Fitting of the In Vitro Gas Production Technique to the Study of High Concentrate Diets
Abstract
Simple Summary
Abstract
1. The Gas Production Technique for Estimating Rumen Fermentation
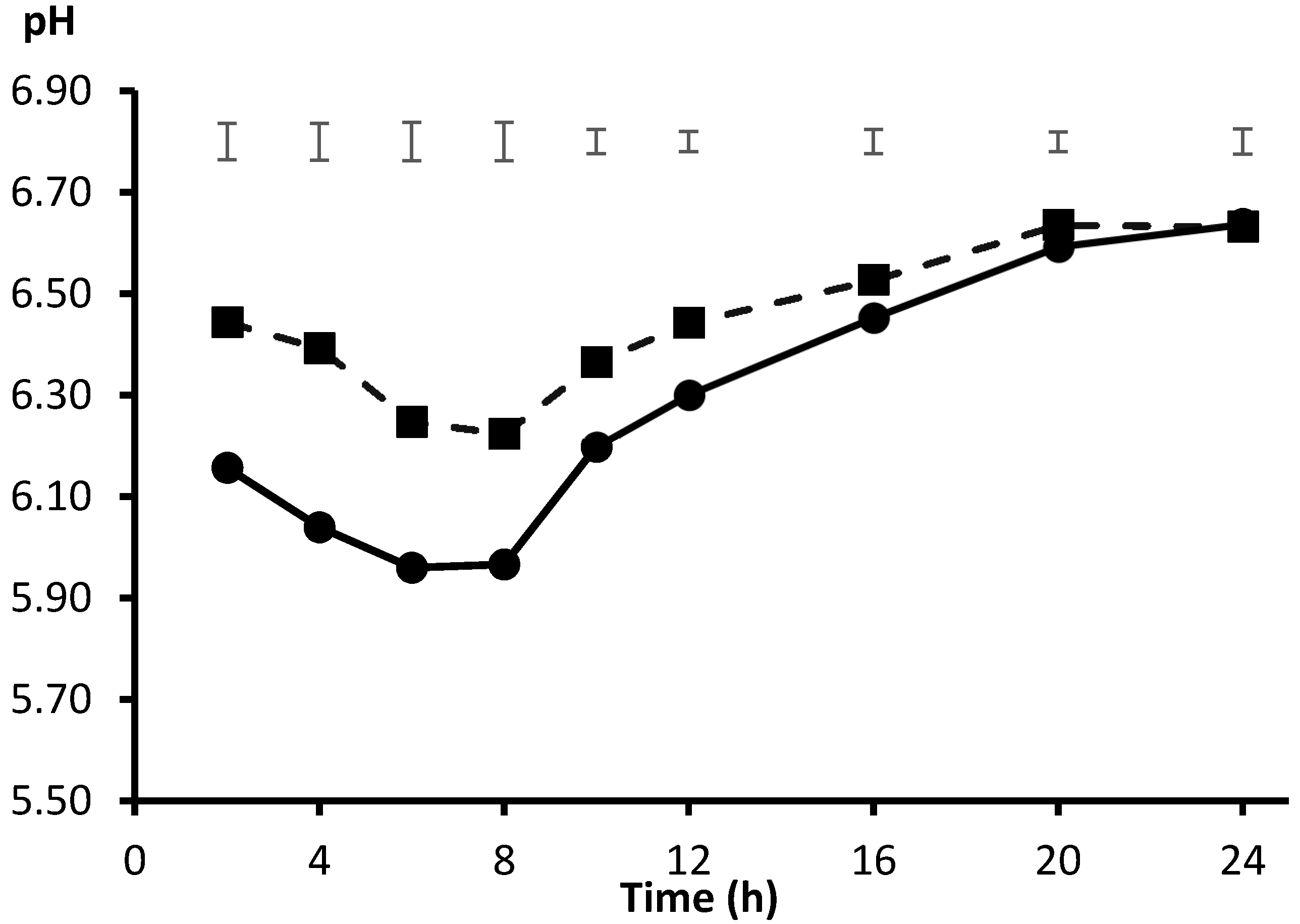
2. Source of Inoculum and Fitting of Incubation pH
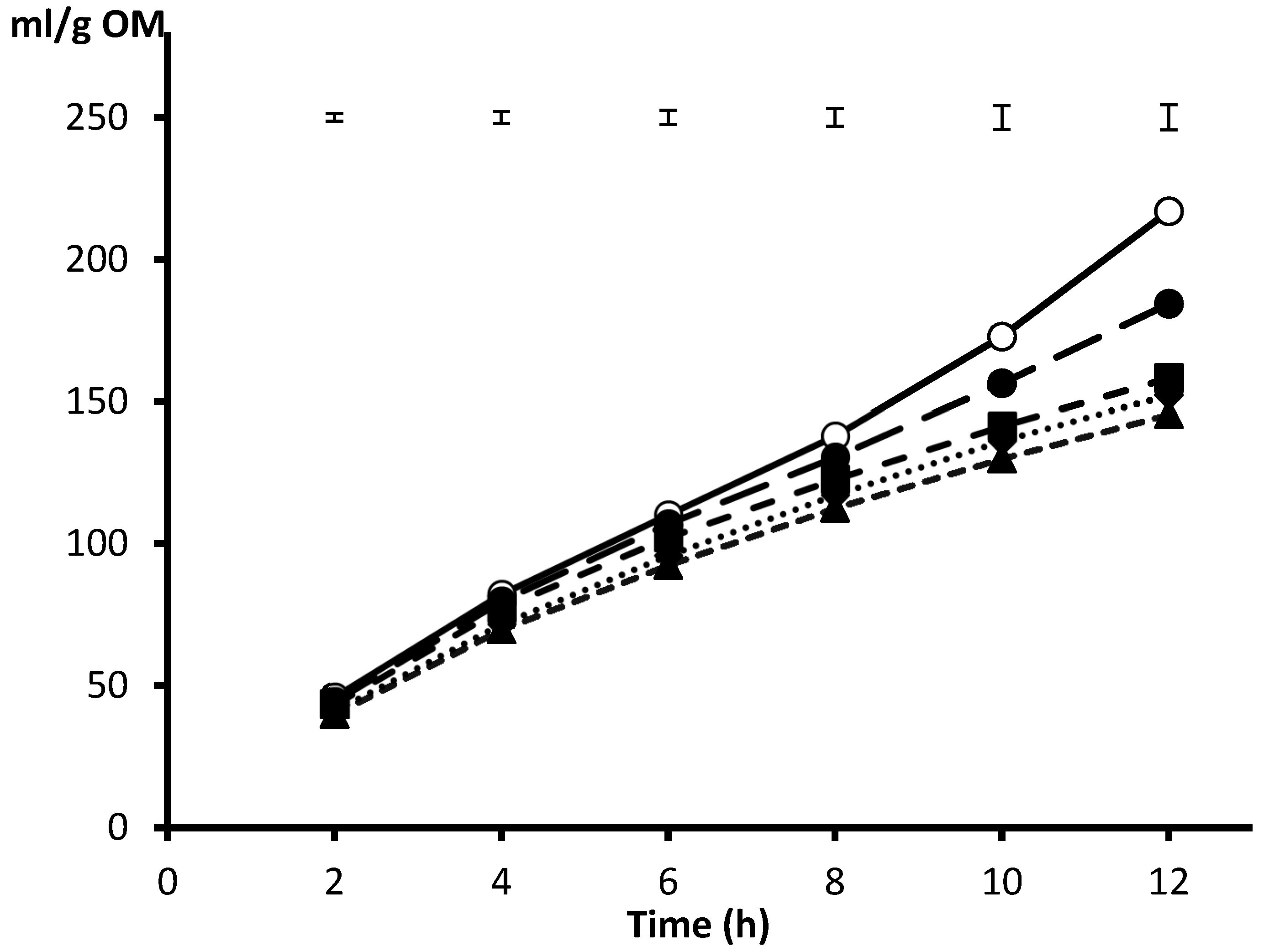
3. Adapting the In Vitro Model to High Concentrate Feeding Conditions
4. Origin of Gas and Partition into Direct and Indirect Gas
5. Adaptation of Gas Production to a Semicontinuous System for Estimating Fermentation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deaville, E.R.; Owen, E.; Adesogan, A.T.; Rymer, C.; Huntington, J.A.; Lawrence, T.L.J. In Vitro Techniques for Measuring Nutrient Supply to Ruminants; BSAS Occasional Publication: Edinburgh, UK, 1998; Volume 22. [Google Scholar]
- Rymer, C.; Huntington, J.A.; Williams, B.A.; Givens, D.I. In vitro cumulative gas production techniques: History, methodological considerations and challenges. Anim. Feed Sci. Technol. 2005, 123, 9–30. [Google Scholar] [CrossRef]
- Getachew, G.; Blümmel, M.; Makkar, H.P.S.; Becker, K. In vitro gas measuring techniques for assessment of nutritional quality of feeds: A review. Anim. Feed Sci. Technol. 1998, 72, 261–281. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energy feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Kim, E.T.; Min, K.S.; Kim, C.H.; Moon, Y.H.; Kim, S.C.; Lee, S.S. The effect of plant extracts on in-vitro ruminal fermentation, methanogenesis and methane-related microbes in the rumen. Asian Australas. J. Anim. Sci. 2013, 26, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Gosselink, J.M.J.; Dulphy, J.P.; Poncet, C.; Jailler, M.; Tamminga, S.; Cone, J.W. Prediction of forage digestibility in ruminants using in situ and in vitro techniques. Anim. Feed Sci. Technol. 2004, 115, 227–246. [Google Scholar] [CrossRef]
- Cone, J.W.; Rodrigues, M.A.M.; Guedes, C.M.; Blok, M.C. Comparison of protein fermentation characteristics in rumen fluid determined with the gas production technique and the nylon bag technique. Anim. Feed Sci. Technol. 2009, 153, 28–38. [Google Scholar] [CrossRef]
- Yáñez-Ruiz, D.R.; Bannink, A.; Dijkstra, J.; Kebreab, E.; Morgavi, D.P.; O’Kiely, P.; Reynolds, C.K.; Schwarm, A.; Shingfield, K.J.; Yu, Z.; et al. Design, implementation and interpretation of in vitro batch culture experiments to assess enteric methane mitigation in ruminants—A review. Anim. Feed Sci. Technol. 2016, 216, 1–18. [Google Scholar] [CrossRef]
- Bodas, R.; López, S.; Fernández, M.; García-González, R.; Rodríguez, A.B.; Wallace, R.J.; González, J.S. In vitro screening of the potential of numerous plant species as antimethanogenic feed additives for ruminants. Anim. Feed Sci. Technol. 2008, 145, 245–258. [Google Scholar] [CrossRef]
- Durmic, Z.; Hutton, P.; Revell, D.K.; Emms, J.; Hughes, S.; Vercoe, P.E. In vitro fermentative traits of Australian woody perennial plant species that may be considered as potential sources of feed for grazing ruminants. Anim. Feed Sci. Technol. 2010, 160, 98–109. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of digestibility and metabolizable energy content of ruminant feedstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 193, 217–225. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Pell, A.N.; Schofield, P. Computerised monitoring of gas production to measure forage digestion. J. Dairy Sci. 1993, 76, 1063–1073. [Google Scholar] [CrossRef]
- Cone, J.W.; van Gelder, A.H.; Visscher, G.J.W.; Oudshoorn, L. Influence of rumen fluid and substrate concentration on rumen fermentation kinetics measured with a fully automated time related gas production apparatus. Anim. Feed Sci. Technol. 1996, 61, 113–128. [Google Scholar] [CrossRef]
- Davies, Z.S.; Mason, D.; Brooks, A.E.; Griffith, G.W.; Merry, R.J.; Theodorou, M.K. An automated system for measuring gas production from forages inoculated with rumen fluid and its use in determining the effect of enzymes on grass silage. Anim. Feed Sci. Technol. 2000, 83, 205–221. [Google Scholar] [CrossRef]
- Cornou, C.; Drejer-Storm, I.M.L.; Hindrichsen, I.K.; Worgan, H.; Bakewell, E.; Yáñez-Ruiz, D.R.; Abecia, L.; Tagliapetra, F.; Cattani, M.; Ritz, C.; et al. A ring test of a wireless in vitro gas production system. Anim. Prod. Sci. 2013, 53, 585–592. [Google Scholar] [CrossRef]
- Fondevila, M.; Perez-Espés, B. A new in vitro system to study the effect of liquid phase turnover and pH on microbial fermentation of concentrate diets for ruminants. Anim. Feed Sci. Technol. 2008, 144, 196–211. [Google Scholar] [CrossRef]
- Blümmel, M.; Makkar, H.P.S.; Becker, K. In vitro gas production: A technique revisited. J. Anim. Physiol. Anim Nutr. 1997, 77, 24–34. [Google Scholar] [CrossRef]
- Jouany, J.P.; Lassalas, B. Gas pressure inside a rumen in vitro system stimulates the use of hydrogen. In Proceedings of the 3rd Joint RRI-INRA Gastrointestinal Tract Microbiology Symposium Beyond Antimicrobials: The Future of Gut Microbiology, Aberdeen, UK, 12–15 June 2002. [Google Scholar]
- Tagliapietra, F.; Cattani, M.; Bailoni, L.; Schiavon, S. In vitro rumen fermentation: Effect of headspace pressure on the gas production kinetics of cornmeal and meadow hay. Anim. Feed Sci. Technol. 2010, 158, 197–201. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Lowman, R.S.; Davies, Z.S.; Cuddeford, D.; Owen, E. Principles of techniques that rely on gas measurements in ruminant nutrition. In In Vitro Techniques for Measuring Nutrient Supply To Ruminants; Deaville, E.R., Owen, E., Adesogan, A.T., Rymer, C., Huntington, J.A., Lawrence, T.L.J., Eds.; BSAS Occasional Publication: Edinburgh, UK, 1998; Volume 22, pp. 55–63. [Google Scholar]
- Hidayat; Hillman, K.; Newbold, C.J.; Stewart, C.S. The contribution of bacteria and protozoa to ruminal forage fermentation, as determined by microbial gas production. Anim. Feed Sci. Technol. 1993, 42, 111–118. [Google Scholar] [CrossRef]
- Mould, F.L.; Morgan, R.; Kliem, K.E.; Krystallidou, E. A review and simplification of the in vitro incubation medium. Anim. Feed Sci. Technol. 2005, 123, 155–172. [Google Scholar] [CrossRef]
- Amanzougarene, Z.; Yuste, S.; Fondevila, M. Fermentation pattern of several carbohydrate sources incubated in an in vitro semicontinuous system with inocula from ruminants given either forage or concentrate-based diets. Animals 2020, 10, 261. [Google Scholar] [CrossRef]
- Martínez, M.E.; Ranilla, M.J.; Tejido, M.L.; Ramos, S.; Carro, M.D. The effect of the diet fed to donor sheep on in vitro methane production and ruminal fermentation of diets of variable composition. Anim. Feed Sci. Technol. 2010, 158, 126–135. [Google Scholar] [CrossRef]
- Amanzougarene, Z.; Yuste, S.; Castrillo, C.; Fondevila, M. In vitro acidification potential and fermentation pattern of different cereal grains incubated with inoculum from animals given forage or concentrate-based diets. Anim. Prod. Sci. 2018, 58, 2300–2307. [Google Scholar] [CrossRef]
- Counotte, G.H.M.; Van’t Klooster, A.T.; van der Kuilen, J.; Prins, R.A. An analysis of the buffer system in the rumen of dairy cattle. J. Anim. Sci. 1979, 49, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Erdman, R.A. Dietary buffering requirements of the lactating dairy cow: A review. J. Dairy Sci. 1988, 71, 3246–3266. [Google Scholar] [CrossRef]
- Goering, H.K.; van Soest, P.J. Forage fiber analyses (apparatus, reagents, procedures and some applications). In USDA Agriculture Handbook; USDA-ARS: Washington, DC, USA, 1970; Volume 379. [Google Scholar]
- McDougall, E.J. Studies on ruminant saliva, 1. The composition and output of sheep’s saliva. Biochem. J. 1948, 43, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Beuvink, J.M.; Spoelstra, S.F. Interactions between substrate, fermentation end-products, buffering systems and gas production upon fermentation of different carbohydrates by mixed rumen microorganisms in vitro. Appl. Microbiol. Technol. 1992, 37, 505–509. [Google Scholar] [CrossRef]
- Kohn, R.A.; Dunlap, T.F. Calculation of the buffering capacity of bicarbonate in the rumen and in vitro. J. Anim. Sci. 1998, 76, 1702–1709. [Google Scholar] [CrossRef]
- Huntington, J.A.; Rymer, C.; Givens, D.I. The effect of host diet on the gas production profile of hay and high-temperature dried grass. Anim. Sci. 1998, 67, 59–64. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Titgemeyer, E.C. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 2007, 90, E17–E38. [Google Scholar] [CrossRef]
- Getachew, G.; Crovetto, G.M.; Fondevila, M.; Krishnamoorthy, U.; Singh, B.; Spanghero, M.; Steingass, H.; Robinson, P.H.; Kailas, M.M. Laboratory variation of 24 h in vitro gas production and estimated metabolizable energy values of ruminant feeds. Anim. Feed Sci. Technol. 2002, 102, 169–180. [Google Scholar] [CrossRef]
- Cardozo, P.; Calsamiglia, S.; Ferret, A.; Kamel, C. Screening for the effects of natural plant extracts at different pH on in vitro rumen microbial fermentation of a high-concentrate diet for beef cattle. J. Anim. Sci. 2005, 83, 2572–2579. [Google Scholar] [CrossRef] [PubMed]
- Mateos, I.; Ranilla, M.J.; Tejido, M.L.; Saro, C.; Kamel, C.; Carro, M.D. The influence of diet type (dairy versus intensive fattening) on the effectiveness of garlic oil and cinnamaldehyde to manipulate in vitro ruminal fermentation and methane production. Anim. Prod. Sci. 2012, 53, 299–307. [Google Scholar] [CrossRef]
- Bertipaglia, L.M.A.; Fondevila, M.; van Laar, H.; Castrillo, C. Effect of pelleting and pellet size of a concentrate for intensively reared beef cattle on in vitro fermentation by two different approaches. Anim. Feed Sci. Technol. 2010, 159, 88–95. [Google Scholar] [CrossRef]
- Opatpatanakit, Y.; Kellaway, R.C.; Lean, I.J.; Annison, G.; Kirby, A. Microbial fermentation of cereal grains in vitro. Aust. J. Agric. Res. 1994, 45, 1247–1263. [Google Scholar] [CrossRef]
- Amanzougarene, Z.; Fondevila, M. Fitting of pH conditions for the study of concentrate feeds fermentation by the in vitro gas production technique. Anim. Prod. Sci. 2018, 58, 1751–1757. [Google Scholar] [CrossRef]
- Chai, W.Z.; van Gelder, A.H.; Cone, J.W. Relationship between gas production and starch degradation in fed samples. Anim. Feed Sci. Technol. 2004, 114, 195–204. [Google Scholar] [CrossRef]
- Lanzas, C.; Fox, D.G.; Pell, A.N. Digestion kinetics of dried cereal grains. Anim. Feed Sci. Technol. 2007, 136, 265–280. [Google Scholar] [CrossRef]
- Tahir, M.N.; Hetta, M.; Larsen, M.; Lund, P.; Huhtanen, P. In vitro estimations of the rate and extent of ruminal digestion of starch rich feed fractions compared to in vivo data. Anim. Feed Sci. Technol. 2013, 179, 36–45. [Google Scholar] [CrossRef]
- Hoover, W.H.; Crooker, B.A.; Sniffen, C.J. Effects of differential solid–liquid removal rates on protozoa numbers in continuous cultures of rumen contents. J. Anim. Sci. 1976, 43, 528–534. [Google Scholar] [CrossRef]
- Czerkawski, J.W.; Breckenridge, G. Design and development of a long-term rumen simulation technique (Rusitec). Br. J. Nutr. 1977, 38, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Amanzougarene, Z.; Yuste, S.; de Vega, A.; Fondevila, M. Differences in nutritional characteristics of three varieties of sorghum grain determine their in vitro rumen fermentation. Span. J. Agric. Res. 2018, 16. [Google Scholar] [CrossRef]
- Kliem, K.; Morgan, R.; Mould, F.L. The effect of Lactuca sativa and Urtica dioica on in vitro acidosis. Proc. Br. Soc. Anim. Sci. 2005, 226. [Google Scholar] [CrossRef]
- Rymer, C.; Moss, A.R.; Deaville, E.R.; Givens, D.I. Factors affecting the amount of indirect gas produced by the in vitro gas production technique. In In Vitro Techniques for Measuring Nutrient Supply to Ruminants; Deaville, E.R., Owen, E., Adesogen, A.T., Rymer, C., Huntington, J.A., Lawrence, T.L.J., Eds.; BSAS: Edinburgh, UK, 1998; Volume 22, pp. 89–91. [Google Scholar]
- Amanzougarene, Z.; Yuste, S.; de Vega, A.; Fondevila, M. In vitro fermentation pattern and acidification, potential of different sources of carbohydrates by ruminants giving high concentrate diets. Span. J. Agric. Res. 2017, 15, e0602. [Google Scholar] [CrossRef]
- Kim, S.H.; Mamuad, L.L.; Kim, E.J.; Sung, H.G.; Bae, G.S.; Cho, K.K.; Lee, C.; Lee, S.S. Effect of different concentrate diet levels on rumen fluid inoculum used for determination of in vitro rumen fermentation, methane concentration, and methanogen abundance and diversity. Ital. J. Anim. Sci. 2018, 17, 359–367. [Google Scholar] [CrossRef]
- Broudiscou, L.P.; Offner, A.; Sauvant, D. Effects of inoculum source, pH, redox potential and headspace dihydrogen on rumen in vitro fermentation yields. Animal 2014, 8, 931–937. [Google Scholar] [CrossRef]
- Nagadi, S.; Herrero, M.; Jessop, N.S. The influence of diet of the donor animal on the initial bacterial concentration of ruminal fluid and in vitro gas production degradability parameters. Anim. Feed Sci. Technol. 2000, 87, 231–239. [Google Scholar] [CrossRef]
- Raab, L.; Cafantaris, B.; Jilg, T.; Menke, K.H. Rumen protein degradation and biosynthesis. A new method for determination of protein degradation in rumen fluid in vitro. Br. J. Nutr. 1983, 50, 569–582. [Google Scholar] [CrossRef]
- González Ronquillo, M.; Fondevila, M.; Barrios-Urdaneta, A.; Newman, Y. In vitro gas production from buffel grass (Cenchrus ciliaris L.) fermentation in relation to the cutting interval, the level of nitrogen fertilization and the season of growth. Anim. Feed Sci. Technol. 1998, 72, 19–32. [Google Scholar] [CrossRef]
- Cone, J.W.; van Gelder, A.H. Influence of protein fermentation on gas production profiles. Anim. Feed Sci. Technol. 1999, 76, 251–264. [Google Scholar] [CrossRef]
- Wu, Z.; Palmquist, D.L. Synthesis and biohydrogenation of fatty acids by ruminal microorganisms in vitro. J. Dairy Sci. 1991, 74, 3035–3046. [Google Scholar] [CrossRef]
- Moore, J.A.; Swingle, R.S.; Hale, W.H. Effect of whole cottonseed, cottonseed oil or animal fat on digestibility of wheat straw diets by steers. J. Anim. Sci. 1986, 63, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Tesfa, A.T. Effect of rapeseed oil on rumen enzyme activity and in sacco degradation of grass silage. Anim. Feed Sci. Technol. 1992, 36, 77–89. [Google Scholar] [CrossRef]
- Blümmel, M.; Orskov, E.R. Comparison of in vitro gas production and nylon bag degradability of roughages in predicting feed intake in cattle. Anim. Feed Sci. Technol. 1993, 40, 109–119. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Blümmel, M.; Becker, K. Formation of complexes between poly vinyl pyrrolidones or polyethylene glycols and their implication in gas production and true digestibility in vitro techniques. Br. J. Nutr. 1995, 73, 897–913. [Google Scholar] [CrossRef]
- Wolin, M.J. A theoretical rumen fermentation balance. J. Dairy Sci. 1960, 43, 1452–1459. [Google Scholar] [CrossRef]
- Hungate, R.E. The Rumen and Its Microbes; Academic Press: New York, NY, USA, 1966. [Google Scholar]
- Mauricio, R.M.; Vitti, D.M.S.S.; Mould, F.L.; Owen, E.; Dhanoa, M.S.; Theodorou, M.K. Determination of total gas production and gas profile release from a bicarbonate buffer during addition of acetic acid to a carbonate-buffered medium. In Proceedings of the British Society of Animal Science (BSAS), Scarborough, UK, 31 July 1998; p. 58. [Google Scholar] [CrossRef]
- Teather, R.M.; Sauer, F.D. A naturally compartmented rumen simulation system for the continuous culture of rumen bacteria and protozoa. J. Dairy Sci. 1988, 71, 666–673. [Google Scholar] [CrossRef]
- Muetzel, S.; Lawrence, P.; Hoffman, E.M.; Becker, K. Evaluation of stratified continuous rumen incubation system. Anim. Feed Sci. Technol. 2009, 151, 32–43. [Google Scholar] [CrossRef]
- Prates, A.; de Oliveira, J.A.; Abecia, L.; Fondevila, M. Effects of preservation procedures of rumen inoculum on in vitro microbial diversity and fermentation. Anim. Feed Sci. Technol. 2010, 155, 186–193. [Google Scholar] [CrossRef]

| Adjusted pH Value | HCO3− in Buffer (mol) | Buffer Composition (NaHCO3 + NH4HCO3, g/L) | HCO3− in Total Medium (mol/L) |
|---|---|---|---|
| 6.80 | 0.111 | 35.00 + 4.00 | 0.0238 |
| 6.50 | 0.058 | 18.30 + 1.90 | 0.0124 |
| 6.25 | 0.032 | 10.30 + 1.07 | 0.0068 |
| 6.00 | 0.018 | 5.70 + 0.60 | 0.0039 |
| 5.75 | 0.010 | 3.17 + 0.25 | 0.0021 |
| 5.50 | 0.006 | 1.91 + 0.12 | 0.0013 |
| VFA, mmol | Direct Gas, mmol | Indirect Gas, mmol | Total Gas, mmol |
|---|---|---|---|
| 2 acetate | 2 CO2 | 2 CO2 | 4 CO2 |
| 2 propionate | − | 2 CO2 | 2 CO2 |
| 1 butyrate | 2 CO2 | 1 CO2 | 3 CO2 |
| 2 lactate | − | 2 CO2 | 2 CO2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amanzougarene, Z.; Fondevila, M. Fitting of the In Vitro Gas Production Technique to the Study of High Concentrate Diets. Animals 2020, 10, 1935. https://doi.org/10.3390/ani10101935
Amanzougarene Z, Fondevila M. Fitting of the In Vitro Gas Production Technique to the Study of High Concentrate Diets. Animals. 2020; 10(10):1935. https://doi.org/10.3390/ani10101935
Chicago/Turabian StyleAmanzougarene, Zahia, and Manuel Fondevila. 2020. "Fitting of the In Vitro Gas Production Technique to the Study of High Concentrate Diets" Animals 10, no. 10: 1935. https://doi.org/10.3390/ani10101935
APA StyleAmanzougarene, Z., & Fondevila, M. (2020). Fitting of the In Vitro Gas Production Technique to the Study of High Concentrate Diets. Animals, 10(10), 1935. https://doi.org/10.3390/ani10101935





