Evaluation of Stylosanthes scabra Accessions as Forage Source for Ruminants: Growth Performance, Nutritive Value and In Vitro Ruminal Fermentation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
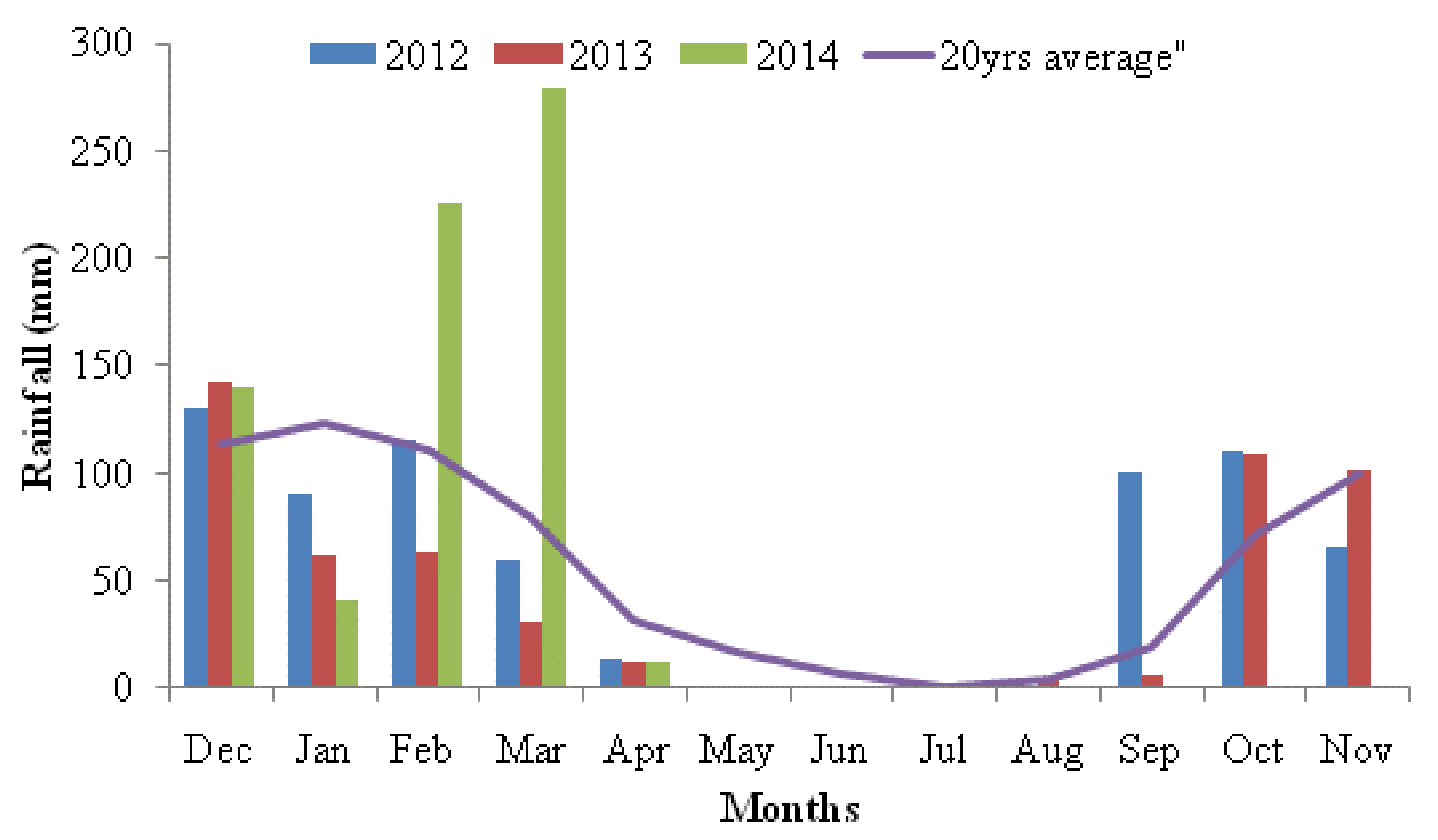
2.1. Study Site
2.2. Experimental Design and Measurements
2.2.1. Growth Performance
2.2.2. Nutritive Value
2.2.3. In Vitro Ruminal Fermentation
2.3. Data Calculation and Statistical Analysis
- μ = Overall mean.
- Si = the ith effect of S. scabra accessions as treatments (I = 1 to 15).
- Bj = effect of the jth block (j = 1, 2, 3).
- εij = random error.
3. Results
3.1. General Observation
3.2. Multivariate Analysis and General Groupings
3.3. Growth Performance
3.4. Chemical Analysis and Phenolic Compounds
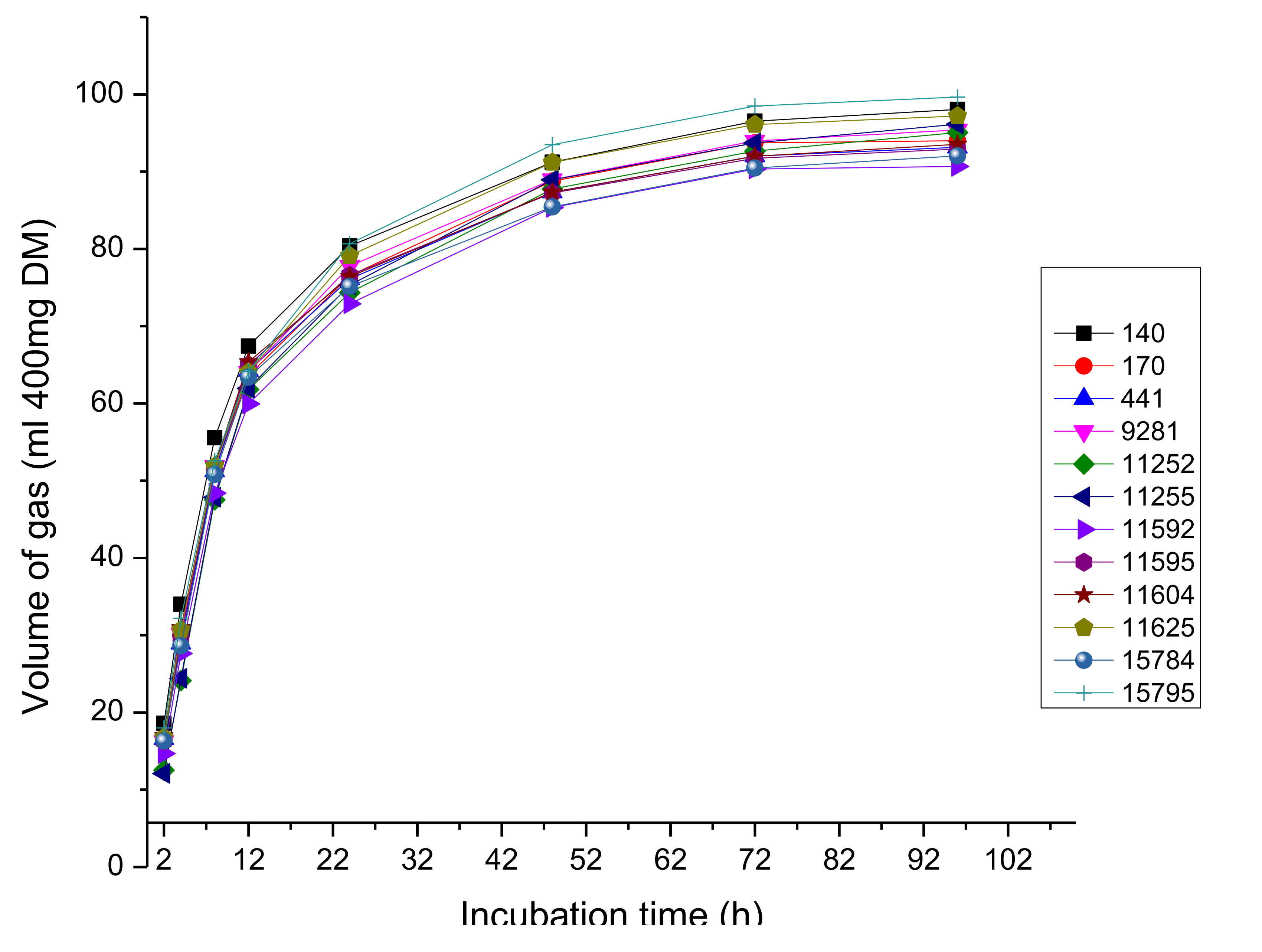
3.5. Ruminal Fermentation
3.6. Feeding Value
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akinola, J.O.; Iji, P.A.; Onifade, O.S. Effects of seedling rate, row spacing and nitrogen and phosphorus fertilizer on forage yield and quality of Stylosanthes scabra cv. Seca and Fitzroy in South-western Nigeria. Trop. Grassl. 2010, 44, 282–288. [Google Scholar]
- Rebero, E.; Mupenzi, M. Comparison of nutrient composition and in vitro digestion characteristics of four forage legumes from two agro-ecological zones of Rwanda. Agric. J. 2012, 7, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Ogunbosoye, D.O.; Babayemi, O.J. Potential values of some non-leguminous browse plants as dry season feed for ruminants in Nigeria. Afr. J. Biotechnol. 2010, 9, 2720–2726. [Google Scholar] [CrossRef]
- Anele, U.Y.; Südekum, K.; Aigbede, O.M.; Welp, G.; Oni, A.O.; Olanite, J.A.; Ojo, O.V. Agronomic performance and nutritive quality of some commercial and improved dual-purpose cowpea (Vigna unguiculata L. Walp) varieties on marginal land in Southwest Nigeria. Grassl. Sci. 2011, 57, 211–218. [Google Scholar] [CrossRef]
- Mendieta-Araica, B.; Spӧrndly, E.; Reyes-Sánchez, N.; Norell, L.; Spӧrndly, R. Silage quality when Moringa oleifera is ensiled in mixtures with Elephant grass, sugar cane and molasses. Grass Forage Sci. 2009, 64, 364–373. [Google Scholar] [CrossRef]
- Ajayi, D.A.; Adeneye, J.A.; Ajayi, F.T. Intake and nutrient utilization of West African dwarf goats fed Mango (Mangifera indica), Ficus (Ficus thionningii), Gliricidia (Gliricidia sepium) forages and concentrates as supplements to basal diet of Guinea grass (Panicum maximum). World J. Agric. Sci. 2005, 1, 184–189. [Google Scholar]
- Patra, A. Responses of intake, digestibility and nitrogen utilisation in goats fed low-quality roughages supplemented with tree foliages. J. Food Sci. Agric. 2009, 89, 1462–1472. [Google Scholar] [CrossRef]
- Kumara-Mahipala, M.B.P.; Krebs, G.L.; McCafferty, P.; Gunaratne, L.H.P. Chemical composition, biological effects of tannins and in vitro nutritive value of selected browse species grown in the West Australia Mediterranean environment. Anim. Feed Sci. Technol. 2009, 153, 203–215. [Google Scholar] [CrossRef]
- Katsande, S.; Baloyi, J.J.; Nherera-Chokuda, F.V.; Ngongoni, N.T.; Matope, G.; Zvinorova, P.I.; Gusha, J. Apparent digestibility and microbial protein yield of Desmodium uncinatum, Mucuna pruriens and Vigna unguiculata forage legumes in goats. Afr. J. Range For. Sci. 2016, 33, 53–58. [Google Scholar] [CrossRef]
- Trytsman, M.; Masemula, E.L.; Müller, F.L.; Calitz, F.J.; van Wyk, A.E. Assessing legumes indigenous to South Africa, Lesotho and Swaziland for their pasture potential. Afr. J. Range For. Sci. 2019, 36, 27–40. [Google Scholar] [CrossRef]
- Schultze-Kraft, R.; Reid, R.; Williams, R.J.; Carodin, L. The existing Stylosanthes collection. In The Biology and Agronomy of Stylosanthes; Stace, H.M., Edye, L.A., Eds.; Academic Press: Sydney, Australia, 1984; pp. 23–28. [Google Scholar]
- Pathak, P.S.; Ramesh, C.R.; Bhatt, R.K. Stylosanthes in the reclamation and development of degraded soils in India. In High-Yielding Anthracnose Resistant Stylosanthes for Agricultural Systems; Chakraborty, S., Ed.; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 2004; pp. 85–96. [Google Scholar]
- Akinlade, J.A.; Farinu, G.O.; Agboola, O.O.; Akingbade, A.A.; Ojebiyi, O.O.; Aderinola, O.A. Research note: Nutritive value of four Accessions of Stylosanthes scabra in the derived savannah zone of Nigeria. Trop. Grassl. 2008, 42, 120–123. [Google Scholar]
- Tropical Forages. Available online: https://www.tropicalforages.info (accessed on 15 March 2011).
- Hassen, A. Characterization and Evaluation of Indigofera Species as Potential Forage and Cover Crops for Semi-Arid and Arid Ecosystems. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2006. [Google Scholar]
- Tarawali, S.A.; Tarawali, G.; Larbi, A.; Hanson, J. Methods for the Evaluation of Forage Legumes, Grasses and Fodder Trees for Use as Livestock Feed; International Livestock Research Institute: Nairobi, Kenya, 1995. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Tilley, J.M.A.; Terry, R.A. A two-stage technique for in vitro digestion of forage crops. J. Br. Grassl. Soc. 1963, 8, 104–111. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Goering, H.K.; van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Procedures and Some Application); Agric Handbook No. 379 Series; ARS-USDA: Washingtom, DC, USA, 1970; pp. 1–20. [Google Scholar]
- Mould, F.L.; Morgan, R.; Kliem, K.E.; Krystallidou, E. A review and simplification of the in vitro incubation medium. Anim. Feed Sci. Technol. 2005, 123–124, 155–172. [Google Scholar] [CrossRef]
- Mpanza, T.D.E. Biomass Yield and Nutritive Value of Stylosanthes scabra Accessions as Forage Source for Goats. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2015. [Google Scholar]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllen, A.B.; France, J. A simple gas production method using pressure transducers to determine the fermentation kinetics of ruminant feed. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Akanmu, A.M.; Hassen, A. The use of certain medicinal plant extracts reduced in vitro methane production while improving in vitro organic matter digestibility. Anim. Prod. Sci. 2018, 58, 900–908. [Google Scholar] [CrossRef] [Green Version]
- Ørskov, E.R.; Mcdonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. Camb. 1979, 92, 499–503. [Google Scholar] [CrossRef] [Green Version]
- SAS. Statistical Analysis Systems User’s Guide: Statistics, Version 9.0; SAS Institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of digestibility and metabolizable energy content of ruminant feedstuffs from the gas production when they incubated with rumen liquor in vitro. J. Agric. Sci. Camb. 1979, 92, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Rootman, G.; Mabistela, M.; Pengelly, B. Selecting Potential Fodder Bank Legumes in Semi-arid Northern South Africa. In Tropical Legumes for Sustainable Farming Systems in Southern Africa and Australia; Whitbread, A.M., Pengelly, B.C., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 2004. [Google Scholar]
- Ciotti, E.M.; Tomei, C.E.; Castelan, M.E. Research note: The adaptation and production of some Stylosanthes scabra species in Corrientes, Argentina. Trop. Grassl. 1999, 33, 165–169. [Google Scholar]
- Hall, T.J.; Edye, L.A.; Middleton, C.H.; Messer, W.B.; Walker, R.W. Evaluation of thirteen Stylosanthes scabra Accessions in five dry tropical environments. Trop. Grassl. 1995, 29, 169–176. [Google Scholar]
- Ajayi, F.T.; Babayemi, O.J. Comparative in vitro evaluation of mixtures of Panicum maximum cv Ntchisi with stylo (Stylosanthes guianensis) 3304 Lablab (Lablab purpureus), Centro (Centosema pubescens) and Histrix (Aeschynomene histrix). Livest. Res. Rural Dev. 2008, 20, 83. Available online: http://www.lrrd.org/lrrd20/6/ajay20083.htm (accessed on 7 May 2012).
- Poppi, D.P.; McLennan, S.R. Protein and energy utilization by ruminants at pasture. J. Anim. Sci. 1995, 73, 278–290. [Google Scholar] [CrossRef]
- Schingoethe, D.J. Feeding dairy cows. In Livestock Feeds and Feeding; Kellems, R.O., Church, D.C., Eds.; Prentice Hall: Upper Saddle River, NJ, USA, 1998. [Google Scholar]
- Meissner, H.H.; Viljoen, M.O.; Van Niekerk, W.A. Intake and digestibility by sheep of Anthephora, Panicum, Rhodes and Smooth finger grass. In Proceedings of the IV International Rangeland Congress, Montpellier, France, 22–26 April 1991; pp. 648–649. [Google Scholar]
- Nastis, A.S.; Malachek, J.C. Digestion and utilization of nutrients in oak browse by goats in Nigeria. J. Anim. Sci. 1981, 52, 283–288. [Google Scholar] [CrossRef]
- Barry, T.N.; McNabb, W.C. The implications on condensed tannins on the nutritive value of temperate forages fed to ruminants. Br. J. Nutr. 1999, 81, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Babayemi, O.J.; Demeyer, D.; Fievez, V. In vitro rumen fermentation of tropical browse seeds in relation to their content of secondary metabolites. J. Anim. Feed Sci. 2004, 13 (Suppl. S1), 31–34. [Google Scholar] [CrossRef]
- Njidda, A.A.; Nasiru, A. In vitro gas production and dry matter digestibility of tannin-containing forages of semi-arid region of North-Eastern Nigeria. Pak. J. Nutr. 2010, 9, 60–66. [Google Scholar] [CrossRef]
- Abegunde, T.O.; Babayemi, O.J.; Akinsoyinu, A.O. Nutritive value assessment of Ficus polita and Panicum maximum at varying proportions using an in vitro gas production method in the dry and we seasons. Pak. J. Nutr. 2011, 10, 35–39. [Google Scholar] [CrossRef] [Green Version]
- Getachew, G.P.; Robinson, H.; DePeters, E.J.; Taylor, S.J. Relationships between chemical composition, dry matter degradation and in vitro gas production of several ruminant feeds. Anim. Feed Sci. Technol. 2004, 111, 57–71. [Google Scholar] [CrossRef]
- Kafilzadeh, F.; Heidary, N. Chemical composition, in vitro digestibility and kinetics of fermentation of whole-crop forage from 18 different varieties of oat (Avena sativa L.). J. Appl. Anim. Res. 2013, 41, 61–68. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, F.; Nan, Z. Comparative grain yield, straw yield, chemical composition, carbohydrate and protein fractions, in vitro digestibility and rumen degradability of four common Vetch varieties grown on the Qinghai-Tibetan plateau. Animals 2019, 9, 505. [Google Scholar] [CrossRef] [Green Version]
- Abas, I.; Özpinar, H.; Kutay, H.C.; Kahraman, R. Determination of the metabolizable energy (ME) and net energy lactation (NEL) contents of some feed in the Marmara region by in vitro gas technique. Turk. J. Vet. Anim. Sci. 2005, 29, 751–757. [Google Scholar]
- EL-Waziry, A.M. Nutritive value assessment of ensiling or mixing Acacia and Atriplex using in vitro gas production technique. Res. J. Agric. Biol. Sci. 2007, 3, 605–614. [Google Scholar]
- Niemeläinen, O.; Kontturi, M.; Jauhiainen, L.; Nissinen, O. Estimated metabolizable energy yields of perennial and annual grass swards compared with those of spring barley and oat. Agric. Food Sci. Finl. 2001, 10, 335–346. [Google Scholar] [CrossRef]


| Soil Depth (cm) | ||
|---|---|---|
| Properties | 0–20 | 20–40 |
| pH (H2O) a | 6.39 | 6.21 |
| Total N (%) | 0.02 | 0.02 |
| Carbon (%) | 0.46 | 0.52 |
| C:N ratio | 23.17 | 23.50 |
| Available P (kg ha−1) b | 0.63 | 0.42 |
| Cations c (cmol kg−1) | ||
| Ca | 1.94 | 1.92 |
| Mg | 1.05 | 1.03 |
| K | 0.13 | 0.12 |
| Na | 0.04 | 0.05 |
| CEC 1 | 3.47 | 3.84 |
| Soil texture d (%) | ||
| Sand | 70.7 | 66.0 |
| Silt | 5.3 | 6.0 |
| Clay | 24.0 | 28.0 |
| Parameters | PC 1 × 100 | PC 2 × 100 | PC 3 × 100 |
|---|---|---|---|
| Plant height | 3.50 | 25.99 | 50.75 |
| Canopy | −0.19 | 20.16 | 5.65 |
| Tillering | −0.61 | −10.97 | −19.38 |
| Day to 100% flowering | −0.64 | 26.47 | −7.86 |
| Survival | 0.97 | −5.18 | 23.09 |
| Mean forage yield | −0.19 | 0.61 | 1.50 |
| Ash | 4.97 | −7.01 | 70.35 |
| Crude protein | 12.06 | 84.94 | −13.37 |
| Neutral detergent fiber | 99.04 | −10.99 | −3.98 |
| Total phenols | −0.59 | 6.86 | 3.31 |
| Total tannins | 0.22 | 4.60 | 4.94 |
| Total condensed tannins | −0.26 | 5.31 | 2.42 |
| Insoluble but slowly fermentable fraction | −1.81 | −10.25 | −5.95 |
| Rate constant of gas production | 0.01 | −0.009 | 0.03 |
| Effective gas production | −0.92 | −2.35 | 11.25 |
| Gas volume in 24 h | −0.66 | −13.10 | −1.50 |
| In vitro organic matter digestibility | −0.28 | −16.54 | −2.93 |
| Metabolizable energy | 0.16 | 0.50 | −0.26 |
| Short chain fatty acid | 0.00 | −0.11 | 0.12 |
| Metabolizable energy yield | −0.38 | 3.98 | 31.59 |
| Eigenvalue | 4662.67 | 87.57 | 49.35 |
| % variance | 95.534 | 1.79 | 1.01 |
| Accessions (ILRI no) * | Plant Height (cm) | Canopy Spread (cm) | Tillering Capacity | Days to 100% Flowering | % Survival after 3 Years |
|---|---|---|---|---|---|
| 140 | 34.8 bc | 34.1 bcd | 14.1 bcd | 56.0 cd | 94.8 a |
| 170 | 43.9 a | 39.7 ab | 12.3 bcd | 63.7 abc | 94.5 a |
| 441 | 41.3 ab | 32.9 bcd | 13.3 bcd | 61.0 cd | 86.4 a |
| 9268 | 39.2 abc | 43.6 a | 12.3 bcd | 53.0 d | 85.4 a |
| 9281 | 35.8 bc | 31.6 d | 14.9 b | 56.3 cd | 95.1 a |
| 11,252 | 33.3 c | 39.4 abc | 13.5 bcd | 61.5 cd | 98.1 a |
| 11,255 | 32.8 c | 39.5 abc | 14.7 bc | 58.5 cd | 89.0 a |
| 11,591 | 41.5 ab | 39.9 ab | 13.9 bcd | 70.7 ab | None |
| 11,592 | 34.5 c | 32.1 cd | 13.3 bcd | 71.3 ab | 88.5 a |
| 11,595 | 36.8 bc | 43.2 a | 12.3 bcd | 56.3 cd | 95.8 a |
| 11,604 | 34.1 c | 36.9 abc | 11.6 cd | 60.3 cd | 95.7 a |
| 11,625 | 35.4 bc | 33.8 bcd | 11.1 d | 72.0 a | 89.9 a |
| 12,555 | 32.4 c | 39.93 ab | 14.3 bc | 59.3 cd | 41.3 b |
| 15,784 | 34.3 c | 38.3 abc | 11.7 cd | 63.0 bc | 93.0 a |
| 15,795 | 22.0 d | 35.3 bcd | 19.9 a | 56.7 cd | 88.3 a |
| SEM | 2.34 | 2.57 | 1.05 | 3.03 | 5.11 |
| Accessions (ILRI no) * | Forage Yield (t ha−1DM) | |||
|---|---|---|---|---|
| Year-2012 | Year-2013 | Year-2014 | Mean | |
| 140 | 4.2 bcA | 5.3 bA | 5.3 abA | 4.9 |
| 170 | 4.9 bcA,B | 5.6 abA | 3.6 bcB | 4.7 |
| 441 | 5.3 abcA | 5.0 bA,B | 3.8 abcB | 3.5 |
| 9268 | 4.4 bcB | 6.3 aA | 1.8 cC | 4.1 |
| 9281 | 4.6 bcA | 5.0 bA | 5.4 abA | 5.0 |
| 11,252 | 5.4 abcA | 5.4 abA | 4.5 abcA | 5.1 |
| 11,255 | 5.8 abA | 5.4 abA | 3.3 bcB | 4.8 |
| 11,591 | 6.7 a | - | - | - |
| 11,592 | 5.2 abcA | 5.6 abA | 3.3 bcB | 4.7 |
| 11,595 | 5.0 abcA | 5.3 bA | 6.6 aA | 5.6 |
| 11,604 | 4.8 bcA | 5.2 bA | 5.4 abA | 5.1 |
| 11,625 | 4.8 bcA | 5.3 bA | 4.5 abcA | 4.9 |
| 12,555 | 4.4 bcA | 5.1 bA | 2.9 bcB | 4.1 |
| 15,784 | 3.9 cB | 5.6 abA | 4.7 abA | 4.7 |
| 15,795 | 4.6 bcA | 5.3 bA | 3.3 bcB | 4.4 |
| SEM | 0.14 | 0.28 | 0.78 | |
| Year effects | 4.9 A | 5.3 A | 4.3 B | |
| Accessions (ILRI no) * | Chemical Analysis (g kg−1 DM) | |||||
|---|---|---|---|---|---|---|
| Ash | CP | NDF | TP | THT | TCT | |
| 140 | 92.3 | 196.5 b | 501.7 ab | 6.7 c | 3.2 ab | nd |
| 170 | 93.5 | 230.9 a | 559.2 a | 6.9 c | 4.5 ab | 0.5 f |
| 441 | 97.5 | 206.7 b | 539.2 ab | 7.8 bc | 3.4 ab | 1.9 cd |
| 9281 | 88.3 | 195.3 b | 469.0 ab | 6.8 c | 4.2 ab | nd |
| 11,252 | 86.5 | 193.4 b | 439.9 ab | 8.8 ab | 3.5 ab | 3.1 a |
| 11,255 | 82.5 | 198.0 b | 345.3 b | 9.8 a | 3.5 ab | 2.3 bc |
| 11,592 | 84.8 | 210.9 ab | 511.1 ab | 9.8 a | 4.4 ab | 2.3 bc |
| 11,595 | 86.8 | 211.7 ab | 483.3 ab | 8.5 b | 5.6 a | 2.9 ab |
| 11,604 | 94.5 | 197.9 b | 498.0 ab | 6.9 c | 3.0 b | 1.0 ef |
| 11,625 | 82.5 | 189.7 b | 353.9 b | 7.8 bc | 3.2 ab | 1.3 de |
| 15,784 | 80.0 | 212.9 ab | 529.7 ab | 7.2 c | 2.4 b | 1.8 cd |
| 15,795 | 76.3 | 196.3 b | 446.7 ab | 5.7 d | 2.6 b | nd |
| SEM | 7.60 | 6.87 | 68.08 | 0.36 | 0.75 | 0.25 |
| Accession (ILRI no) * | b (mL 400 mg−1) | c (h−1) | EGP (mL 400 mg−1) |
|---|---|---|---|
| 140 | 90.2 ab | 0.097 | 59.0 |
| 170 | 88.8 ab | 0.091 | 56.9 |
| 441 | 88.6 ab | 0.096 | 57.8 |
| 9281 | 90.2 ab | 0.093 | 58.1 |
| 11,252 | 89.2 ab | 0.086 | 59.0 |
| 11,255 | 90.8 ab | 0.086 | 59.8 |
| 11,592 | 86.2 b | 0.087 | 54.5 |
| 11,595 | 82.0 b | 0.099 | 58.9 |
| 11,604 | 87.8 ab | 0.098 | 57.9 |
| 11,625 | 91.4 a | 0.088 | 57.8 |
| 15,784 | 87.3 ab | 0.097 | 57.2 |
| 15,795 | 91.7 a | 0.084 | 56.7 |
| SEM | 3.79 | 0.0099 | 1.76 |
| Accessions (ILRI no) * | In Vitro Parameters | ||||
|---|---|---|---|---|---|
| GV24h (mL 400 mg−1) | IVOMD (% DM) | ME (MJ kg−1 DM) | SCFA (µmol g−1 DM) | ME Yield (GJ ha−1) | |
| 140 | 80.4 | 75.6 a | 9.9 ab | 0.9 | 48.9 ab |
| 170 | 76.5 | 72.3 abc | 10.2 a | 0.9 | 47.1 ab |
| 441 | 76.1 | 69.7 bc | 9.8 ab | 0.8 | 45.8 ab |
| 9281 | 77.8 | 76.5 a | 9.7 ab | 0.9 | 48.9 ab |
| 11,252 | 75.6 | 69.8 bc | 9.5 b | 0.8 | 48.4 ab |
| 11,255 | 74.9 | 71.6 abc | 9.5 b | 0.8 | 45.9 ab |
| 11,592 | 72.9 | 67.4 c | 9.6 ab | 0.8 | 45.4 ab |
| 11,595 | 76.6 | 68.4 c | 9.9 ab | 0.9 | 55.5 a |
| 11,604 | 76.4 | 71.8 abc | 9.6 ab | 0.9 | 48.5 ab |
| 11,625 | 79.1 | 71.1 abc | 9.7 ab | 0.9 | 49.7 ab |
| 15,784 | 75.1 | 71.4 abc | 9.8 ab | 0.8 | 44.7 ab |
| 15,795 | 80.7 | 74.7 ab | 9.9 ab | 0.9 | 42.6 b |
| SEM | 2.45 | 1.62 | 0.20 | 0.03 | 3.15 |
| CP | ME | SCFA | IVOMD | NDF | Ash | TP | THT | TCT | |
|---|---|---|---|---|---|---|---|---|---|
| 2 h | −0.088 | 0.583 ** | 0.804 ** | 0.217 | 0.134 | 0.258 | −0.422 * | 0.09 | −0.455 |
| 4 h | −0.058 | 0.651 ** | 0.858 ** | 0.248 | 0.151 | 0.355 | −0.405 * | 0.16 | −0.422 |
| 8 h | −0.056 | 0.684 ** | 0.899 ** | 0.291 | 0.166 | 0.401 | −0.388 | 0.156 | −0.373 |
| 12 h | −0.041 | 0.679 ** | 0.879 ** | 0.295 | 0.171 | 0.448 * | −0.355 | 0.104 | −0.273 |
| 24 h | −0.239 | 0.611 ** | 0.994 ** | 0.453 * | 0.012 | 0.214 | −0.498 * | 0.051 | −0.302 |
| 48 h | −0.261 | 0.509 * | 0.891 ** | 0.505 * | −0.071 | 0.037 | −0.453 * | −0.058 | −0.198 |
| 72 h | −0.171 | 0.507 * | 0.799 ** | 0.528 ** | −0.017 | −0.009 | −0.457 * | −0.089 | −0.195 |
| 96 h | −0.292 | 0.23 | 0.583 ** | 0.599 ** | −0.152 | −0.164 | −0.434 * | −0.178 | −0.055 |
| b | −0.149 | −0.363 | −0.29 | 0.300 | −0.183 | −0.249 | −0.038 | −0.31 | 0.304 |
| c | 0.069 | 0.567 ** | 0.629 ** | 0.005 | 0.210 | 0.564 ** | −0.122 | 0.201 | −0.203 |
| EGP | −0.108 | 0.277 | 0.458 * | 0.379 | 0.056 | 0.439 * | −0.084 | −0.157 | 0.237 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mpanza, T.D.E.; Hassen, A.; Akanmu, A.M. Evaluation of Stylosanthes scabra Accessions as Forage Source for Ruminants: Growth Performance, Nutritive Value and In Vitro Ruminal Fermentation. Animals 2020, 10, 1939. https://doi.org/10.3390/ani10111939
Mpanza TDE, Hassen A, Akanmu AM. Evaluation of Stylosanthes scabra Accessions as Forage Source for Ruminants: Growth Performance, Nutritive Value and In Vitro Ruminal Fermentation. Animals. 2020; 10(11):1939. https://doi.org/10.3390/ani10111939
Chicago/Turabian StyleMpanza, Thamsanqa Doctor Empire, Abubeker Hassen, and Abiodun Mayowa Akanmu. 2020. "Evaluation of Stylosanthes scabra Accessions as Forage Source for Ruminants: Growth Performance, Nutritive Value and In Vitro Ruminal Fermentation" Animals 10, no. 11: 1939. https://doi.org/10.3390/ani10111939
APA StyleMpanza, T. D. E., Hassen, A., & Akanmu, A. M. (2020). Evaluation of Stylosanthes scabra Accessions as Forage Source for Ruminants: Growth Performance, Nutritive Value and In Vitro Ruminal Fermentation. Animals, 10(11), 1939. https://doi.org/10.3390/ani10111939






