Molecular Characterization and Developing a Point-of-Need Molecular Test for Diagnosis of Bovine Papillomavirus (BPV) Type 1 in Cattle from Egypt
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals
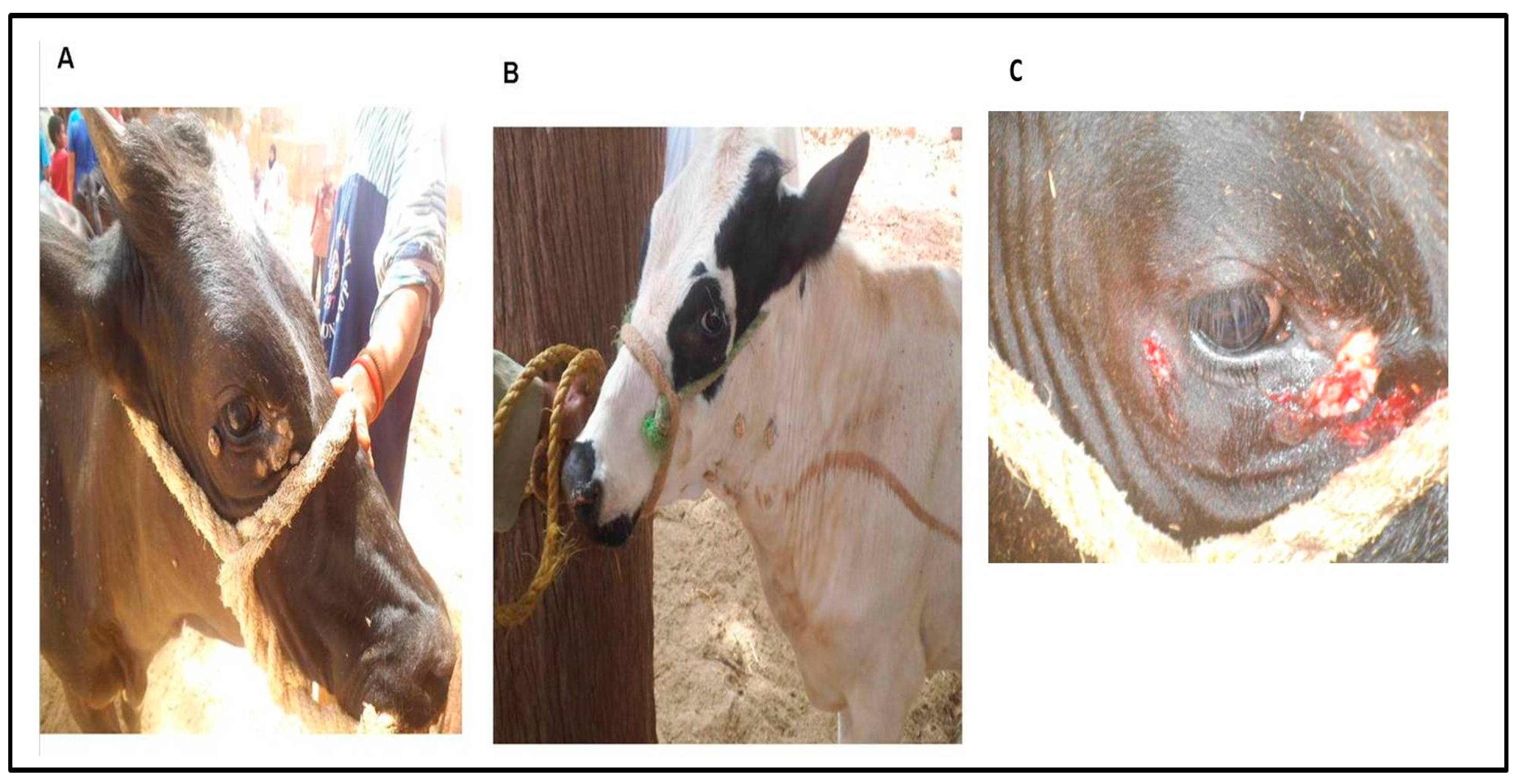
2.3. Surgical Treatment of the Cutaneous Warts and Sampling
2.4. Skin Wart Sample Preparations
2.5. Polymerase Chain Reaction (PCR)
2.6. PCR Product Sequencing and Analysis
2.7. Developing Recombinase Polymerase Amplification-Nucleic Acid Lateral Flow Immunoassays (RPA-NALF) for Point-of-Need Molecular Identification of BPV
2.7.1. Oligonucleotide Primers for RPA
2.7.2. BPV-1 RPA Amplification
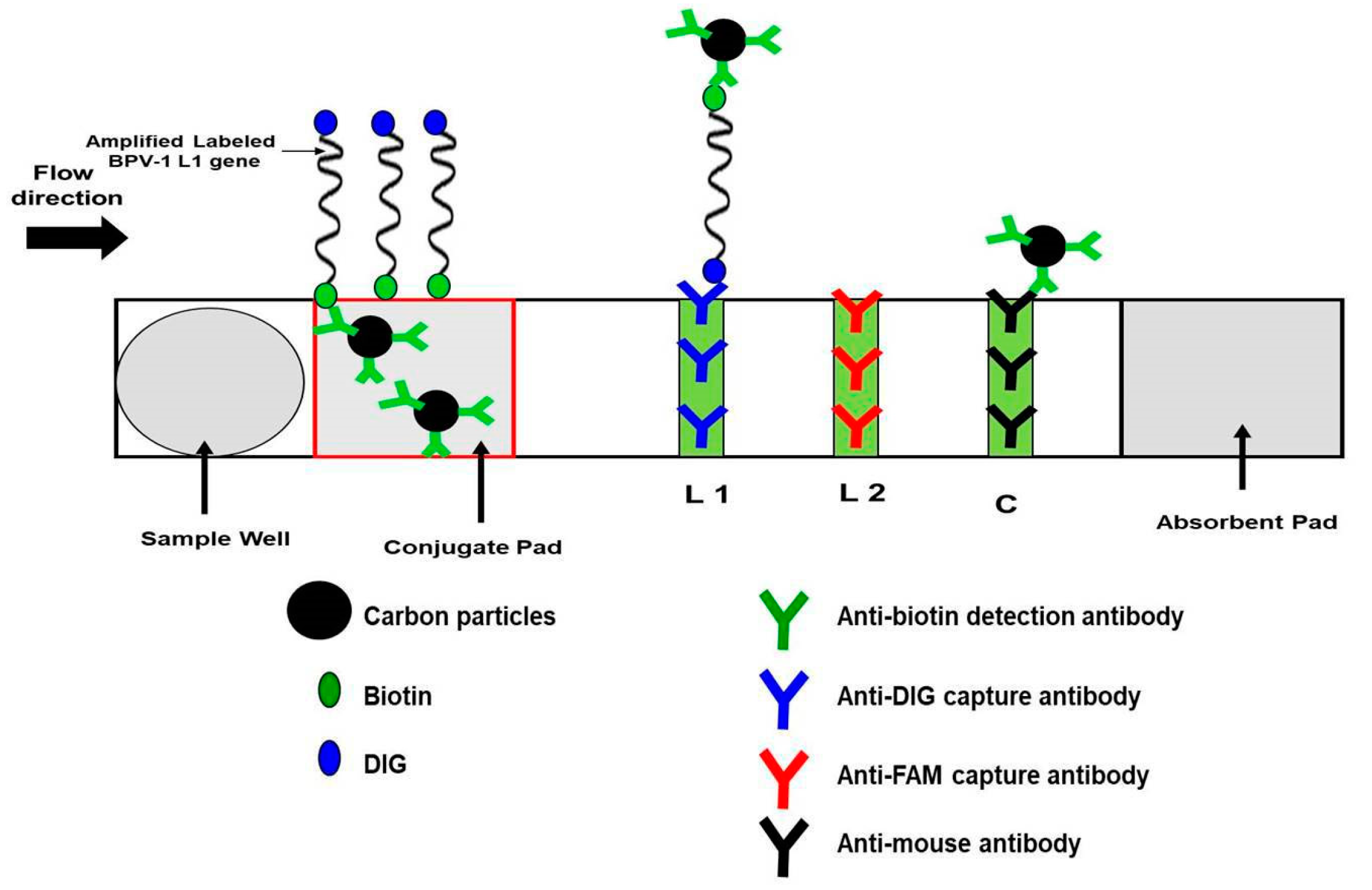
2.7.3. Visualization with Nucleic Acid Lateral Flow (NALF) Strip
2.7.4. The Limit of Detection (LOD)
2.7.5. BPV RPA-NALF Immunoassay Detection Performance
3. Results
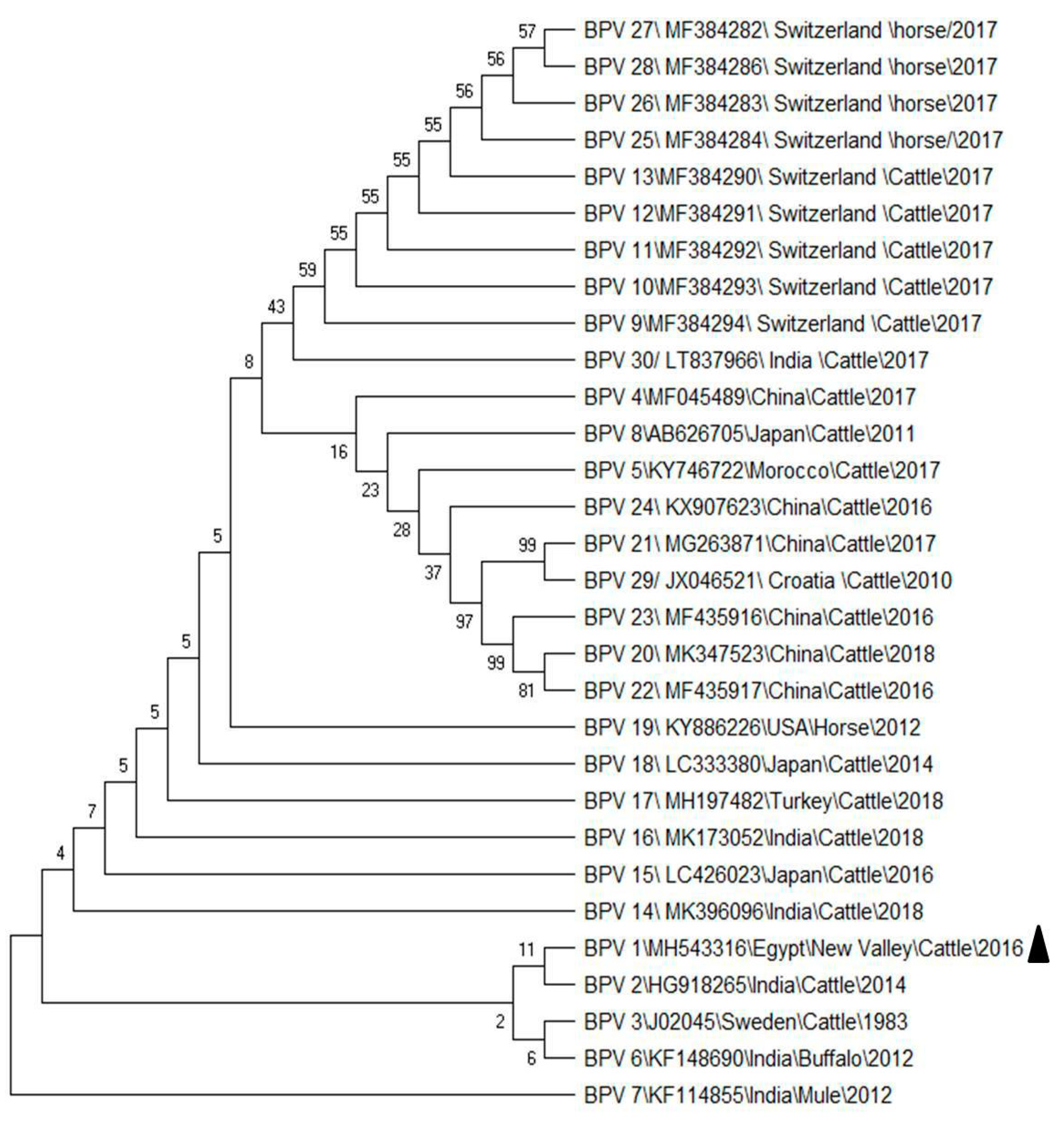
3.1. PCR Detection and Sequence Analysis
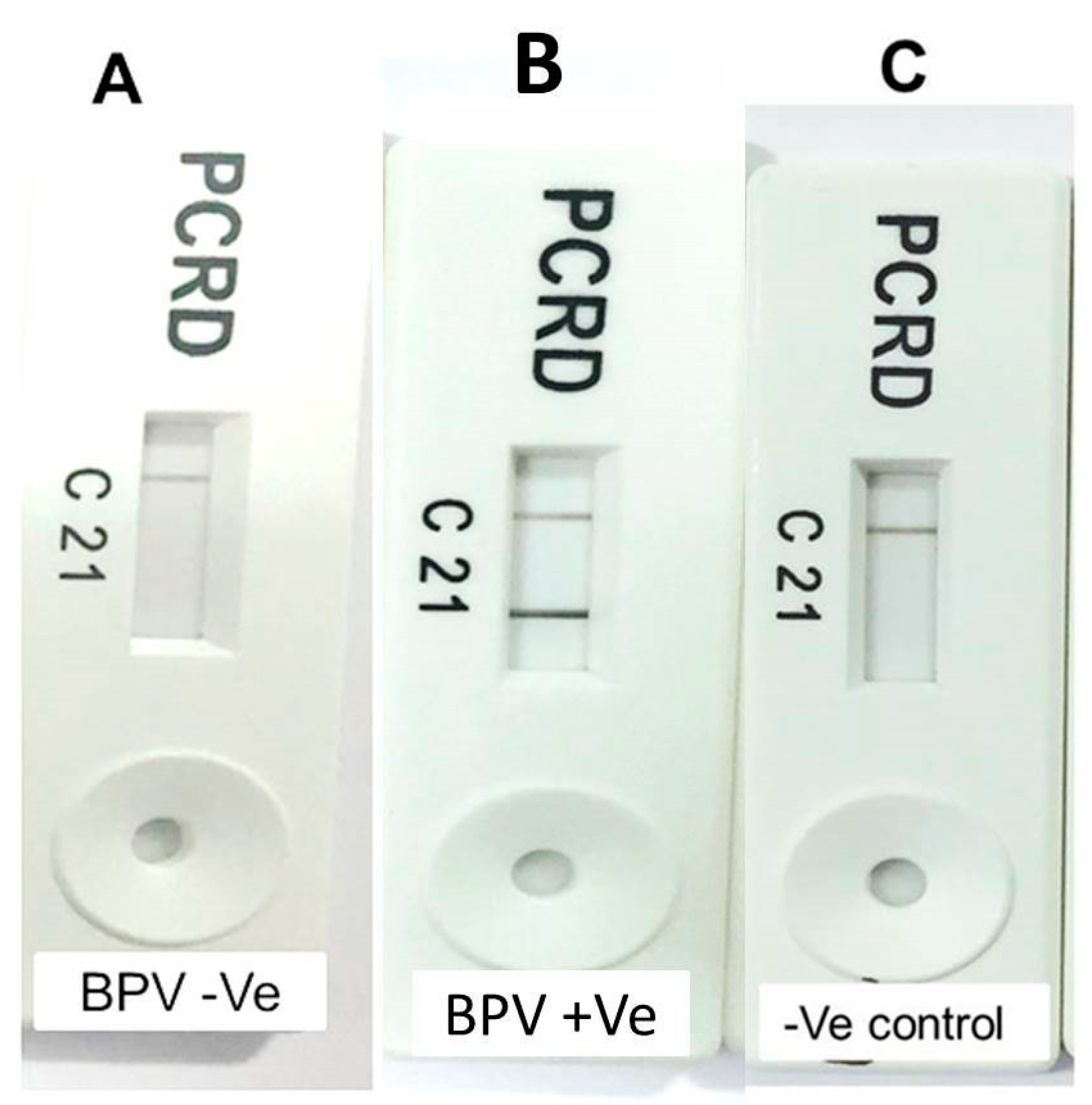
3.2. RPA-NALF Assay Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lunardi, M.; Alfieri, A.A.; Otonel, R.A.A.; Alfieri, A.F. Bovine Papillomaviruses—Taxonomy and Genetic. Features. In Current Issues in Molecular Virology: Viral Genetics and Biotechnological Application; Intechopen: London, UK, 2013; p. 113. [Google Scholar]
- Howley, P.M.; Lowy, D.R. Papillomaviruses. In Fields’ Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2007; pp. 2300–2354. [Google Scholar]
- Campo, M. Papillomas and cancer in cattle. Cancer Surv. 1987, 6, 39–54. [Google Scholar] [PubMed]
- Egawa, K. Eccrine-centred distribution of human papillomavirus 63 infection in the epidermis of the plantar skin. Br. J. Dermatol. 2005, 152, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Radostits, O.; Gay, C.; Hinchcliff, K.; Constable, P.; Jacobs, D.; Ikede, B.; McKenzie, R.; Colwell, D.; Osweiler, G.; Bildfell, R. Veteriary Medicine: A Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses, 10th ed.; Saunders Ltd.: Philadelphia, PA, USA, 2007. [Google Scholar]
- McBride, A.A. Mechanisms and strategies of papillomavirus replication. Biol. Chem. 2017, 398, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Campo, M.S. Papillomavirus and disease in humans and animals. Vet. Comp. Oncol. 2003, 1, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Bernard, H.-U.; Burk, R.D.; Chen, Z.; Van Doorslaer, K.; Zur Hausen, H.; de Villiers, E.-M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010, 401, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Dempsey, D.M.; Dutilh, B.E.; Harrach, B.; Harrison, R.L.; Hendrickson, R.C.; Junglen, S.; et al. Changes to virus taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2019). Arch. Virol. 2019, 164, 2417–2429. [Google Scholar] [CrossRef]
- Hamad, M.A.; Al-Shammari, A.M.; Odisho, S.M.; Yaseen, N.Y. Molecular epidemiology of bovine papillomatosis and identification of three genotypes in central Iraq. Intervirology 2017, 60, 156–164. [Google Scholar] [CrossRef]
- Borzacchiello, G.; Roperto, F. Bovine papillomaviruses, papillomas and cancer in cattle. Vet. Res. 2008, 39, 1. [Google Scholar] [CrossRef]
- Munday, J.S.; Thomson, N.; Dunowska, M.; Knight, C.G.; Laurie, R.E.; Hills, S. Genomic characterisation of the feline sarcoid-associated papillomavirus and proposed classification as Bos taurus papillomavirus type 14. Vet. Microbiol. 2015, 177, 289–295. [Google Scholar] [CrossRef]
- Daudt, C.; Da Silva, F.; Lunardi, M.; Alves, C.; Weber, M.; Cibulski, S.; Alfieri, A.; Alfieri, A.; Canal, C. Papillomaviruses in ruminants: An update. Transbound. Emerg. Dis. 2018, 65, 1381–1395. [Google Scholar] [CrossRef]
- Corteggio, A.; Altamura, G.; Roperto, F.; Borzacchiello, G. Bovine papillomavirus E5 and E7 oncoproteins in naturally occurring tumors: Are two better than one? Infect. Agent Cancer 2013, 8, 1. [Google Scholar] [CrossRef]
- Lunardi, M.; de Alcântara, B.K.; Otonel, R.A.A.; Rodrigues, W.B.; Alfieri, A.F.; Alfieri, A.A. Bovine papillomavirus type 13 DNA in equine sarcoids. J. Clin. Microbiol. 2013, 51, 2167–2171. [Google Scholar] [CrossRef] [PubMed]
- Bocaneti, F.; Altamura, G.; Corteggio, A.; Velescu, E.; Roperto, F.; Borzacchiello, G. Bovine papillomavirus: New insights into an old disease. Transbound. Emerg. Dis. 2016, 63, 14–23. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, N.J.; Dubovi, E.J. (Eds.) Chapter 11—Papillomaviridae and Polyomaviridae. In Fenner’s Veterinary Virology (Fourth Edition); Academic Press: San Diego, CA, USA, 2011; pp. 213–223. [Google Scholar]
- Salib, F.A.; Farghali, H.A. Clinical, epidemiological and therapeutic studies on Bovine Papillomatosis in Northern Oases, Egypt in 2008. Vet. World 2011, 4, 53. [Google Scholar]
- Ata, E.B.; Mahmoud, M.A.E.; Madboli, A.A. Molecular detection and immunopathological examination of Deltapapillomavirus 4 in skin and udder of Egyptian cattle. Vet. World 2018, 11, 915–920. [Google Scholar] [CrossRef]
- Özsoy, Ş.Y.; Özyıldız, Z.; Güzel, M. Clinical, pathological and immunohistochemical findings of bovine cutaneous papillomatosis. Ankara Üniv. Vet. Fak. Derg. 2011, 58, 161–165. [Google Scholar]
- Silva, M.; Pontes, N.; Da Silva, K.; Guerra, M.; Freitas, A. Detection of bovine papillomavirus type 2 DNA in commercial frozen semen of bulls (Bos taurus). Anim. Reprod. Sci. 2011, 129, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Favre, M.; Breitburd, F.; Croissant, O.; Orth, G. Hemagglutinating activity of bovine papilloma virus. Virology 1974, 60, 572–578. [Google Scholar] [CrossRef]
- Kirnbauer, R.; Chandrachud, L.; O’neil, B.; Wagner, E.; Grindlay, G.; Armstrong, A.; McGarvie, G.; Schiller, J.; Lowy, D.; Campo, M. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology 1996, 219, 37–44. [Google Scholar] [CrossRef]
- Pathania, S.; Dhama, K.; Saikumar, G.; Shahi, S.; Somvanshi, R. Detection and quantification of bovine papilloma virus type 2 (BPV-2) by real-time PCR in urine and urinary bladder lesions in enzootic bovine haematuria (EBH)-affected cows. Transbound. Emerg. Dis. 2012, 59, 79–84. [Google Scholar] [CrossRef]
- De Paz, H.; Brotons, P.; Muñoz-Almagro, C. Molecular isothermal techniques for combating infectious diseases: Towards low-cost point-of-care diagnostics. Expert Rev. Mol. Diagn. 2014, 14, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; Armes, N.A. DNA detection using recombination proteins. PLoS Biol. 2006, 4, e204. [Google Scholar] [CrossRef]
- St John, A.; Price, C.P. Existing and emerging technologies for point-of-care testing. Clin. Biochem. Rev. 2014, 35, 155. [Google Scholar] [PubMed]
- El-Tholoth, M.; Branavan, M.; Naveenathayalan, A.; Balachandran, W. Recombinase polymerase amplification–nucleic acid lateral flow immunoassays for Newcastle disease virus and infectious bronchitis virus detection. Mol. Biol. Rep. 2019, 46, 6391–6397. [Google Scholar] [CrossRef] [PubMed]
- Euler, M.; Wang, Y.; Otto, P.; Tomaso, H.; Escudero, R.; Anda, P.; Hufert, F.T.; Weidmann, M. Recombinase polymerase amplification assay for rapid detection of Francisella tularensis. J. Clin. Microbiol. 2012, 50, 2234–2238. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.H.A.; McCloskey, C.; Tong, Y.; Hu, L.; You, Q.; Kelly, C.P.; Kong, H.; Tang, Y.-W.; Tang, W. Application of isothermal helicase-dependent amplification with a disposable detection device in a simple sensitive stool test for toxigenic Clostridium difficile. J. Mol. Diagn. 2008, 10, 452–458. [Google Scholar] [CrossRef]
- Vincent, M.; Xu, Y.; Kong, H. Helicase-dependent isothermal DNA amplification. EMBO Rep. 2004, 5, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, P.P.; Zhang, Y.H.; Tian, K.Y.; Bian, C.Z.; Zhao, J. Development of a reverse transcription recombinase polymerase amplification combined with lateral-flow dipstick assay for avian influenza H9N2 HA gene detection. Transbound. Emerg. Dis. 2019, 66, 546–551. [Google Scholar] [CrossRef]
- Yaguiu, A.; de Carvalho, C.; de Freitas, A.C.; Góes, L.G.B.; Dagli, M.L.Z.; Birgel Jr, E.H.; Beçak, W.; dos Santos, R.d.C.S. Papillomatosis in cattle: In situ detection of bovine papillomavirus DNA sequences in reproductive tissues. J. Morphol. Sci. 2017, 23, 0-0. [Google Scholar]
- Kumar, P.; Nagarajan, N.; Saikumar, G.; Arya, R.; Somvanshi, R. Detection of bovine papilloma viruses in wart-like lesions of upper gastrointestinal tract of cattle and buffaloes. Transbound. Emerg. Dis. 2015, 62, 264–271. [Google Scholar] [CrossRef]
- Viljoen, G.J.; Nel, L.H.; Crowther, J.R. Molecular Diagnostic PCR Handbook; Springer Science & Business Media: Berlin, Germany, 2005. [Google Scholar]
- Shalaby, M.A.; El-Deeb, A.; El-Tholoth, M.; Hoffmann, D.; Czerny, C.-P.; Hufert, F.T.; Weidmann, M.; Abd El Wahed, A. Recombinase polymerase amplification assay for rapid detection of lumpy skin disease virus. BMC Vet. Res. 2016, 12, 244. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.d.; Freitas, A.C.d.; Brunner, O.; Góes, L.G.B.; Cavalcante, A.Y.; Beçak, W.; Santos, R.d.C.S.d. Bovine papillomavirus type 2 in reproductive tract and gametes of slaughtered bovine females. Braz. J. Microbiol. 2003, 34, 82–84. [Google Scholar] [CrossRef]
- Carvalho, R.; Sakata, S.; Giovanni, D.; Mori, E.; Brandão, P.; Richtzenhain, L.; Pozzi, C.; Arcaro, J.; Miranda, M.; Mazzuchelli-de-Souza, J. Bovine papillomavirus in Brazil: Detection of coinfection of unusual types by a PCR-RFLP method. Biomed. Res. Int. 2013, 2013, 270898. [Google Scholar] [CrossRef] [PubMed]
- Catroxo, M.; Martins, A.; Petrella, S.; Souza, F.; Nastari, B. Ultrastructural Study of Bovine Papillomavirus During Outbreaks in Brazil. Int. J. Morphol. 2013, 31, 777–784. [Google Scholar] [CrossRef]
- Campo, M.S. Bovine Papillomavirus: Old System, New Lessons? Caister Academic Press: Norfolk, UK, 2006. [Google Scholar]
- Dos Santos, R.S.; Lindsey, C.J.; Ferraz, O.P.; Pinto, J.R.; Mirandola, R.S.; Benesi, F.J.; Birgel, E.H.; Pereira, C.; Be, W. Bovine papillomavirus transmission and chromosomal aberrations: An experimental model. J. Gen. Virol. 1998, 79, 2127–2135. [Google Scholar] [CrossRef]
- Freitas, A.C.d.; Carvalho, C.d.; Brunner, O.; Birgel-Junior, E.H.; Dellalibera, A.M.M.P.; Benesi, F.J.; Gregory, L.; Beçak, W.; Santos, R.d.C.S.d. Viral DNA sequences in peripheral blood and vertical transmission of the virus: A discussion about BPV-1. Braz. J. Microbiol. 2003, 34, 76–78. [Google Scholar] [CrossRef]
- Araldi, R.; Melo, T.; Diniz, N.; Mazzuchelli-de-Souza, J.; Carvalho, R.; Beçak, W.; Stocco, R. Bovine papillomavirus clastogenic effect analyzed in comet assay. Biomed. Res. Int. 2013, 2013, 630683. [Google Scholar] [CrossRef]
- Campos, S.; Melo, T.; Assaf, S.; Araldi, R.; Mazzuchelli-de-Souza, J.; Sircili, M.; Carvalho, R.; Roperto, F.; Beçak, W.; Stocco, R. Chromosome aberrations in cells infected with bovine papillomavirus: Comparing cutaneous papilloma, esophagus papilloma, and urinary bladder lesion cells. ISRN Oncol. 2013, 2013, 910849. [Google Scholar] [CrossRef]
- Dagalp, S.B.; Dogan, F.; Farzani, T.A.; Salar, S.; Bastan, A. The genetic diversity of bovine papillomaviruses (BPV) from different papillomatosis cases in dairy cows in Turkey. Arch. Virol. 2017, 162, 1507–1518. [Google Scholar] [CrossRef]
- Rojas-Anaya, E.; Cantu-Covarrubias, A.; Alvarez, J.F.; Loza-Rubio, E. Detection and phylogenetic analysis of bovine papillomavirus in cutaneous warts in cattle in Tamaulipas, Mexico. Can. J. Vet. Res. 2016, 80, 262–268. [Google Scholar]
- Olkeba, W.; Sorba, E.; Belay, A.; Deres; Gebremedhin, E. Prevalence of major skin diseases of cattle and associated risk factors around Ambo town, Ethiopia. Anim. Health Prod. 2016, 64, 355–365. [Google Scholar]
- Araldi, R.; Carvalho, R.; Melo, T.; Pessoa, N.; Sant’Ana, T.; Mazzuchelli-de-Souza, J.; Spadacci-Morena, D.; Beçak, W.; Stocco, R. Bovine papillomavirus in beef cattle: First description of BPV-12 and putative type BAPV8 in Brazil. Genet. Mol. Res. 2014, 13, 5644–5653. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.; Horst, R. Physiological changes at parturition and their relationship to metabolic disorders1, 2. J. Dairy Sci. 1997, 80, 1260–1268. [Google Scholar] [CrossRef]
- Otter, A.; Leonard, D. Fibropapillomatosis outbreak in calves. Vet. Rec. 2003, 153, 570–571. [Google Scholar] [PubMed]
- Reetha, T.; Manickam, R.; Boovalingam, P. Detection of Bovine Papillomavirus in Cutaneous Lesions by Polymerase Chain Reaction (PCR) in Cattle. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1611–1616. [Google Scholar] [CrossRef]
- Fagbohun, O. Molecular Detection Of Bovine Papilloma Viruses Associated With Cutaneous Warts In Some Breeds Of Nigerian Cattle. Int. J. Biotechnol. Biochem. 2016, 12, 123–130. [Google Scholar]
- Han, S.-H.; Park, Y.-S.; Seo, J.-P.; Kang, T.-Y. PCR-based Detection of Bovine Papillomavirus DNA from the Cutaneous Papillomas and Surrounding Environments in the Korean Native Cattle, Hanwoo. J. Vet. Clin. 2016, 33, 346. [Google Scholar] [CrossRef]
- Peng, H.; Wu, C.; Li, J.; Li, C.; Chen, Z.; Pei, Z.; Tao, L.; Gong, Y.; Pan, Y.; Bai, H.; et al. Detection and genomic characterization of Bovine papillomavirus isolated from Chinese native cattle. Transbound. Emerg. Dis. 2019, 66, 2197–2203. [Google Scholar] [CrossRef]
- Mark, D.; Haeberle, S.; Roth, G.; Von Stetten, F.; Zengerle, R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. In Microfluidics Based Microsystems; Springer: New York, NY, USA, 2010; pp. 305–376. [Google Scholar]
- Abd El Wahed, A.; Weidmann, M.; Hufert, F.T. Diagnostics-in-a-Suitcase: Development of a portable and rapid assay for the detection of the emerging avian influenza A (H7N9) virus. J. Clin. Virol. 2015, 69, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Faye, O.; Faye, O.; Soropogui, B.; Patel, P.; Abd El Wahed, A.; Loucoubar, C.; Fall, G.; Kiory, D.; Magassouba, N.F.; Keita, S. Development and deployment of a rapid recombinase polymerase amplification Ebola virus detection assay in Guinea in 2015. Eurosurveillance 2015, 20, 30053. [Google Scholar] [CrossRef]
- La Marca, A.; Capuzzo, M.; Paglia, T.; Roli, L.; Trenti, T.; Nelson, S.M. Testing for SARS-CoV-2 (COVID-19): A systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reprod. Biomed. Online 2020, 41, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.O.S.; Vilela, E.G.; Neves, M.S.; Lobato, F.C.F. Evaluation of three enzyme immunoassays and a nucleic acid amplification test for the diagnosis of Clostridium difficile-associated diarrhea at a university hospital in Brazil. Rev. Soc. Bras. Med. Trop. 2014, 47, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Macdonald, J.; von Stetten, F. A comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst 2018, 144, 31–67. [Google Scholar] [CrossRef] [PubMed]
| Farm Number | Age Range (Year) | Total Number of Animals/Farm | Sex of Examined Animals | No, Sex, and Age of Animals Showed Clinical Signs and Positive Result by BPV-1-PCR | ||
|---|---|---|---|---|---|---|
| Female | Male | |||||
| 1 | 1–4 | 15 | 10 | 5 | 0 | |
| 2 | 1–5 | 19 | 11 | 8 | 1 male (13 month) | |
| 3 | 1–3 | 13 | 9 | 4 | 0 | |
| 4 | 1–5 | 21 | 13 | 8 | 1 Female (18 months) 1 male (24 month) | |
| 5 | 1–5 | 16 | 10 | 6 | 0 | |
| 6 | 1–3 | 10 | 8 | 2 | 1 male (23 month) | |
| 7 | 1–4 | 15 | 11 | 4 | 0 | |
| 8 | 1–3 | 18 | 12 | 6 | 0 | |
| 9 | 1–3 | 12 | 8 | 4 | 0 | |
| 10 | 1–5 | 14 | 10 | 4 | 1 Female (14 month) | |
| 11 | 1–4 | 16 | 11 | 5 | 1 Female (13 month) | |
| 12 | 1–5 | 15 | 10 | 5 | 0 | |
| 13 | 1–3 | 20 | 12 | 8 | 0 | |
| 14 | 1–4 | 14 | 10 | 4 | 1 Female (20 month) | |
| 15 | 1–4 | 17 | 11 | 6 | 1 Female (15 month) | |
| 16 | 1–5 | 10 | 8 | 2 | 0 | |
| 17 | 1–4 | 19 | 12 | 7 | 1 Female (14 month) | |
| 18 | 1–5 | 19 | 13 | 6 | 0 | |
| 19 | 1–3 | 12 | 9 | 3 | 1 Female (17 month) | |
| 20 | 1–5 | 13 | 9 | 4 | 1 Female (22 month) | |
| Total (%) | 308 (100%) | 207 (67.2%) | 101 (32.8%) | 11 (3.6%) | ||
| 8 females (2.6%) | 3 males (1.0%) | |||||
| Isolate Number | Country of Isolation | Year of Identification | Host Species | Accession Number |
|---|---|---|---|---|
| BPV 1 | Egypt\New Valley Governorate (This study) | 2016 | Cattle | MH543316 |
| BPV 2 | India | 2014 | Cattle | HG918265 |
| BPV 3 | Sweden | 1983 | Cattle | J02045 |
| BPV 4 | China | 2017 | Cattle | MF045489 |
| BPV 5 | Morocco | 2017 | Cattle | KY746722 |
| BPV 6 | India | 2012 | Buffalo | KF148690 |
| BPV 7 | India | 2012 | Equine | KF114855 |
| BPV 8 | Japan | 2011 | Cattle | AB626705 |
| BPV 9 | Switzerland | 2017 | Cattle | MF384294 |
| BPV 10 | Switzerland | 2017 | Cattle | MF384293 |
| BPV 11 | Switzerland | 2017 | Cattle | MF384292 |
| BPV 12 | Switzerland | 2017 | Cattle | MF384291 |
| BPV 13 | Switzerland | 2017 | Cattle | MF384290 |
| BPV 14 | India | 2018 | Cattle | MK396096 |
| BPV 15 | Japan | 2016 | Cattle | LC426023 |
| BPV 16 | India | 2018 | Cattle | MK173052 |
| BPV 17 | Turkey | 2018 | Cattle | MH197482 |
| BPV 18 | Japan | 2014 | Cattle | LC333380 |
| BPV 19 | USA | 2012 | Equine | KY886226 |
| BPV 20 | China | 2018 | Cattle | MK347523 |
| BPV 21 | China | 2017 | Cattle | MG263871 |
| BPV 22 | China | 2016 | Cattle | MF435917 |
| BPV 23 | China | 2016 | Cattle | MF435916 |
| BPV 24 | China | 2016 | Cattle | KX907623 |
| BPV 25 | Switzerland | 2017 | Equine | MF384284 |
| BPV 26 | Switzerland | 2017 | Equine | MF384283 |
| BPV 27 | Switzerland | 2017 | Equine | MF384282 |
| BPV 28 | Switzerland | 2017 | Equine | MF384286 |
| BPV 29 | Croatia | 2010 | Cattle | JX046521 |
| BPV 30 | India | 2017 | Cattle | LT837966 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Tholoth, M.; Mauk, M.G.; Elnaker, Y.F.; Mosad, S.M.; Tahoun, A.; El-Sherif, M.W.; Lokman, M.S.; Kassab, R.B.; Abdelsadik, A.; Saleh, A.A.; et al. Molecular Characterization and Developing a Point-of-Need Molecular Test for Diagnosis of Bovine Papillomavirus (BPV) Type 1 in Cattle from Egypt. Animals 2020, 10, 1929. https://doi.org/10.3390/ani10101929
El-Tholoth M, Mauk MG, Elnaker YF, Mosad SM, Tahoun A, El-Sherif MW, Lokman MS, Kassab RB, Abdelsadik A, Saleh AA, et al. Molecular Characterization and Developing a Point-of-Need Molecular Test for Diagnosis of Bovine Papillomavirus (BPV) Type 1 in Cattle from Egypt. Animals. 2020; 10(10):1929. https://doi.org/10.3390/ani10101929
Chicago/Turabian StyleEl-Tholoth, Mohamed, Michael G. Mauk, Yasser F. Elnaker, Samah M. Mosad, Amin Tahoun, Mohamed W. El-Sherif, Maha S. Lokman, Rami B. Kassab, Ahmed Abdelsadik, Ayman A. Saleh, and et al. 2020. "Molecular Characterization and Developing a Point-of-Need Molecular Test for Diagnosis of Bovine Papillomavirus (BPV) Type 1 in Cattle from Egypt" Animals 10, no. 10: 1929. https://doi.org/10.3390/ani10101929
APA StyleEl-Tholoth, M., Mauk, M. G., Elnaker, Y. F., Mosad, S. M., Tahoun, A., El-Sherif, M. W., Lokman, M. S., Kassab, R. B., Abdelsadik, A., Saleh, A. A., & Elmahallawy, E. K. (2020). Molecular Characterization and Developing a Point-of-Need Molecular Test for Diagnosis of Bovine Papillomavirus (BPV) Type 1 in Cattle from Egypt. Animals, 10(10), 1929. https://doi.org/10.3390/ani10101929






