The Relevance of Caseous Lymphadenitis as a Cause of Culling in Adult Sheep
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Studied Farms
2.2. Animal Management
2.3. Ancillary Tests
2.4. Microbiological Analysis
2.5. Statistical Analysis
3. Results
3.1. Clinical Examination
3.2. Ancillary Test
3.3. CLA Post-Mortem Findings
3.4. Caseous Lymphadenitis Clinical Presentations
3.4.1. Superficial or Cutaneous Presentation
3.4.2. Visceral Presentation
3.4.3. Combined Presentations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Braga, W.U. Protection in alpacas against Corynebacterium pseudotuberculosis using different bacterial components. Vet. Microbiol. 2007, 119, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Belchior, S.G.E.; Gallardo, A.A.; Abalos, M.A.; Alvarez, L.A.; Nuñez, N.C.; Guevara, D.; Jensen, O. Corynebacterium pseudotuberculosis, potencial agente zoonótico. Revisión de casos. REDVET. Rev. Electron. de Vet. 2009, 10, 1–16. Available online: https://www.redalyc.org/comocitar.oa?id=63617128018 (accessed on 16 September 2020).
- Baird, G.J.; Fontaine, M.C. Corynebacterium pseudotuberculosis and its role in ovine caseous lymphadenitis. J. Comp. Pathol. 2007, 137, 179–210. [Google Scholar] [CrossRef] [PubMed]
- Oreiby, A.F. Diagnosis of caseous lymphadenitis in sheep and goat. Small Rumin. Res. 2015, 123, 160–166. [Google Scholar] [CrossRef]
- Osman, A.Y.; Nordin, M.L.; Kadir, A.A.; Saharee, A.A. The Epidemiology and Pathophysiology of Caseous Lymphadenitis: A Review. J. Vet. Med. Res. 2018, 5, 1129. Available online: https://www.jscimedcentral.com/VeterinaryMedicine/veterinarymedicine-5-1129.pdf (accessed on 10 September 2020).
- Hussain, R.; Khaliq, S.A.; Siddique, A.B.; Khan, I.A.; Hassan, M.F.; Younus, M. Clinico-pathological and bacteriological studies on caseous lymphadenitis in small ruminants. Pak. J. Agri. Sci. 2017, 54, 437–442. [Google Scholar] [CrossRef]
- Bernheimer, A.W.; Campbell, B.J.; Forrester, L.J. Comparative toxicology of Loxosceles reclusa and Corynebacterium pseudotuberculosis. Science 1985, 228, 590–591. [Google Scholar] [CrossRef]
- Pépin, M.; Paton, M. Caseous lymphadenitis in sheep and goats. In Infectious and Parasitic Diseases of Livestock, 1st ed.; Lefevre, P.C., Blancou, J., Chermette, R., Uilenberg, G., Eds.; Lavoisier: Paris, France, 2010; pp. 1153–1165. [Google Scholar]
- Williamson, L.H. Caseous lymphadenitis in small ruminants. Vet. Clin. North. Am. Food. Anim. Pract. 2001, 17, 359–371. [Google Scholar] [CrossRef]
- Paton, M.W. The Epidemiology and Control of Caseous Lymphadenitis in Australian Sheep Flocks. Ph.D. Thesis, Murdoch University, Perth, Australia, January 2010. [Google Scholar]
- Windsor, P.A. Control of caseous lymphadenitis. Vet. Clin. N. Am. Food. Anim. 2011, 27, 193–202. [Google Scholar] [CrossRef]
- Fontaine, M.C.; Baird, G.J. Caseous lymphadenitis. Small Rumin. Res. 2008, 76, 42–48. [Google Scholar] [CrossRef]
- Al-Gaabary, M.H.; Osman, S.A.; Ahmed, M.S.; Oreiby, A.F. Abattoir survey on caseous lymphadenitis in sheep and goats in Tanta, Egypt. Small Rumin. Res. 2010, 94, 117–124. [Google Scholar] [CrossRef]
- Al-Gaabary, M.H.; Osman, S.A.; Oreiby, A.F. Caseous lymphadenitis in sheep and goats: Clinical, epidemiological and preventive studies. Small Rumin. Res. 2009, 87, 116–121. [Google Scholar] [CrossRef]
- Oreiby, A.F.; Hegazy, Y.M. Diagnosis of ovine caseous lymphadenitis by blood and milk gamma interferon assays. Small Rumin.Res. 2016, 144, 109–112. [Google Scholar] [CrossRef]
- Valli, V.E.O.; Parry, B.W. Caseous lymphadenitis. In Pathology of Domestic Animals, 4th ed.; Jubb, K.V.F., Kennedy, P.C., Palmer, N., Eds.; Academic Press: San Diego, CA, USA, 1993; Volume 3, pp. 238–240. [Google Scholar]
- Guimarães, A.S.; Carmo, F.B.; Heinemann, M.B.; Portela, R.W.; Meyer, R.; Lage, A.P.; Seyffert, N.; Miyoshi, A.; Azevedo, V.; Gouveia, A.M. High sero-prevalence of caseous lymphadenitis identified in slaughterhouse samples as a consequence of deficiencies in sheep farm management in the state of Minas Gerais, Brazil. BMC Vet. Res. 2011, 7, 1–5. Available online: https://bmcvetres.biomedcentral.com/articles/10.1186/1746-6148-7-68 (accessed on 15 October 2020). [CrossRef] [PubMed]
- O’Reilly, K.M.; Green, L.E.; Malone, F.E.; Medley, G.F. Parameter estimation and simulations of a mathematical model of Corynebacterium pseudotuberculosis transmission in sheep. Prev. Vet. Med. 2008, 83, 242–259. [Google Scholar] [CrossRef]
- Zavoshti, F.R.; Khoojine, A.B.S.; Helan, J.A.; Hassanzadeh, B.; Heydari, A.A. Frequency of caseous lymphadenitis (CLA) in sheep slaughtered in an abattoir in Tabriz: Comparison of bacterial culture and pathological study. Comp. Clin. Pat. 2012, 21, 667–671. [Google Scholar] [CrossRef]
- Stoops, S.G.; Renshaw, H.W.; Thilsted, J.P. Ovine caseous lymphadenitis: Disease prevalence, lesion distribution, and thoracic manifestations in a population of mature culled sheep from western United States. Am. J. Vet. Res. 1984, 45, 557–561. [Google Scholar] [PubMed]
- Paton, M.W.; Sutherland, S.S.; Rose, I.R.; Hart, R.A.; Mercy, A.R.; Ellis, T.M. The spread of Corynebacterium pseudotuberculosis infection to unvaccinated and vaccinated sheep. Aust. Vet. J. 1995, 72, 266–269. [Google Scholar] [CrossRef]
- Brogden, K.A.; Cutlip, R.C.; Lehmkuhl, H.D. Comparison of protection induced in lambs by Corynebacterium pseudotuberculosis whole cell and cell wall vaccine. Am. J. Vet. Res. 1984, 45, 2393–2395. [Google Scholar]
- Fontaine, M.C.; Baird, G.; Connor, K.M.; Rudge, K.; Sales, J.; Donachie, W. Vaccination confers significant protection of sheep against infection with a virulent United Kingdon strain of Corynebacterium pseudotuberculosis. Vaccine 2006, 24, 5986–5996. [Google Scholar] [CrossRef]
- Batey, R.G. Aspects of pathogenesis in a mouse model of infection by Corynebacterium pseudotuberculosis. Aust. J. Exp. Biol. Med. Sci. 1986, 64, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Middleton, M.J.; Epstein, W.M.; Gregory, G.G. Caseous lymphadenitis on Flinders Island: Prevalence and management surveys. Aust. Vet. J. 1991, 68, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, J.; Girard, C.; Dubreuil, P.; Daignault, D.; Galarneau, J.R.; Boisclair, J.; Simard, C.; Bélanger, D. Prevalence of and carcass condemnation from maedi–visna, paratuberculosis and caseous lymphadenitis in culled sheep from Quebec, Canada. Prev. Vet. Med. 2003, 59, 67–81. [Google Scholar] [CrossRef]
- Alves, J.R.A.; de Farias, A.E.M.; da Silva, J.D.; Viana, M.P.; Lima, A.M.C.; Faccioli-Martins, P.Y.; Pinheiro, R.R.; Alves, F.S.F.; de Azevedo, S.S.; Alves, C.J. Factors associated with the seroprevalence of caseous lymphadenitis in sheep from Northeastern Brazil. Prev. Vet. Med. 2020, 182, 105098. [Google Scholar] [CrossRef] [PubMed]
- Severini, M.; Ranucci, D.; Miraglia, D.; Goga, B.C. Pseudotuberculosis in sheep as a concern of veterinary public health. Vet. Res. Commun. 2003, 27, 315–318. [Google Scholar] [CrossRef]
- Branciari, R.; Mammoli, R.; Ranucci, D.; Miraglia, D.; Gorziglia, G.; Feliziani, F.; Avellini, P. The slaughterhouse as an epidemiological observatory for the surveillance of caseous lymphadenitis in sheep. In Food Safety Assurance and Veterinary Public Health, 1st ed.; Smulders, F.J.M., Ed.; Wageningen Academic Publishers: Gemeente Wageningen, The Netherlands, 2006; Volume 4, pp. 247–249. [Google Scholar] [CrossRef]
- Baird, G.; Synge, B.; Dercksen, D. Survey of caseous lymphadenitis seroprevalence in British terminal sire sheep breeds. Vet Record 2004, 154, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Listos, P.; Gryzinska, M.; Martychiewicz, M.; Pointing, S.; Barton, A.; Dylewska, M. Caseous Lymphadenitis in Sheep in the Falkland Islands. Acta Vet. 2016, 66, 406–412. [Google Scholar] [CrossRef]
- Paton, M.W.; Walker, S.B.; Rose, I.R.; Watt, G.F. Prevalence of caseous lymphadenitis and usage of caseous lymphadenitis vaccines in sheep flocks. Aust. Vet. J. 2003, 81, 91–95. [Google Scholar] [CrossRef]
- Komala, T.S.; Ramlan, M.; Yeoh, N.N.; Surayani, A.R.; Hamidah, S.M.S. A survey of caseous lymphadenitis in small ruminant farms from two districts in Perak, Malaysia-Kinta and Hilir Perak. Trop Biomed 2008, 25, 196–201. [Google Scholar]
- High, R. Culling the Sheep Flock. The Ohio State University. College of Food, Agricultural and Environmental Sciences. 2008. Available online: https://u.osu.edu/sheep/2008/08/29/culling-thesheep-flock/ (accessed on 10 September 2020).
- Ferrer, L.M.; Lacasta, D.; Chacón, G.; Ramos, J.J.; Villa, A.; Gómez, P.; Latre, M.V. Clinical diagnosis of visceral caseous lymphadenitis in a Salz ewe. Small Rumin. Res. 2009, 87, 126–127. [Google Scholar] [CrossRef]
- Musa, N.; Babiker, A.; Eltom, K.; Rodwan, K.; El Sanousi, S.M. Prevalence of Staphylococcus aureus subsp. anaerobius in Sub-Clinical Abscess Cases of Sheep. Microbiol. Res. J. Int. 2012, 2, 131–136. [Google Scholar] [CrossRef]
- Luján, L.; Pérez, M.; de Andrés, D.; Reina, R. Pulmonary lentivirus infection in sheep. Small Rumin. Res. 2019, 181, 87–90. [Google Scholar] [CrossRef]
- Leask, R.; Blignaut, D.J.; Grobler, M.J. Corynebacterium pseudotuberculosis associated with otitis media-interna in goats. J. S. Afr. Vet. Assoc. 2013, 84, 1–3. Available online: http://www.scielo.org.za/scielo.php?script=sci_arttext&pid=S1019-91282013000100035 (accessed on 15 September 2020). [CrossRef]
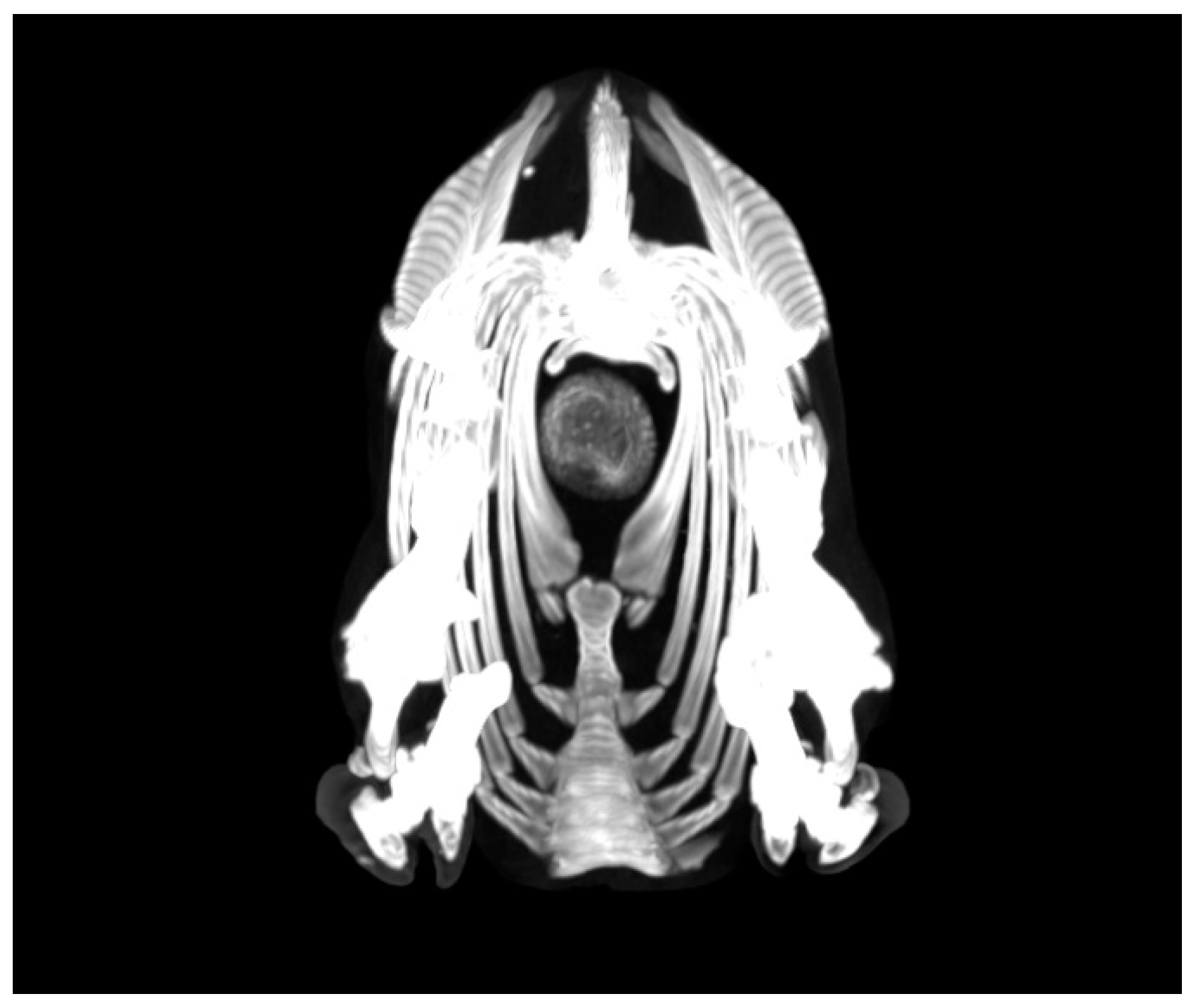
- Castells, E.; Lacasta, D.; Climent, M.; Pérez, M.; Sanromán, F.; Jiménez, C.; Ferrer, L.M. Diagnostic imaging techniques of the respiratory tract of sheep. Small Rumin. Res. 2019, 180, 112–126. [Google Scholar] [CrossRef]
- Ferrer, L.M.; Ramos, J.J.; Castells, E.; Ruíz, H.; Climent, M.; Lacasta, D. Use of Computed Tomography and Thermography for the Diagnosis of Respiratory Disorders in Adult Sheep. In Sheep Farming; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Grosso, F.V.; Tinkler, S.; Sola, M.; Miller, M.; Heng, H.G. Radiographic and computed tomographic appearance of caseous lymphadenitis in a goat. Vet. Radiol. Ultrasound. 2020, 61, E1–E6. [Google Scholar] [CrossRef]
- Voigt, K.; Baird, G.J.; Munro, F.; Murray, F.; Brülisauer, F. Eradication of caseous lymphadenitis under extensive management conditions on a Scottish hill farm. Small Rumin. Res. 2012, 106, 21–24. [Google Scholar] [CrossRef]
- Gascoigne, E.; Ogen, N.; Lovatt, F.; Davies, P. Update on caseous lymphadenitis in sheep. In Pract. 2020, 42, 105–114. [Google Scholar] [CrossRef]
- Brown, C.C.; Olander, H.J. Caseous lymphadenitis of goats and sheep: A review. Vet. Bull. 1987, 57, 1–11. [Google Scholar]

| CLA Presentations | Num. of CLA-Affected Animals of Total Studied (498) | Percentage of Total Studied Animals | Num. of Affected Animals of Total CLA-Affected (147) | Percentage of CLA-Affected Animals |
|---|---|---|---|---|
| CLA | 147/498 | 29.52% | ||
| Visceral form | 107/498 | 21.48% | 107/147 | 72.79% |
| Superficial form | 32/498 | 6.43% | 32/147 | 21.77% |
| Combined form | 8/498 | 1.61% | 8/147 | 5.44% |
| Unique CLA lesion | 84/498 | 16.87% | 84/147 | 57.14% |
| Unique visceral presentation | 65/498 | 13.05% | 65/147 | 44.21% |
| Unique superficial presentation | 12/498 | 2.41% | 12/147 | 8.16% |
| Unique combined presentation | 7/498 | 1.41% | 7/147 | 4.76% |
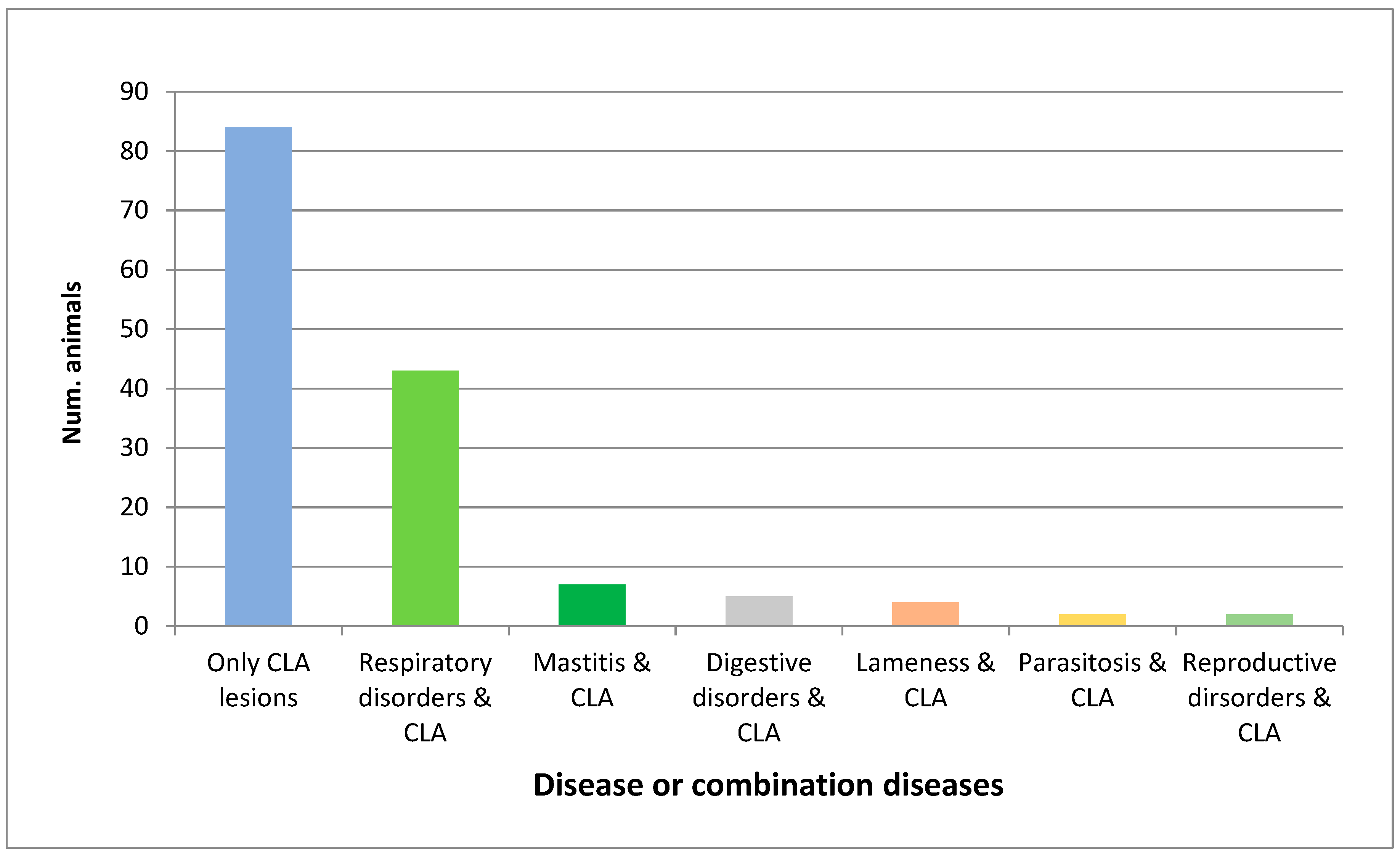
| CLA + concomitant diseases | 63/498 | 12.65% | 63/147 | 42.86% |
| Affected Lymph Nodes | Number | Percentage |
|---|---|---|
| Retropharyngeal LN | 13/32 | 40.63% |
| Mammary LN | 10/32 | 31.25% |
| Prescapular LN | 5/32 | 15.63% |
| Precrural LN | 2/32 | 6.25% |
| Prescapular + retropharyngeal LN | 1/32 | 3.12% |
| Retropharyngeal + parotid LN | 1/32 | 3.12% |
| Affected Lymph Nodes | Number | Percentage |
|---|---|---|
| Mediastinal LN | 36/107 | 33.65% |
| Lung parenchyma | 27/107 | 25.23% |
| Mediastinal LN + lung parenchyma | 12/107 | 11.21% |
| Liver + respiratory system | 9/107 | 8.41% |
| Mesenteric LN + respiratory system | 4/107 | 3.74% |
| Kidney + respiratory system | 3/107 | 2.80% |
| Liver | 5/107 | 4.67% |
| Liver + mesenteric LN | 3/107 | 2.80% |
| Liver + kidney + brain | 1/107 | 0.94% |
| Mesenteric LN | 1/107 | 0.94% |
| Mesenteric LN + heart | 1/107 | 0.94% |
| Kidney | 2/107 | 1.87% |
| Atypical presentations | 3/107 | 2.80% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz, H.; Ferrer, L.M.; Ramos, J.J.; Baselga, C.; Alzuguren, O.; Tejedor, M.T.; de Miguel, R.; Lacasta, D. The Relevance of Caseous Lymphadenitis as a Cause of Culling in Adult Sheep. Animals 2020, 10, 1962. https://doi.org/10.3390/ani10111962
Ruiz H, Ferrer LM, Ramos JJ, Baselga C, Alzuguren O, Tejedor MT, de Miguel R, Lacasta D. The Relevance of Caseous Lymphadenitis as a Cause of Culling in Adult Sheep. Animals. 2020; 10(11):1962. https://doi.org/10.3390/ani10111962
Chicago/Turabian StyleRuiz, Héctor, Luis Miguel Ferrer, Juan José Ramos, Cristina Baselga, Oihane Alzuguren, María Teresa Tejedor, Ricardo de Miguel, and Delia Lacasta. 2020. "The Relevance of Caseous Lymphadenitis as a Cause of Culling in Adult Sheep" Animals 10, no. 11: 1962. https://doi.org/10.3390/ani10111962
APA StyleRuiz, H., Ferrer, L. M., Ramos, J. J., Baselga, C., Alzuguren, O., Tejedor, M. T., de Miguel, R., & Lacasta, D. (2020). The Relevance of Caseous Lymphadenitis as a Cause of Culling in Adult Sheep. Animals, 10(11), 1962. https://doi.org/10.3390/ani10111962






