Morphometric Characteristics of the Spermatozoa of Blue Fox (Alopex lagopus) and Silver Fox (Vulpes vulpes)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Yij—value of feature
- µ—mean for population
- ai—effect of ith level of factor (species)
- eij—sampling error
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farstad, W. Reproduction in foxes: Current research and future challenges. Anim. Reprod. Sci. 1998, 53, 35–42. [Google Scholar] [CrossRef]
- Boue, F.; Delhomme, A.; Chaffaux, S. Reproductive management of silver foxes (Vulpes vulpes) in captivity. Theriogenology 2000, 53, 1717–1728. [Google Scholar] [CrossRef]
- Stasiak, K.; Janicki, B.; Kupcewicz, B. Biologic parameters of polar fox (Alopex lagopus L.) semen during the breeding season. Turk. J. Vet. Anim. Sci. 2010, 34, 327–331. [Google Scholar] [CrossRef]
- Maree, L.; du Plessis, S.S.; Menkveld, R.; Van der Horst, G. Morphometric dimensions of the human sperm head depend on the staining method used. Hum. Reprod. 2010, 25, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Lasiene, K.; Gedrimas, V.; Vitkus, A.; Glinskyte, S.; Lasys, V.; Valanciute, A.; Sienkiewicz, W. Evaluation of morphological criteria of sperm quality before in vitro fertilization and intracytoplasmic sperm injection. Pol. J. Vet. Sci. 2013, 16, 773–785. [Google Scholar] [CrossRef] [Green Version]
- Soler, C.; Contell, J.; Bori, L.; Sancho, M.; García-Molina, A.; Valverde, A.; Segarvall, J. Sperm kinematic, head morphometric and kinetic- morphometric subpopulations in the blue fox (Alopex lagopus). Asian J. Androl. 2017, 19, 154–159. [Google Scholar] [CrossRef]
- Jalkanen, L. Sperm abnormalities in silver fox (Vulpes vulpes) semen selected for artificial insemination. J. Reprod. Fertil. Suppl. 1993, 47, 287–290. [Google Scholar]
- Lavara, R.; Vicente, J.S.; Baselga, M. Genetic variation in head morphometry of rabbit sperm. Theriogenology 2013, 80, 313–318. [Google Scholar] [CrossRef]
- Vicente-Fiel, S.; Palacin, I.; Santolaria, P.; Yániz, J.L. A comparative study of sperm morphometric subpopulations in cattle, goat, sheep and pigs using a computer-assisted fluorescence method (CASMA-F). Anim. Reprod. Sci. 2013, 139, 182–189. [Google Scholar] [CrossRef]
- Yániz, J.L.; Capistros, S.; Vicente-Fiel, S.; Hidalgo, C.O.; Santolaria, P. A comparative study of the morphometry of sperm head components in cattle, sheep, and pigs with a computer-assisted fluorescence method. Asian J. Androl. 2016, 18, 840–843. [Google Scholar] [CrossRef]
- Casey, P.J.; Gravance, C.G.; Davis, R.O.; Chabot, D.D.; Liu, I.K. Morphometric differences in sperm head dimensions of fertile and subfertile stallions. Theriogenology 1997, 47, 575–582. [Google Scholar] [CrossRef]
- Núñez-Martínez, I.; Moran, J.M.; Peña, F.J. Do computer-assisted, morphometric-derived sperm characteristics reflect DNA status in canine spermatozoa? Reprod. Domest. Anim. 2005, 40, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Martínez, I.; Moran, J.M.; Peña, F.J. Sperm indexes obtained using computer-assisted morphometry provide a forecast of the freezability of canine sperm. Int. J. Androl. 2007, 30, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Ramon, M.; Soler, A.J.; Ortiz, J.A.; García-Álvarez, O.; Maroto-Morales, A.; Roldan, E.R.; Garde, J.J. Sperm population structure and male fertility: An intraspecific study of sperm design and velocity in red deer. Biol. Reprod. 2013, 89, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Maroto-Morales, A.; Ramon, M.; García-Álvarez, O.; Montoro, V.; Soler, A.J.; Fernández-Santos, M.R.; Roldan, E.R.; Pérez-Guzmán, M.D.; Garde, J.J. Sperm head phenotype and male fertility in ram semen. Theriogenology 2015, 84, 1536–1541. [Google Scholar] [CrossRef] [Green Version]
- Katz, D.F.; Overstreet, J.W.; Samuels, S.J.; Niswander, P.W.; Bloom, T.D.; Lewis, E.L. Morphometric analysis of spermatozoa in the assessment of human male fertility. J. Androl. 1986, 7, 203–210. [Google Scholar] [CrossRef]
- Gravance, C.G.; Liu, I.K.M.; Davis, R.O.; Hughes, J.P.; Casey, P.J. Quantification of normal head morphometry of stallion spermatozoa. J. Reprod. Fertil. 1996, 108, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Andraszek, K.; Banaszewska, D.; Szostek, M.; Wójcik, E.; Czubaszek, M.; Walczak-Jędrzejowska, R. Use of silver nitrate for the assessment of sperm measurements in selected farm and free-living animal species. Bull. Vet. Inst. Pulawy 2014, 58, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Humphries, S.; Evans, J.P.; Simmons, L.W. Sperm competition: Linking from to function. BMC Evol. Biol. 2008, 8, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzpatrick, J.L.; Garcia-Gonzalez, F.; Evans, J.P. Linking sperm length and velocity: The importance of intramale variation. Biol. Lett. 2010, 6, 797–799. [Google Scholar] [CrossRef] [Green Version]
- Yániz, J.L.; Capistros, S.; Vicente-Fiel, S.; Soler, C.; Núnez de Murga, J.; Santolaria, P. Study of nuclear and acrosomal sperm morphometry in ram using a computer-assisted sperm morphometry analysis fluorescence (CASMA-F) method. Theriogenology 2014, 82, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Falzone, N.; Huyser, C.; Becker, P.; Leszczynski, D.; Franken, D.R. The effect of pulsed 900-MHz GSM mobile phone radiation on the acrosome reaction, head morphometry and zona binding of human spermatozoa. Int. J. Androl. 2011, 34, 20–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andraszek, K.; Smalec, E. The use of silver nitrate for the identification of spermatozoon structure in selected mammals. Can. J. Anim. Sci. 2011, 91, 239–246. [Google Scholar] [CrossRef]
- Bugno-Poniewierska, M.; Pawlina, K.; Orszulak-Wolny, N.; Woźniak, B.; Wnuk, M.; Jakubczak, A.; Jeżewska-Witkowska, G. Cytogenetic characterization of the genome of interspecies hybrids (Alopex-Vulpes). Ann. Anim. Sci. 2015, 15, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Mäkinen, A. The standard karyotype of the blue fox (Alopex lagopus L.). Committee for the standard karyotype of Alopex lagopus L. Hereditas 1985, 103, 33–38. [Google Scholar]
- Mäkinen, A. The standard karyotype of the silver fox (Vulpes fulvus Desm.). Committee for the standard karyotype of Vulpes fulvus Desm. Hereditas 1985, 103, 171–176. [Google Scholar]
- Wurster-Hill, D.H.; Ward, O.G.; Davis, B.H.; Park, J.P.; Moyzis, R.K.; Meyne, J. Fragile sites, telomeric DNA sequences, B chromosomes, and DNA content in raccoon dogs, Nyctereutes procyonoides, with comparative notes on foxes, coyote, wolf, and raccoon. Cytogenet. Cell Genetics 1988, 49, 278–281. [Google Scholar] [CrossRef]
- Basheva, E.A.; Torgasheva, A.A.; Sakaeva, G.R.; Bidau, C.; Borodin, P.M. A- and B-chromosome pairing and recombination in male meiosis of the silver fox (Vulpes vulpes L., 1758, Carnivora, Canidae). Chromosome Res. 2010, 18, 689–696. [Google Scholar] [CrossRef]
- Howell, W.M.; Black, D.A. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: A 1-step method. Experentia 1980, 36, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Czubaszek, M.; Andraszek, K.; Banaszewska, D.; Walczak-Jędrzejowska, R. The effect of the staining technique on morphological and morphometric parameters of boar sperm. PLoS ONE 2019, 14, e0214243. [Google Scholar] [CrossRef] [Green Version]
- Ellis, D.J.; Shadan, S.; James, P.S.; Henderson, R.M.; Edwardson, J.M.; Hutchings, A.; Jones, R. Post-testicular development of a novel membrane structure within the equatorial segment of ram, bull, boar, and goat spermatozoa as viewed of by atomic force microscopy. J. Struct. Biol. 2002, 138, 187–198. [Google Scholar] [CrossRef]
- Bielańska-Osuchowska, Z.; Tischner, M. Mammalian Sperm—an Amazing Cell; Wydawnictwo Uniwersytetu Rolniczego w: Krakow, Poland, 2018. [Google Scholar]
- Andraszek, K.; Banaszewska, D.; Szeleszczuk, O.; Niedbała, P.; Kuchta-Gładysz, M. Comparison of the structure of chinchilla sperm isolated from semen and from the tail of the epididymis. Reprod. Domest. Anim. 2020, 55, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Banaszewska, D.; Andraszek, K.; Szostek, M.; Danielewicz, A.; Wójcik, E.; Walczak-Jędrzejowska, R. Analysis of stallion semiologic semen parameters. Med. Weter. 2015, 71, 563–567. [Google Scholar]
- Banaszewska, D.; Andraszek, K.; Zdrowowicz, E.; Danielewicz, A. The effect of selected staining techniques on stallion sperm morphometry. Livestock Sci. 2015, 175, 128–132. [Google Scholar] [CrossRef]
- Banaszewska, D.; Czubaszek, M.; Walczak-Jędrzejowska, R.; Andraszek, K. Morphometric dimensions of the stallion sperm head depending. Bull. Vet. Inst. Pulawy 2015, 59, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Andraszek, K.; Banaszewska, D.; Biesiada-Drzazga, B. The use of two staining methods for identification of spermatozoon structure in roosters. Poultry Sci. 2018, 97, 2575–2581. [Google Scholar] [CrossRef]
- Gontarz, A.; Banaszewska, D.; Gryzińska, M.; Andraszek, K. Differences in drone sperm morphometry and activity at the beginning and end of the season. Turk. J. Veter. Anim. Sci. 2016, 40, 1511–1516. [Google Scholar] [CrossRef] [Green Version]
- Świtoński, M. Study of the centric fusion in the blue fox (Alopex lagopus). Genetica 1985, 68, 65–68. [Google Scholar] [CrossRef]
- Andraszek, K.; Ceranka, M.; Gryzińska, M.; Larisch, A. Structure of nucleoli in first-order spermatocytes of selected free-living animal species. Anim. Reprod. Sci. 2015, 161, 16–22. [Google Scholar] [CrossRef]
- Gage, M.J. Mammalian sperm morphometry. Proc. Biol. Sci. 1998, 265, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Gago, C.; Perez-Sanchez, F.; Yeung, C.H.; Tablado, L.; Cooper, T.G.; Soler, C. Standardization of sampling and staining methods for the morphometric evaluation of sperm heads in the Cynomolgus monkey (Macaca fascicularis) using computer-assisted image analysis. Int. J. Androl. 1998, 21, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Kruger, T.F.; Van der Merwe, F.; Van Waart, J. The Tygerberg strict criteria: What are the clinical thresholds for in vitro fertilization, intrauterine insemination, and in vivo fertilization? In Atlas of Human Sperm Morphology Evaluation; Taylor and Francis: London, UK, 2004; pp. 13–18. [Google Scholar]
- Soler, C.; Garcia, A.; Contell, J.; Segervall, J.; Sancho, M. Kinematics and Subpopulations’ Structure Definition of Blue Fox (Alopex lagopus) Sperm Motility using the ISAS® V1 CASA System. Reprod. Dom. Anim. 2014, 49, 560–567. [Google Scholar] [CrossRef]
- World Health Organization. Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Thurston, L.M.; Watson, P.F.; Holt, W.V. Sources of variation in the morphological characteristics of sperm subpopulations assessed objectively by a novel automated sperm morphology analysis system. J. Reprod. Fertil. 1999, 117, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.J.; Saravia, F.; García-Herreros, M.; Núñez-Martínez, I.; Tapia, J.A.; Johannisson, A.; Wallgren, M.; Rodríguez-Martínez, H. Identification of sperm morphometric subpopulations in two different portions of the boar ejaculate and its relation to postthaw quality. J. Androl. 2005, 26, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Mossman, J.; Slate, J.; Humphries, S.; Birkhead, T. Sperm morphology and velocity are genetically codetermined in the zebra finch. Evolution 2009, 63, 2730–2737. [Google Scholar] [CrossRef]
- Dvorakova, K.; Moore, H.D.; Sebkova, N.; Palecek, J. Cytoskeleton localization in the sperm head prior to fertilization. Reproduction 2005, 130, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Barth, A.D.; Bowman, P.A.; Bo, G.A.; Mapletoft, R.J. Effect of narrow sperm head shape on fertility in cattle. Can. Vet. J. 1992, 33, 31–39. [Google Scholar]
- Soler, C.; Pérez-Sánchez, F.; Schulze, H.; Bergmann, M.; Oberpenning, F.; Yeung, C.; Cooper, T.G. Objective evaluation of the morphology of human epididymal sperm heads. Int. J Androl. 2000, 23, 77–84. [Google Scholar] [CrossRef]
- Vilà, C.; Maldonado, J.E.; Wayne, R.K. Phylogenetic relationships, evolution, and genetic diversity of the domestic dog. J. Hered. 1999, 90, 71–77. [Google Scholar] [CrossRef]
- Soler, C.; Sancho, M.; García-Molina, A.; Núñez, J.; Parráguez, V.H.; Contell, J.; Bustos-Obregónet, E. Llama and alpaca comparative sperm head morphometric analysis. J. Camelid Sci. 2014b, 7, 48–58. [Google Scholar]
- van der Horst, G.; Maree, L.; du Plessis, S.S. Current perspectives of CASA applications in diverse mammalian spermatozoa. Reprod. Fertil. Dev. 2018, 30, 875–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgin, C.J.; Colella, J.P.; Kahn, P.L.; Upham, N.S. How many species of mammals are there? J. Mammal. 2018, 99, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Leigh, C.M.; Goodman, S.M.; Bedford, J.M.; Carleton, M.D.; Breed, W.G. Sperm morphology in the Malagasy rodents (Muroidea: Nesomyinae). J. Morphol. 2010, 271, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.F.; de La Sancha, N.U.; Luaces, J.P.; Estevez, D.Y.; Merani, M.S. Morphological description and comparison of sperm from eighteen species of cricetid rodents. J. Mammal. 2018, 99, 1398–1404. [Google Scholar] [CrossRef]
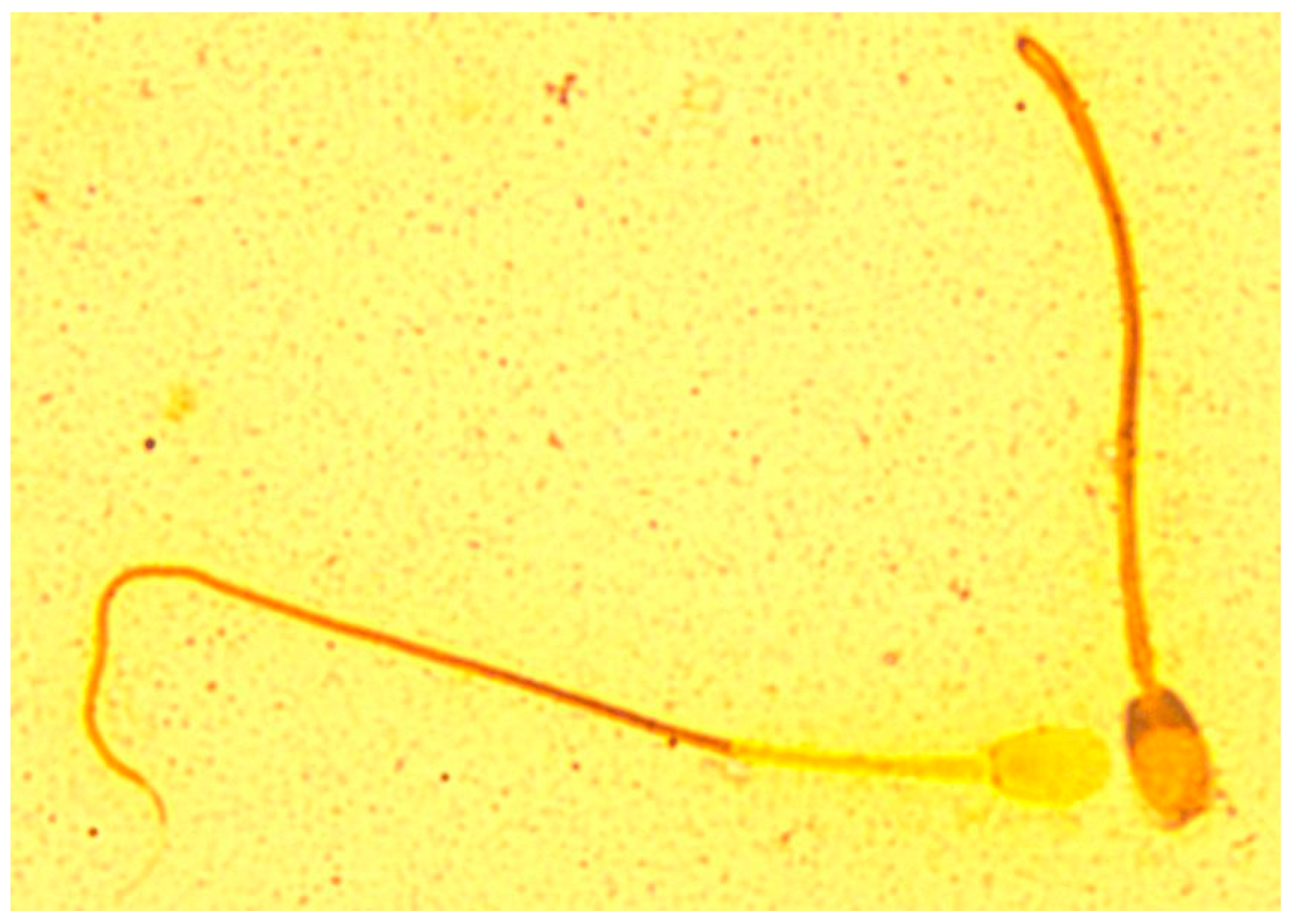
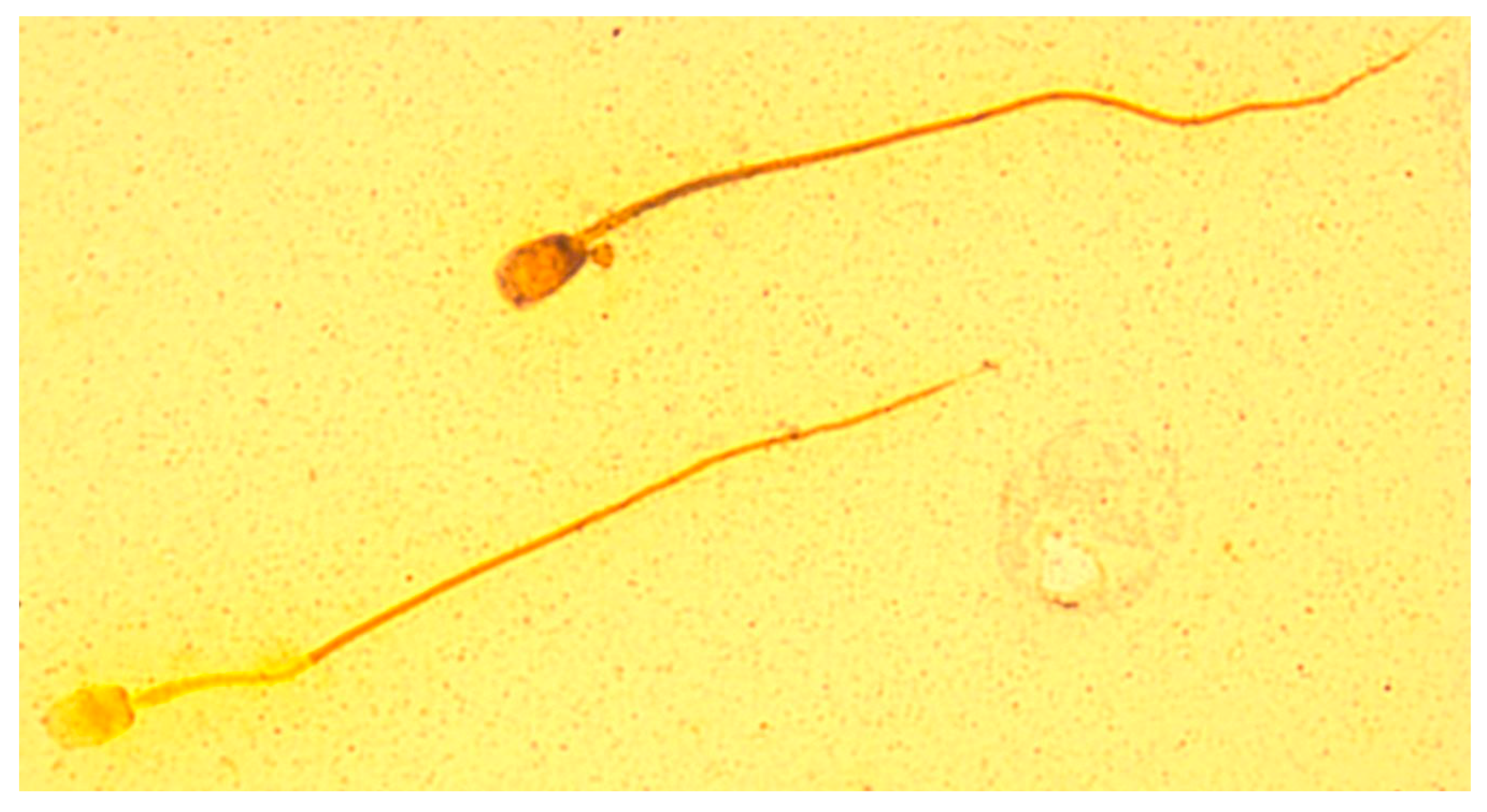
| Parameter | Formula |
|---|---|
| Head length (µm) | L |
| Head width (µm) | W |
| Head perimeter (µm) | P |
| Head area (µm2) | A |
| Head ellipticity | L/W |
| Head elongation | (L − W)/(L + W) |
| Head roughness | 4π(A/P2) |
| Head regularity | π(L × W/4 × A) |
| Morphometric Parameter | Blue Fox | Silver Fox |
|---|---|---|
| Head length (µm) | 6.72 a ± 0.47 | 6.33 b ± 0.47 |
| Head width (µm) | 4.54 a ± 0.40 | 4.21 b ± 0.44 |
| Head perimeter (µm) | 18.11 a ± 0.74 | 17.37 b ± 0.87 |
| Head area (µm2) | 21.94 a ± 0.91 | 21.11 b ± 1.16 |
| Acrosome area (µm2) | 11.50 a ± 0.78 | 10.92 b ± 0.77 |
| Midpiece length (µm) | 12.85 a ± 0.40 | 12.79 a ± 0.43 |
| Tail end piece length (µm) | 3.44 a ± 0.28 | 3.28 b ± 0.28 |
| Tail length (µm) | 65.23 a ± 1.02 | 65.09 a ± 1.10 |
| Acrosome coverage (%) | 52.43 a ± 3.11 | 52.83 a ± 3.99 |
| Midpiece coverage (%) | 19.71 a ± 0.59 | 19.65 a ± 0.59 |
| Sperm length (µm) | 71.95 a ± 1.11 | 71.42 b ± 1.26 |
| Morphometric Parameter | Blue Fox | Silver Fox |
|---|---|---|
| Ellipticity | 1.49 a ± 0.15 | 1.52 a ± 0.19 |
| Elongation | 0.19 a ± 0.05 | 0.20 a ± 0.06 |
| Roughness | 0.84 a ± 0.07 | 0.88 b ± 0.10 |
| Regularity | 1.09 a ± 0.11 | 0.99 b ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andraszek, K.; Banaszewska, D.; Szeleszczuk, O.; Kuchta-Gładysz, M.; Grzesiakowska, A. Morphometric Characteristics of the Spermatozoa of Blue Fox (Alopex lagopus) and Silver Fox (Vulpes vulpes). Animals 2020, 10, 1927. https://doi.org/10.3390/ani10101927
Andraszek K, Banaszewska D, Szeleszczuk O, Kuchta-Gładysz M, Grzesiakowska A. Morphometric Characteristics of the Spermatozoa of Blue Fox (Alopex lagopus) and Silver Fox (Vulpes vulpes). Animals. 2020; 10(10):1927. https://doi.org/10.3390/ani10101927
Chicago/Turabian StyleAndraszek, Katarzyna, Dorota Banaszewska, Olga Szeleszczuk, Marta Kuchta-Gładysz, and Anna Grzesiakowska. 2020. "Morphometric Characteristics of the Spermatozoa of Blue Fox (Alopex lagopus) and Silver Fox (Vulpes vulpes)" Animals 10, no. 10: 1927. https://doi.org/10.3390/ani10101927
APA StyleAndraszek, K., Banaszewska, D., Szeleszczuk, O., Kuchta-Gładysz, M., & Grzesiakowska, A. (2020). Morphometric Characteristics of the Spermatozoa of Blue Fox (Alopex lagopus) and Silver Fox (Vulpes vulpes). Animals, 10(10), 1927. https://doi.org/10.3390/ani10101927





