Perfluorooctane Sulfonate (PFOS) and Perfluorohexane Sulfonate (PFHxS) Alters Protein Phosphorylation, Increase ROS Levels and DNA Fragmentation during In Vitro Capacitation of Boar Spermatozoa
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Boar Spermatozoa Samples and Incubation Media
- (1)
- For the LC50 determination.
- T0, spermatozoa washed and not capacitated.
- Controls, incubated only in capacitation medium, and to ensure that nontoxic effect of DMSO, used as a diluent of PFCs, 10 µL of DMSO was added in another aliquot.
- Capacitated in the presence of PFOS (1000, 2000 and 3000 µM) and PFHxS (1000, 2500 and 5000 µM).
- (2)
- For CTC stain and acrosome status by FITC-PNA.
- T0, spermatozoa washed and not capacitated.
- Controls, incubated only in capacitation medium, and to ensure that nontoxic effect of DMSO, used as a diluent of PFCs, 10 µL of DMSO was added in another aliquot.
- Capacitated in the presence of sub-lethal fractions (⅕ LC50, ½ LC50, LC50) of PFOS and PFHxS.
- (3)
- For tyrosine phosphorylation, reactive oxygen species (ROS), and comet assays only LC50 concentration of each PFCs were tested. The controls in these last tests were spermatozoa capacitated without the toxics.
- (4)
- Incubation times for experiments above were of 4 h, except in the ROS-determination experiments, where the samples were incubated for 30 and 120 min (Figure 1).
- (5)
- For microscopic studies, at least 200 cells were analyzed per slide.
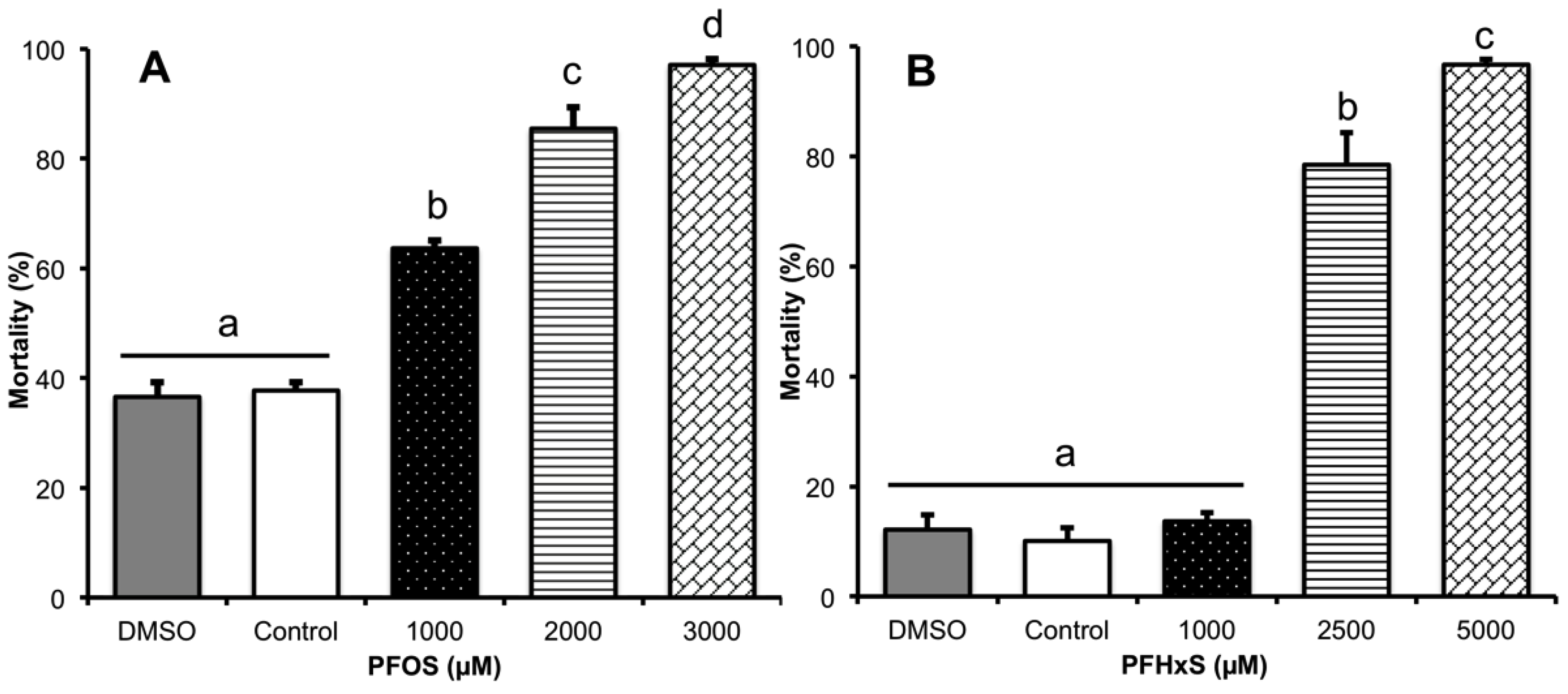
2.2. Determination of Mean Lethal Concentration (LC50)
2.3. Spermatozoa Viability
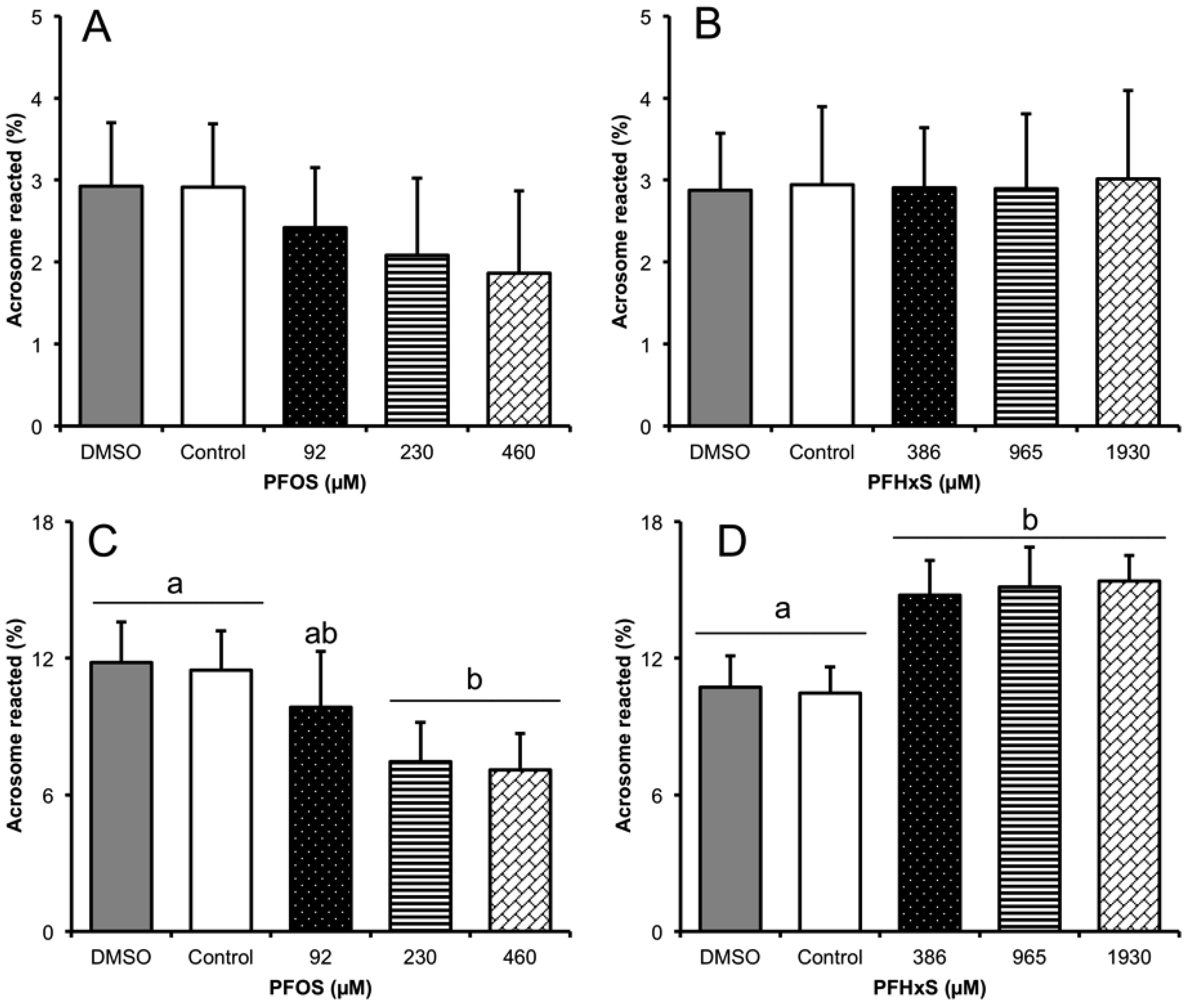
2.4. Capacitation and the Spontaneous Acrosome Reaction (sAR)
2.5. Evaluation of Tyrosine Phosphorylation
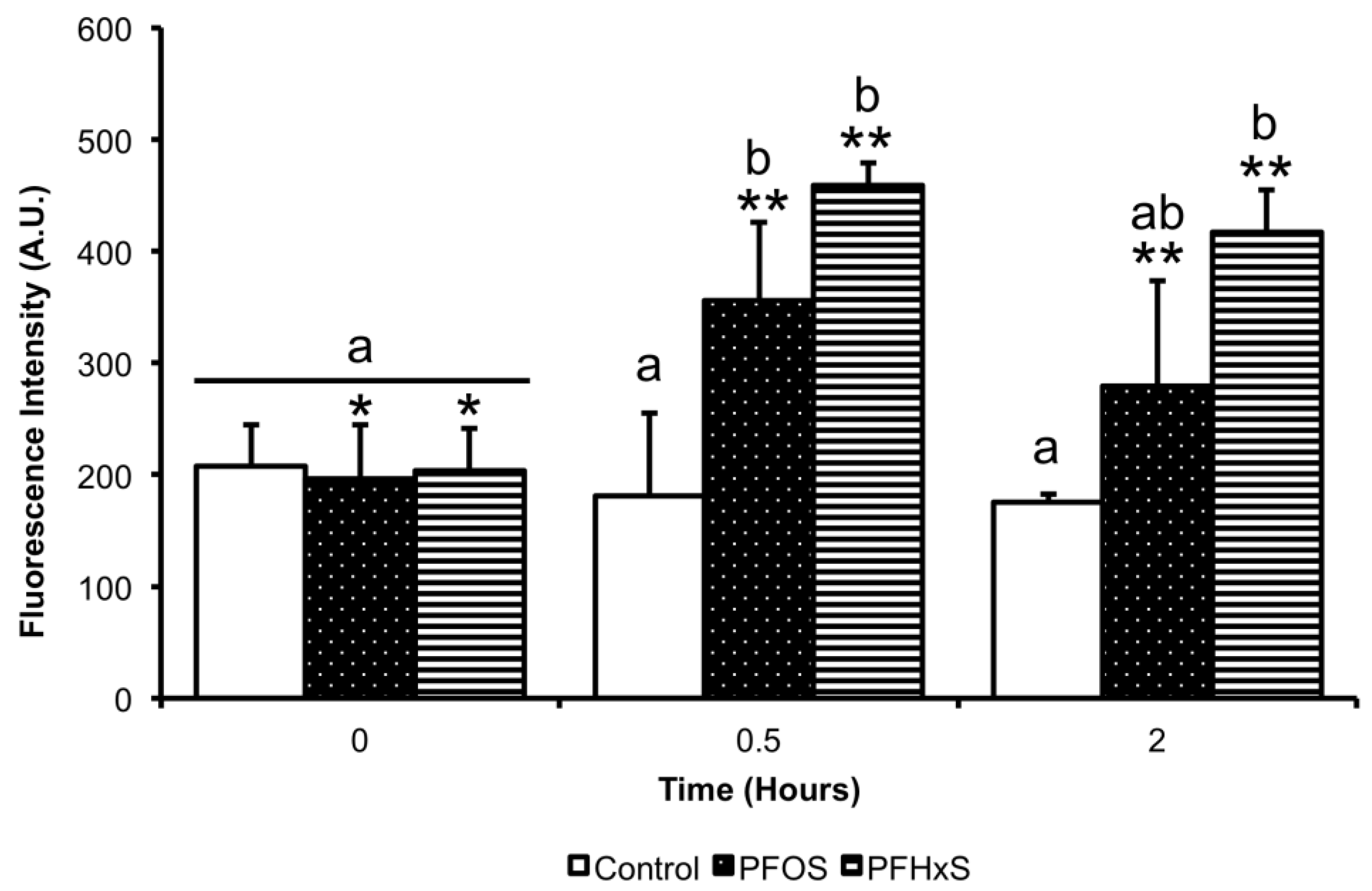
2.6. Intracellular Reactive Oxygen Species (ROS) Levels
2.7. Single-Cell Gel Electrophoresis (SCGE), or the Comet Assay
2.8. Flow Cytometry
2.9. Fluorescence Microscopy
2.10. Statistical Analyses
3. Results
3.1. Determination of the LC50 of PFOS and PFHxS
3.2. Effect of Sub-Lethal Fractions on Boar Spermatozoa Capacitation
3.3. Subsection Effect of Sub-Lethal Concentrations of PFCs on the sAR Process
3.4. Tyrosine Phosphorylation Determination
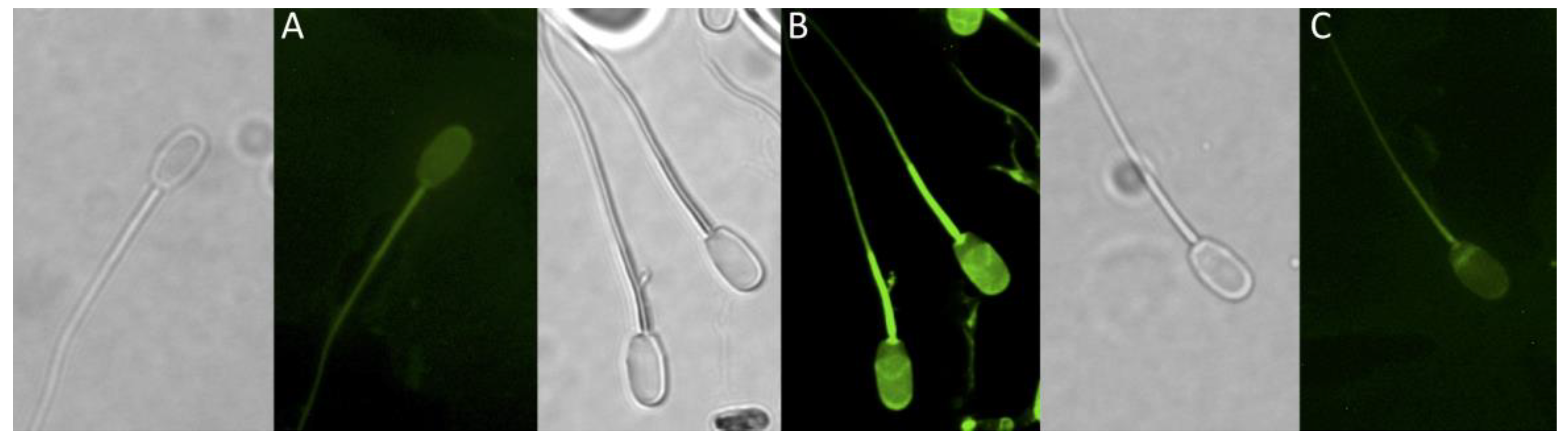
3.4.1. Flow Cytometry
3.4.2. Fluorescence Microscopy
3.5. Effect of PFCs on ROS Production
3.6. Effect of PFCs on DNA Fragmentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petrovic, M.; Farré, M.; Eljarrat, E.; Diaz, S.; Barceló, D. Chapter 14—Environmental Analysis: Emerging Pollutants. In Liquid Chromatography; Fanali, S., Haddad, P.R., Poole, C.F., Schoenmakers, P., Lloyd, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 389–410. [Google Scholar] [CrossRef]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl Acids: A Review of Monitoring and Toxicological Findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerranti, C.; Ancora, S.; Bianchi, N.; Perra, G.; Fanello, E.L.; Corsolini, S.; Fossi, M.C.; Focardi, S.E. Perfluorinated compounds in blood of Caretta caretta from the Mediterranean Sea. Mar. Pollut. Bull. 2013, 73, 98–101. [Google Scholar] [CrossRef]
- Kato, K.; Wong, L.-Y.; Basden, A.; Calafat, A.M. Effect of temperature and duration of storage on the stability of polyfluoroalkyl chemicals in human serum. Chemosphere 2012, 91, 115–117. [Google Scholar] [CrossRef] [Green Version]
- Dorman, F.L.; Reiner, E.J. Chapter 28—Emerging and Persistent Environmental Compound Analysis. In Gas Chromatography; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 647–677. [Google Scholar] [CrossRef]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated Compounds: Past, Present, and Future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef]
- Reiner, E.J.; Jobst, K.J.; Megson, D.; Dorman, F.L.; Focant, J.-F. Chapter 3—Analytical Methodology of POPs. In Environmental Forensics for Persistent Organic Pollutants; O’Sullivan, G., Sandau, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 59–139. [Google Scholar] [CrossRef]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-Life of Serum Elimination of Perfluorooctanesulfonate, Perfluorohexanesulfonate, and Perfluorooctanoate in Retired Fluorochemical Production Workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Guruge, K.S.; Manage, P.M.; Yamanaka, N.; Miyazaki, S.; Taniyasu, S.; Yamashita, N. Species-specific concentrations of perfluoroalkyl contaminants in farm and pet animals in Japan. Chemosphere 2008, 73, S210–S215. [Google Scholar] [CrossRef] [PubMed]
- Vestergren, R.; Orata, F.; Berger, U.; Cousins, I.T. Bioaccumulation of perfluoroalkyl acids in dairy cows in a naturally contaminated environment. Environ. Sci. Pollut. Res. 2013, 20, 7959–7969. [Google Scholar] [CrossRef]
- Stahl, T.; Falk, S.; Failing, K.; Berger, J.; Georgii, S.; Brunn, H. Perfluorooctanoic Acid and Perfluorooctane Sulfonate in Liver and Muscle Tissue from Wild Boar in Hesse, Germany. Arch. Environ. Contam. Toxicol. 2011, 62, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Bassols, A.; Costa, C.; Eckersall, P.D.; Osada, J.; Sabrià, J.; Tibau, J. The pig as an animal model for human pathologies: A proteomics perspective. Proteom. Clin. Appl. 2014, 8, 715–731. [Google Scholar] [CrossRef]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The pig: A model for human infectious diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef]
- Mordhorst, B.R.; Prather, R.S. Pig Models of Reproduction. In Animal Models and Human Reproduction; Constantinescu, G., Schatten, H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 213–234. [Google Scholar] [CrossRef]
- Joensen, U.N.; Bossi, R.; Leffers, H.; Jensen, A.A.; Skakkebæk, N.E.; Jørgensen, N. Do Perfluoroalkyl Compounds Impair Human Semen Quality? Environ. Health Perspect. 2009, 117, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Joensen, U.N.; Veyrand, B.; Antignac, J.-P.; Jensen, M.B.; Petersen, J.H.; Marchand, P.; Skakkebæk, N.E.; Andersson, A.-M.; Le Bizec, B.; Jørgensen, N. PFOS (perfluorooctanesulfonate) in serum is negatively associated with testosterone levels, but not with semen quality, in healthy men. Human Reprod. 2012, 28, 599–608. [Google Scholar] [CrossRef] [Green Version]
- Toft, G.; Jönsson, B.; Lindh, C.; Giwercman, A.; Spano, M.; Heederik, D.; Lenters, V.; Vermeulen, R.; Rylander, L.; Pedersen, H.; et al. Exposure to perfluorinated compounds and human semen quality in arctic and European populations. Human Reprod. 2012, 27, 2532–2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, H.T.; Zhao, Y.G.; Wong, M.H.; Lee, K.-F.; Yeung, W.S.; Giesy, J.P.; Wong, C.K.C. Testicular Signaling is the Potential Target of Perfluorooctanesulfonate-Mediated Subfertility in Male Mice1. Biol. Reprod. 2011, 84, 1016–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, H.-T.; Mruk, D.D.; Wong, C.K.C.; Cheng, C.Y. Perfluorooctanesulfonate (PFOS) Perturbs Male Rat Sertoli Cell Blood-Testis Barrier Function by Affecting F-Actin Organization via p-FAK-Tyr407: An in Vitro Study. Endocrinology 2014, 155, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, H.; Xiao, X.; Lui, W.Y.; Lee, W.M.; Mruk, D.D.; Cheng, C.Y. Perfluorooctanesulfonate (PFOS)-induced Sertoli cell injury through a disruption of F-actin and microtubule organization is mediated by Akt1/2. Sci. Rep. 2017, 7, 1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Wang, X.; Ge, X.; Wang, D.; Wang, T.; Zhang, L.; Tanguay, R.L.; Simonich, M.; Huang, C.; Dong, Q. Chronic perfluorooctanesulphonic acid (PFOS) exposure produces estrogenic effects in zebrafish. Environ. Pollut. 2016, 218, 702–708. [Google Scholar] [CrossRef]
- Ickowicz, D.; Finkelstein, M.; Breitbart, H. Mechanism of sperm capacitation and the acrosome reaction: Role of protein kinases. Asian J. Androl. 2012, 14, 816–821. [Google Scholar] [CrossRef] [Green Version]
- Meyers, S.A. Chapter 5—Sperm Physiology. In Equine Breeding Management and Artificial Insemination, 2nd ed.; Samper, J.C., Ed.; W.B. Saunders: Saint Louis, MO, USA, 2009; pp. 47–55. [Google Scholar] [CrossRef]
- Alkmin, D.V.; Martinez-Alborcia, M.J.; Parrilla, I.; Vazquez, J.M.; Martinez, E.A.; Roca, J. The nuclear DNA longevity in cryopreserved boar spermatozoa assessed using the Sperm-Sus-Halomax. Theriogenology 2013, 79, 1294–1300. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M.; Ishizaki, Y.; Kawagishi, H. Blastocyst formation by pig embryos resulting from in-vitro fertilization of oocytes matured in vitro. J. Reprod. Fertil. 1990, 88, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Maravilla-Galván, R.; Fierro, R.; González-Márquez, H.; Gomez-Arroyo, S.; Jiménez, I.; Betancourt, M. Effects of Atrazine and Fenoxaprop-Ethyl on Capacitation and the Acrosomal Reaction in Boar Sperm. Int. J. Toxicol. 2009, 28, 24–32. [Google Scholar] [CrossRef]
- Ded, L.; Dostalova, P.; Zatecka, E.; Dorosh, A.; Dvořáková-Hortová, K.; Peknicova, J. Fluorescent analysis of boar sperm capacitation process in vitro. Reprod. Biol. Endocrinol. 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Fraser, L.R.; Herod, J.E. Expression of capacitation-dependent changes in chlortetracycline fluorescence patterns in mouse spermatozoa requires a suitable glycolysable substrate. J. Reprod. Fertil. 1990, 88, 611–621. [Google Scholar] [CrossRef]
- Fàbrega, A.; Puigmulé, M.; Yeste, M.; Casas, I.; Bonet, S.; Pinart, E. Impact of epididymal maturation, ejaculation and in vitro capacitation on tyrosine phosphorylation patterns exhibited of boar (Sus domesticus) spermatozoa. Theriogenology 2011, 76, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, H.D.; Welch, G.R. Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in Percoll-treated viable boar sperm using fluorescence-activated flow cytometry. J. Anim. Sci. 2006, 84, 2089–2100. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cerezales, S.; Laguna-Barraza, R.; De Castro, A.C.; Sánchez-Calabuig, M.J.; Cano-Oliva, E.; De Castro-Pita, F.J.; Montoro-Buils, L.; Pericuesta, E.; Fernández-González, R.; Gutiérrez-Adán, A. Sperm selection by thermotaxis improves ICSI outcome in mice. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Końca, K.; Lankoff, A.; Banasik, A.; Lisowska, H.; Kuszewski, T.; Góźdź, S.; Koza, Z.; Wojcik, A. A cross-platform public domain PC image-analysis program for the comet assay. Mutat. Res. Mol. Mech. Mutagen. 2003, 534, 15–20. [Google Scholar] [CrossRef]
- Brownlee, K.A.; Finney, D.J.; Tattersfield, F. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve. J. Am. Stat. Assoc. 1952, 47, 687. [Google Scholar] [CrossRef]
- Louis, G.M.B.; Chen, Z.; Schisterman, E.F.; Kim, S.; Sweeney, A.M.; Sundaram, R.; Lynch, C.D.; Gore-Langton, R.E.; Barr, D.B. Perfluorochemicals and Human Semen Quality: The LIFE Study. Environ. Health Perspect. 2015, 123, 57–63. [Google Scholar] [CrossRef]
- Slotkin, T.A.; MacKillop, E.A.; Melnick, R.L.; Thayer, K.A.; Seidler, F.J. Developmental Neurotoxicity of Perfluorinated Chemicals Modeled in Vitro. Environ. Health Perspect. 2008, 116, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, I.; Fierro, R.; González-Márquez, H.; Mendoza-Hernández, G.; Romo, S.; Betancourt, M. Carbohydrate Affinity Chromatography Indicates that Arylsulfatase-A from Capacitated Boar Sperm has Mannose and N-Acetylglucosamine/Sialic Acid Residues. Arch. Androl. 2006, 52, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.R.; Storey, B.T. Determination of the time course of capacitation in mouse spermatozoa using a chlortetracycline fluorescence assay. Dev. Biol. 1984, 104, 287–296. [Google Scholar] [CrossRef]
- Pan, Y.; Qin, H.; Liu, W.; Zhang, Q.; Zheng, L.; Zhou, C.; Quan, X. Effects of chlorinated polyfluoroalkyl ether sulfonate in comparison with perfluoroalkyl acids on gene profiles and stemness in human mesenchymal stem cells. Chemosphere 2019, 237, 124402. [Google Scholar] [CrossRef]
- Liao, C.-Y.; Li, X.-Y.; Wu, B.; Duan, S.; Jiang, G.-B. Acute Enhancement of Synaptic Transmission and Chronic Inhibition of Synaptogenesis Induced by Perfluorooctane Sulfonate through Mediation of Voltage-Dependent Calcium Channel. Environ. Sci. Technol. 2008, 42, 5335–5341. [Google Scholar] [CrossRef] [PubMed]
- Wassarman, P.M. Fertilization, Mammalian. In Encyclopedia of Genetics; Brenner, S., Miller, J.H., Eds.; Academic Press: New York, NY, USA, 2001; pp. 690–695. [Google Scholar] [CrossRef]
- Kopf, G.S. Acrosome. In Encyclopedia of Genetics; Brenner, S., Miller, J.H., Eds.; Academic Press: New York, NY, USA, 2001; pp. 3–4. [Google Scholar] [CrossRef]
- Rajabi-Toustani, R.; Akter, Q.S.; Almadaly, E.A.; Hoshino, Y.; Adachi, H.; Mukoujima, K.; Murase, T. Methodological improvement of fluorescein isothiocyanate peanut agglutinin (FITC-PNA) acrosomal integrity staining for frozen-thawed Japanese Black bull spermatozoa. J. Veter. Med Sci. 2019, 81, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K.; Rajesh, P.B. Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod. Biol. Endocrinol. 2004, 2, 75. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.; James, P.S.; Oxley, D.; Coadwell, J.; Suzuki-Toyota, F.; Howes, E.A. The Equatorial Subsegment in Mammalian Spermatozoa Is Enriched in Tyrosine Phosphorylated Proteins. Biol. Reprod. 2008, 79, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.; Qian, Y.; Liu, Z.; Wang, C.; Qu, J.; Wang, X.; Wang, S. Perfluorooctane sulfonate (PFOS) disrupts blood-testis barrier by down-regulating junction proteins via p38 MAPK/ATF2/MMP9 signaling pathway. Toxicology 2016, 373, 1–12. [Google Scholar] [CrossRef]
- Chen, N.; Li, J.; Li, D.; Yang, Y.; He, D. Chronic Exposure to Perfluorooctane Sulfonate Induces Behavior Defects and Neurotoxicity through Oxidative Damages, In Vivo and In Vitro. PLoS ONE 2014, 9, e113453. [Google Scholar] [CrossRef] [Green Version]
- Khansari, M.R.; Yousefsani, B.S.; Kobarfard, F.; Faizi, M.; Pourahmad, J. In vitro toxicity of perfluorooctane sulfonate on rat liver hepatocytes: Probability of distructive binding to CYP 2E1 and involvement of cellular proteolysis. Environ. Sci. Pollut. Res. 2017, 24, 23382–23388. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, S.-Y.; Yang, J.-H. PFHxS induces apoptosis of neuronal cells via ERK1/2-mediated pathway. Chemosphere 2014, 94, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ford, W. Regulation of sperm function by reactive oxygen species. Human Reprod. Updat. 2004, 10, 387–399. [Google Scholar] [CrossRef]
- Awda, B.J.; Mackenzie-Bell, M.; Buhr, M. Reactive Oxygen Species and Boar Sperm Function1. Biol. Reprod. 2009, 81, 553–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocuzza, M.; Sikka, S.C.; Athayde, K.S.; Agarwal, A. Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: An evidence based analysis. Int. Braz. J. Urol. 2007, 33, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Kothari, S.; Thompson, A.; Agarwal, A.; Du Plessis, S.S. Free radicals: Their beneficial and detrimental effects on sperm function. Indian J. Exp. Boil. 2010, 48, 425–435. [Google Scholar]





| Samples at T0 | Assay (%) | ||||
|---|---|---|---|---|---|
| Eosin-Nigrosin | CTC Stain | FITC-PNA | |||
| Variable | Mortality | Abnormalities | Capacitated | sAR | sAR |
| n = 6 | 5.69 ± 1.64 | 1.86 ± 0.38 | 6 ± 1.33 | 1.86 ± 0.56 | 2.31 ± 0.33 |
| Treatment | Tyrosine Phosphorylation Patterns | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NFH | AF | EHZ | AE | |||||||||||||
| T0 | 9.51 | ± | 1.03 | a | 14.42 | ± | 0.75 | a | 75.62 | ± | 0.98 | a | 0.45 | ± | 0.79 | a |
| Control | 12.15 | ± | 0.80 | ab | 5.24 | ± | 1.48 | bc | 65.38 | ± | 1.98 | b | 17.23 | ± | 0.74 | b |
| PFOS | 12.69 | ± | 1.50 | b | 3.00 | ± | 1.46 | bc | 71.06 | ± | 2.50 | a | 13.26 | ± | 0.73 | c |
| PFHxS | 9.81 | ± | 0.69 | a | 1.28 | ± | 1.29 | c | 81.6 | ± | 1.87 | c | 7.32 | ± | 1.25 | d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oseguera-López, I.; Pérez-Cerezales, S.; Ortiz-Sánchez, P.B.; Mondragon-Payne, O.; Sánchez-Sánchez, R.; Jiménez-Morales, I.; Fierro, R.; González-Márquez, H. Perfluorooctane Sulfonate (PFOS) and Perfluorohexane Sulfonate (PFHxS) Alters Protein Phosphorylation, Increase ROS Levels and DNA Fragmentation during In Vitro Capacitation of Boar Spermatozoa. Animals 2020, 10, 1934. https://doi.org/10.3390/ani10101934
Oseguera-López I, Pérez-Cerezales S, Ortiz-Sánchez PB, Mondragon-Payne O, Sánchez-Sánchez R, Jiménez-Morales I, Fierro R, González-Márquez H. Perfluorooctane Sulfonate (PFOS) and Perfluorohexane Sulfonate (PFHxS) Alters Protein Phosphorylation, Increase ROS Levels and DNA Fragmentation during In Vitro Capacitation of Boar Spermatozoa. Animals. 2020; 10(10):1934. https://doi.org/10.3390/ani10101934
Chicago/Turabian StyleOseguera-López, Iván, Serafín Pérez-Cerezales, Paola Berenice Ortiz-Sánchez, Oscar Mondragon-Payne, Raúl Sánchez-Sánchez, Irma Jiménez-Morales, Reyna Fierro, and Humberto González-Márquez. 2020. "Perfluorooctane Sulfonate (PFOS) and Perfluorohexane Sulfonate (PFHxS) Alters Protein Phosphorylation, Increase ROS Levels and DNA Fragmentation during In Vitro Capacitation of Boar Spermatozoa" Animals 10, no. 10: 1934. https://doi.org/10.3390/ani10101934






