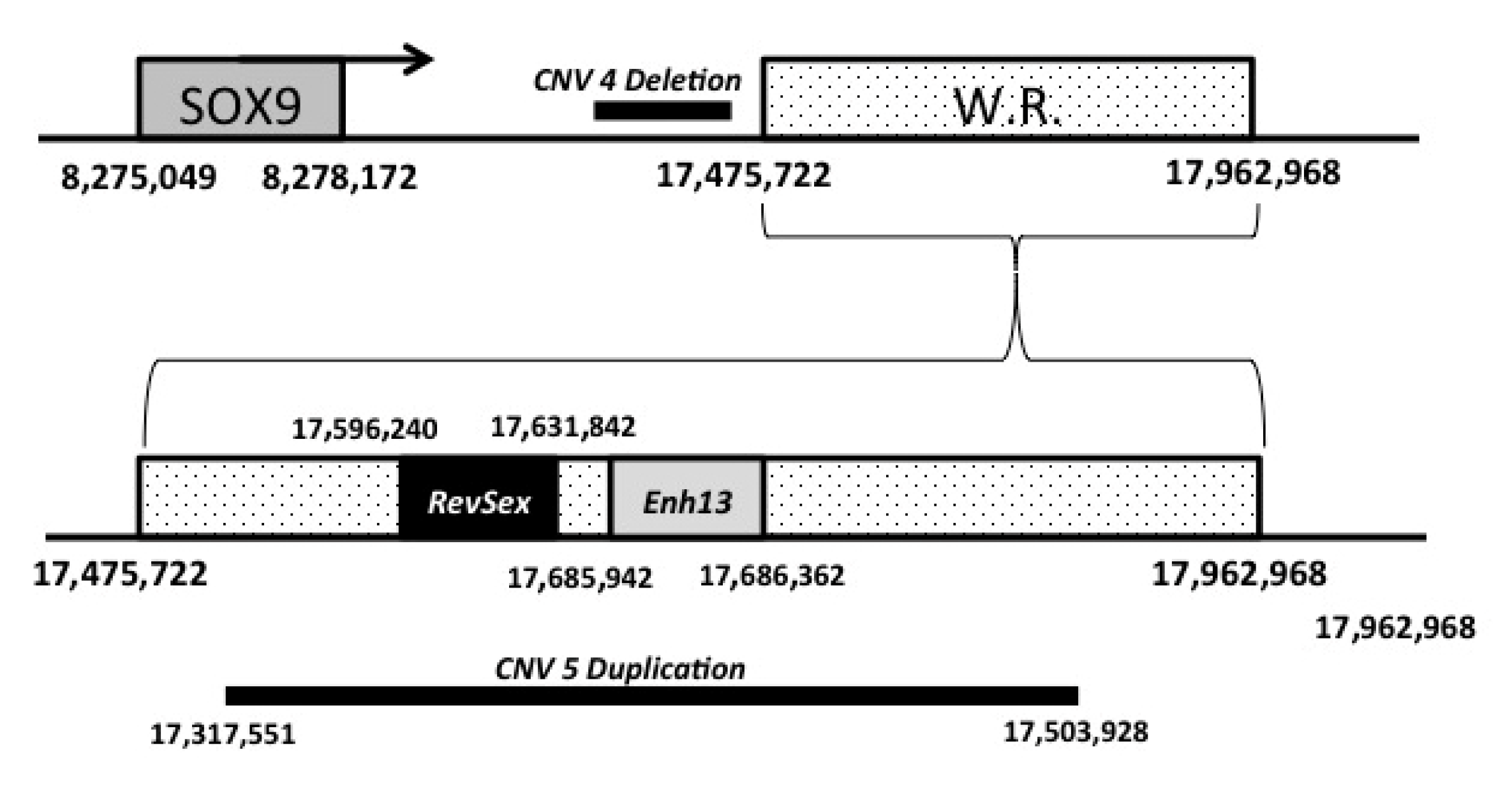
3.5. Array-CGH Analyses
3.6. qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albarella, S.; De Lorenzi, L.; Catone, G.; Magi, G.E.; Petrucci, L.; Vullo, C.; D’Anza, E.; Parma, P.; Raudsepp, T.; Ciotola, F.; et al. Diagnosis of XX/XY Blood Cell Chimerism at a Low Percentage in Horses. J. Equine Vet. Sci. 2018, 69, 129–135. [Google Scholar] [CrossRef]
- Szczerbal, I.; Nowacka-Woszuk, J.; Albarella, S.; Switonski, M. Technical note: Droplet digital PCR as a new molecular method for a simple and reliable diagnosis of freemartinism in cattle. J. Dairy Sci. 2019, 102, 10100–10104. [Google Scholar] [CrossRef]
- Capel, B. To Be or Not To Be a Testis. Reproduction 2019. [Google Scholar] [CrossRef] [PubMed]
- Parma, P.; Veyrunes, F.; Pailhoux, E. Sex Reversal in Non-Human Placental Mammals. Sex Dev. 2016, 10, 326–344. [Google Scholar] [CrossRef] [PubMed]
- Pailhoux, E.; Popescu, P.; Parma, P.; Boscher, J.; Legault, C.; Molteni, L.; Fellous, M.; Cotinot, C. Genetic analysis of 38,XX males with genital ambiguities and true hermaphrodites in pigs. Anim. Genet. 1994, 25, 299–305. [Google Scholar] [CrossRef]
- Vaiman, D.; Koutita, O.; Oustry, A.; Elsen, J.M.; Manfredi, E.; Fellous, M.; Cribiu, E.P. Genetic mapping of the autosomal region involved in XX sex-reversal and horn development in goats. Mamm. Genome 1996, 7, 133–137. [Google Scholar] [CrossRef]
- Albarella, S.; D’Anza, E.; Galdiero, G.; Esposito, L.; De Biase, D.; Paciello, O.; Ciotola, F.; Peretti, V. Cytogenetic Analyses in Ewes with Congenital Abnormalities of the Genital Apparatus. Animals 2019, 10, 776. [Google Scholar] [CrossRef]
- Pajares, G.; Balseiro, A.; Pérez-Pardal, L.; Gamarra, J.A.; Monteagudo, L.V.; Goyache, F.; Royo, L.J. Sry-negative XX true hermaphroditism in a roe deer. Anim. Reprod. Sci. 2009, 112, 190–197. [Google Scholar] [CrossRef]
- Wilker, C.E.; Meyers-Wallen, V.N.; Schlafer, D.H.; Dykes, N.L.; Kovacs, A.; Ball, B.A. XX sex reversal in a llama. J. Am. Vet. Med. Assoc. 1994, 1, 112–115. [Google Scholar]
- De Lorenzi, L.; Arrighi, S.; Rossi, E.; Grignani, P.; Previderè, C.; Bonacina, S.; Cremonesi, F.; Parma, P. XY (SRY-positive) Ovarian Disorder of Sex Development in Cattle. Sex Dev. 2018, 12, 196–203. [Google Scholar] [CrossRef]
- Albarella, S.; Ciotola, F.; D’Anza, E.; Coletta, A.; Zicarelli, L.; Peretti, V. Congenital Malformations in River Buffalo (Bubalus bubalis). Animals 2017, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Villagómez, D.A.; Lear, T.L.; Chenier, T.; Lee, S.; McGee, R.B.; Cahill, J.; Foster, R.A.; Reyes, E.; St John, E.; King, W.A. Equine disorders of sexual development in 17 mares including XX, SRY-negative, XY, SRY-negative and XY, SRY-positive genotypes. Sex Dev. 2011, 5, 16–25. [Google Scholar] [CrossRef]
- Ciotola, F.; Albarella, S.; Pasolini, M.P.; Auletta, L.; Esposito, L.; Iannuzzi, L.; Peretti, V. Molecular and Cytogenetic Studies in a Case of XX SRY-Negative Sex Reversal in an Arabian Horse. Sex Dev. 2012, 6, 104–107. [Google Scholar] [CrossRef]
- De Lorenzi, L.; Banco, B.; Previderè, C.; Bonacina, S.; Romagnoli, S.; Grieco, V.; Parma, P. Testicular XX (SRY-Negative) Disorder of Sex Development in Cat. Sex Dev. 2017, 11, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Krzemińska, P.; D’Anza, E.; Ciotola, F.; Paciello, O.; Restucci, B.; Peretti, V.; Albarella, S.; Switonski, M. Polymorphisms of MAMLD1, SRD5A2, and AR Candidate Genes in Seven Dogs (78,XY; SRY-Positive) Affected by Hypospadias or Cryptorchidism. Sex Dev. 2019, 13, 92–98. [Google Scholar] [CrossRef]
- Nowacka-Woszuk, J.; Szczerbal, I.; Pausch, H.; Hundi, S.; Hytönen, M.K.; Grzemski, A.; Flisikowski, K.; Lohi, H.; Switonski, M.; Szydlowski, M. Deep sequencing of a candidate region harboring the SOX9 gene for the canine XX disorder of sex development. Anim. Genet. 2017, 48, 330–337. [Google Scholar] [CrossRef] [PubMed]
- d’Ovidio, D.; Melidone, R.; Rossi, G.; Albarella, S.; Noviello, E.; Fioretti, A.; Meomartino, L. Multiple congenital malformations in a ferret (Mustela Putorius Furo). J. Exot. Pet Med. 2015, 24, 92–97. [Google Scholar] [CrossRef]
- Vetro, A.; Dehghani, M.R.; Kraoua, L.; Giorda, R.; Beri, S.; Cardarelli, L.; Merico, M.; Manolakos, E.; Parada-Bustamante, A.; Castro, A.; et al. Testis development in the absence of SRY: Chromosomal rearrangements at SOX9 and SOX3. Eur. J. Hum. Genet. 2015, 23, 1025–1032. [Google Scholar] [CrossRef]
- Gonen, N.; Futtner, C.R.; Wood, S.; Garcia-Moreno, S.A.; Salamone, I.M.; Samson, S.C.; Sekido, R.; Poulat, F.; Maatouk, D.M.; Lovell-Badge, R. Sex reversal following deletion of a single distal enhancer of Sox9. Science 2018, 29, 1469–1473. [Google Scholar] [CrossRef]
- Rossi, E.; Radi, O.; De Lorenzi, L.; Vetro, A.; Groppetti, D.; Bigliardi, E.; Luvoni, G.C.; Rota, A.; Camerino, G.; Zuffardi, O.; et al. Sox9 duplications are a relevant cause of Sry-negative XX sex reversal dogs. PLoS ONE 2014, 10, e101244. [Google Scholar] [CrossRef]
- Parma, P.; Radi, O.; Vidal, V.; Chaboissier, M.C.; Dellambra, E.; Valentini, S.; Guerra, L.; Schedl, A.; Camerino, G. R-spondin 1 is essential in sex determination, skin differentiation and malignancy. Nat. Genet. 2006, 38, 1304–1309. [Google Scholar] [CrossRef]
- Pailhoux, E.; Vigier, B.; Chaffaux, S.; Servel, N.; Taourit, S.; Furet, J.-P.; Fellos, M.; Grosclaude, F.; Cribiu, E.P.; Cotinot, C.; et al. A 11.7-kb deletion triggers intersexuality and polledness in goats. Nat. Genet. 2001, 29, 453–458. [Google Scholar] [CrossRef]
- Sutton, E.; Hughes, J.; White, S.; Sekido, R.; Tan, J.; Arboleda, V.; Rogers, N.; Knower, K.; Rowley, L.; Eyre, H.; et al. Identification of SOX3 as an XX male sex reversal gene in mice and humans. J. Clin. Investig. 2011, 121, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Macrì, F.; Ciotola, F.; Rapisarda, G.; Lanteri, G.; Albarella, S.; Aiudi, G.; Liotta, L.; Marino, F. A rare case of simple syndactyly in a puppy. J. Small Anim. Pract. 2014, 55, 170–173. [Google Scholar] [CrossRef]
- Switoński, M.; Reimann, N.; Bosma, A.A.; Long, S.; Bartnitzke, S.; Pieńkowska, A.; Moreno-Milan, M.M.; Fischer, P. Report on the progress of standardization of the G-banded canine (Canis familiaris) karyotype. Committee for the Standardized Karyotype of the Dog (Canis familiaris). Chromosome Res. 1996, 4, 306–309. [Google Scholar] [CrossRef]
- Yan, S.; Bai, C.; Li, Y.; Li, Y.; Hou, J.; Zhao, Z.; Han, W. Sex identification of dog by PCR based on the differences in the AMELX and AMELY genes. Anim. Genet. 2013, 44, 606. [Google Scholar] [CrossRef]
- De Lorenzi, L.; Groppetti, D.; Arrighi, S.; Pujar, S.; Nicoloso, L.; Molteni, L.; Pecile, A.; Cremonesi, F.; Parma, P.; Meyers-Wallen, V. Mutations in the RSPO1 coding region are not the main cause of canine SRY-negative XX sex reversal in several breeds. Sex Dev. 2008, 2, 84–95. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzi, L.; Kopecna, O.; Gimelli, S.; Cernohorska, H.; Zannotti, M.; Béna, F.; Molteni, L.; Rubes, J.; Parma, P. Reciprocal translocation t(4;7)(q14;q28) in cattle: Molecular characterization. Cytogenet. Genome Res. 2010, 129, 298–304. [Google Scholar] [CrossRef]
- Hubler, M.; Hauser, B.; Meyers-Wallen, V.N.; Arnold, S. Sry-negative XX true hermaphrodite in a Basset hound. Theriogenology 1999, 51, 1391–1403. [Google Scholar] [CrossRef]
- Nowacka-Woszuk, J.; Szczerbal, I.; Stachowiak, M.; Szydlowski, M.; Nizanski, W.; Dzimira, S.; Maslak, A.; Payan-Carreira, R.; Wydooghe, E.; Nowak, T.; et al. Association between polymorphisms in the SOX9 region and canine disorder of sex development (78,XX; SRY-negative) revisited in a multibreed case-control study. PLoS ONE 2019, 14, e0218565. [Google Scholar] [CrossRef] [PubMed]
- Szczerbal, I.; Nowacka-Woszuk, J.; Nizanski, W.; Dzimira, S.; Ligocka, Z.; Jastrzebska, A.; Kabala, B.; Biernacik, M.; Przadka, P.; Switonski, M. Disorders of Sex Development Are an Emerging Problem in French Bulldogs: A Description of Six New Cases and a Review of the Literature. Sex Dev. 2020, 13, 205–211. [Google Scholar] [CrossRef]
- Poth, T.; Breuer, W.; Walter, B.; Hecht, W.; Hermanns, W. Disorders of sex development in the dog-Adoption of a new nomenclature and reclassification of reported cases. Anim. Reprod. Sci. 2010, 121, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Radi, O.; De Lorenzi, L.; Iannuzzi, A.; Camerino, G.; Zuffardi, O.; Parma, P. A Revised Genome Assembly of the Region 5’ to Canine SOX9 Includes the RevSex Orthologous Region. Sex Dev. 2015, 9, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.K.; Swartz, J.D.; Rush, L.J.; Alvarez, C.E. Mapping DNA structural variation in dogs. Genome Res. 2009, 19, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Mortlock, S.A.; Williamson, P.; Khatkar, M.S. Copy number variation and variant discovery in Bullmastiff dogs. Anim. Genet. 2019, 50, 177–181. [Google Scholar] [CrossRef]
- Nicholas, T.J.; Baker, C.; Eichler, E.E.; Akey, J.M. A high-resolution integrated map of copy number polymorphisms within and between breeds of the modern domesticated dog. BMC Genom. 2011, 12, 414. [Google Scholar] [CrossRef]
- Talas, U.; Dunlop, J.; Khalaf, S.; Leigh, I.M.; Kelsell, D.P. Human elastase 1: Evidence for expression in the skin and the identification of a frequent frameshift polymorphism. J. Investig. Derm. 2000, 114, 165–170. [Google Scholar] [CrossRef]
- Bennett, E.P.; Hassan, H.; Mandel, U.; Hollingsworth, M.A.; Akisawa, N.; Ikematsu, Y.; Merkx, G.; Geurts van Kessel, A.; Olofsson, S.; Clausen, H. Cloning and characterization of a close homologue of human UDP-N-acetyl-alpha-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase-T3, designated GalNAc-T6: Evidence for genetic but not functional redundancy. J. Biol. Chem. 1999, 274, 25362–25370. [Google Scholar] [CrossRef]
- Ge, K.; Prendergast, G.C. Bin2, a functionally nonredundant member of the BAR adaptor gene family. Genomics 2000, 67, 210–220. [Google Scholar] [CrossRef]
- Oh, B.; Hwang, S.Y.; Solter, D.; Knowles, B.B. Spindlin, a major maternal transcript expressed in the mouse during the transition from oocyte to embryo. Development 1997, 124, 493–503. [Google Scholar]

| Gene | Primers | Sequence (5′-3′) | Amp Size (bp) | Genome Pos 1 |
|---|---|---|---|---|
| Sry | Sry-F | GCTGGGCGGAGAAATGAGTA | 783 | Not available |
| Sry-R | CCAAGGTTTCCGGACTGTCA | |||
| Amelx/y | DSI-F | ATAATGACAAAGAAAACATGAC | 215/247 | Chrx: 7,828,350–7,828,136 2 |
| DSI-R | CTGCTGAGCTGGCACCAT | |||
| Rspo1 | Rspo1-Ex1-f | GCAGGCGTTAGCAAGAGC | 297 | Chr15: 4,862,841–4,863,138 |
| Rspo1-Ex1-r | ATCTGCAACGGTCATCACG | |||
| Rspo1-Ex2-f | AAGCACGTTCACGTTAGTCTTG | 398 | Chr15: 4,871,649–4,872,047 | |
| Rspo1-Ex2-r | ACCAATGGGTCAAAGCACTC | |||
| Rspo1-Ex3-f | GTCACTCGGGCCTCCTCTA | 478 | Chr15: 4,873,791–4,874,269 | |
| Rspo1-Ex3-r | GCAGAAAAGCTCGGAGACAA | |||
| Rspo1-Ex4-f | ACTGACACTGCCTCCAGCAT | 480 | Chr15: 4,874,276–4,874,754 | |
| Rspo1-Ex4-r | CTGTTGTCTGCCAGCGTCT | |||
| Rspo1-Ex5-f | GGGGACCCTGAGACTGTGTA | 399 | Chr15: 4,875,173-4,875,572 | |
| Rspo1-Ex5-r | TCCAGTTCCGTAAAGCTTCC | |||
| Enh13 | Enh13-f | GCAATGTGCACAGTTTCAGAG | 118 | Chr9: 17,686,202–17,686,320 |
| Enh13-r | TGAGGAATTAGAAGGCCATGA | |||
| RevSex | RevSex-Dog-F | GACACTGTCCTGGGGAGAAA | 100 | Chr9:17,605,471- 17,605,570 |
| RevSex-Dog-R | TGAAGGCCAAGAGGCTAAGA | |||
| Bglr2 | BGLR2-F | GTGGAAGCCTGCAATTGTCT | 203 | Chr6: 734,406–734,609 |
| BGLR2-R | CCGTGAACAGGTGTAATGCT |
| Case Number | Breed | Age at First Clinical Evaluation | Phenotype | Surgical Findings |
|---|---|---|---|---|
| 1 | Staffordshire terrier | 1Y | Female. | Ultrasound showed two ovotestis like structures in the abdomen caudally to the kidneys. |
| 2 | French bulldog | 9M | Female. Presence of a little palpable mass in inguinal region | A gonad with a uterine horn was removed from the abdomen and another gonad was removed from the inguinal region |
| 3 | French bulldog | 6M | Female. Presence of a little palpable mass in inguinal region | One gonad was in inguinal position and connected with a tubular structure. The other gonad was in the abdomen caudally to the kidney and was connected with a tubular structure that showed a fork linked to the vas deferens. Both structures ended in a uterine horn-like structure that lead directly into the vagina. The prostate was absent. Enlarged clitoris with penis bone protruded from an abnormal vulvae opening. |
| 4 | Mongrel | 1Y | Female. 4.8 kg. | Two ovotestis like structures were found in the abdomen connected with uterine horns that merge in a uterine like structure ending in the vagina. |
| CNV | CHR | CanFam3 | END (bp) | Analyzed Subjects | ||||
|---|---|---|---|---|---|---|---|---|
| START (bp) | SIZE (kb) | 1 | 2 | 3 | 4 | |||
| 1 | Chr 4 | 106,352 | 469,199 | 363 | DEL | DEL | DEL | DEL |
| 2 | Chr 5 | 78,189,869 | 78,389,978 | 200 | DEL | DUP | DEL | |
| 3 | Chr 6 | 45,163,433 | 47,125,036 | 1962 | GAIN | GAIN | GAIN | GAIN |
| 4 | Chr 9 | 16,906,864 | 17,317,551 | 411 | DEL | DEL | DEL | |
| 5 | Chr 9 | 17,503,928 | 17,962,221 | 458 | DUP | DUP | DUP | |
| 6 | Chr 9 | 38,978,944 | 38,995,409 | 16 | DEL | DEL | DEL | |
| 7 | Chr 12 | 2,191,427 | 2,270,973 | 80 | DEL | DEL | DEL | |
| 8 | Chr 23 | 20,508,926 | 20,725,281 | 216 | GAIN | GAIN | ||
| 9 | Chr 26 | 27,171,599 | 27,220,687 | 49 | DELHO | DELHO | DELHO | DELHO |
| 10 | Chr 27 | 3,532,831 | 3,573,782 | 41 | GAIN | GAIN | ||
| 11 | Chr X | 71,752,458 | 72,234,092 | 482 | DELHO | DEL | DEL | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albarella, S.; Lorenzi, L.D.; Rossi, E.; Prisco, F.; Riccardi, M.G.; Restucci, B.; Ciotola, F.; Parma, P. Analysis of XX SRY-Negative Sex Reversal Dogs. Animals 2020, 10, 1667. https://doi.org/10.3390/ani10091667
Albarella S, Lorenzi LD, Rossi E, Prisco F, Riccardi MG, Restucci B, Ciotola F, Parma P. Analysis of XX SRY-Negative Sex Reversal Dogs. Animals. 2020; 10(9):1667. https://doi.org/10.3390/ani10091667
Chicago/Turabian StyleAlbarella, Sara, Lisa De Lorenzi, Elena Rossi, Francesco Prisco, Marita Georgia Riccardi, Brunella Restucci, Francesca Ciotola, and Pietro Parma. 2020. "Analysis of XX SRY-Negative Sex Reversal Dogs" Animals 10, no. 9: 1667. https://doi.org/10.3390/ani10091667
APA StyleAlbarella, S., Lorenzi, L. D., Rossi, E., Prisco, F., Riccardi, M. G., Restucci, B., Ciotola, F., & Parma, P. (2020). Analysis of XX SRY-Negative Sex Reversal Dogs. Animals, 10(9), 1667. https://doi.org/10.3390/ani10091667







