Growth Performance, Nutrient Digestibility, Hematological Parameters, and Hepatic Oxidative Stress Response in Juvenile Nile Tilapia, Oreochromis niloticus, Fed Carbohydrates of Different Complexities
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Culture System
2.2. Formulation of Feeds and Preparation
2.3. Sampling and Chemical Analyses
2.4. Antioxidant Enzyme Activity Determinations
2.5. Digestibility Trial
2.6. Calculations and Statistical Analyses
3. Results
3.1. Survival, Feed Intake, Growth Performance, and Feed Utilization
3.2. Nutrients Digestibility
3.3. Slaughter Indices
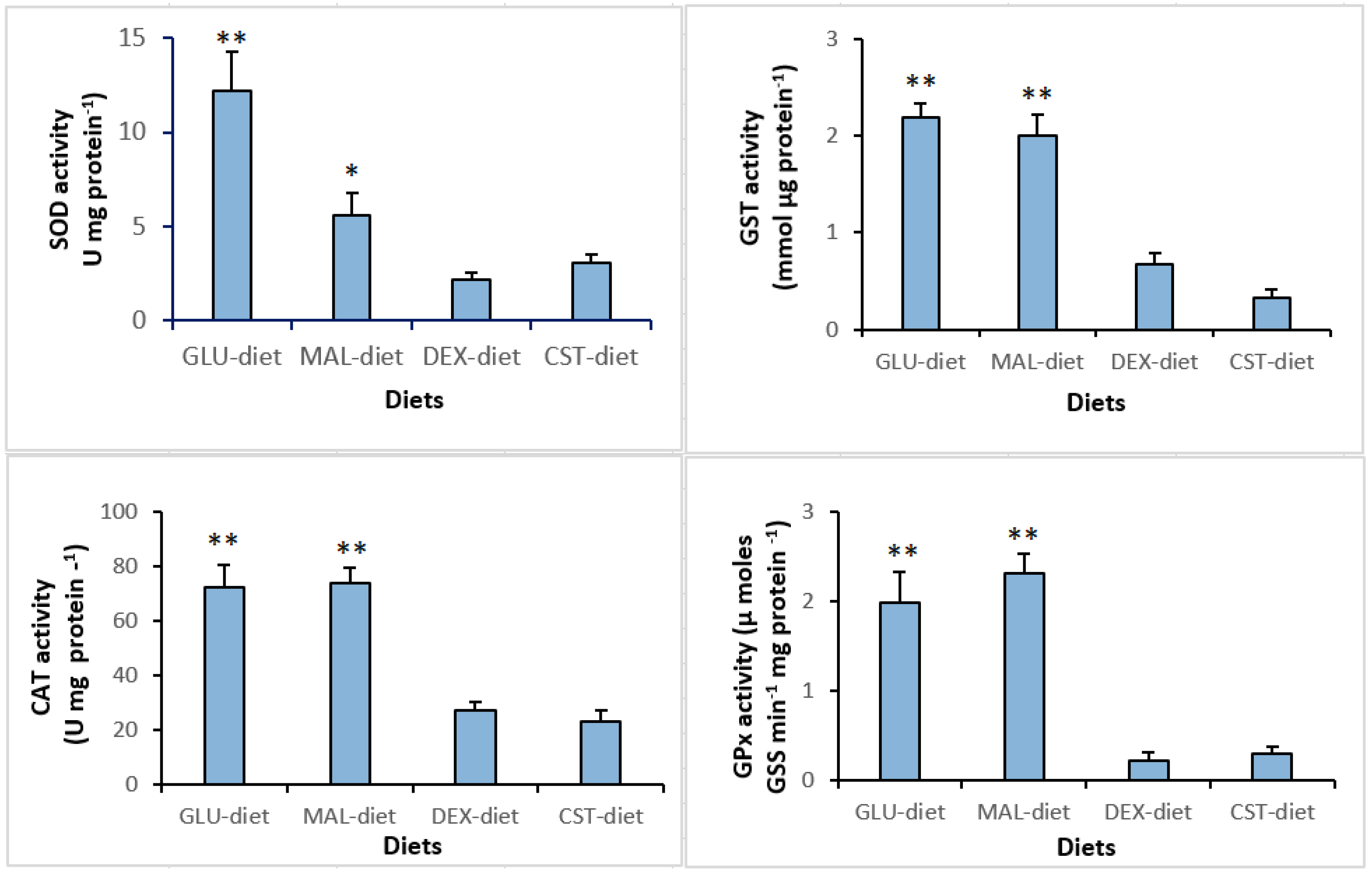
3.4. Hematology and Hepatic Antioxidant Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Azaza, M.S.; Mensi, F.; Wassim, K.; Abdelmouleh, A.; Brini, B.; Kraϊem, M.M. Nutritional evaluation of waste date fruit as partial substitute for soybean meal in practical diets of juvenile Nile tilapia, Oreochromis niloticus L. Aquacult. Nutr. 2009, 15, 262–272. [Google Scholar] [CrossRef]
- Azaza, M.S.; Wassim, K.; Mensi, F.; Abdelmouleh, A.; Brini, B.; Kraϊem, M.M. Evaluation of fava beans (Vicia faba L. var. minuta) as a replacement for soybean meal in practical diets of juvenile Nile tilapia Oreochromis niloticus (L., 1758). Aquaculture 2009, 287, 174–179. [Google Scholar] [CrossRef]
- Azaza, M.S.; Khiari, N.; Dhraief, M.N.; Aloui, N.; Kraϊem, M.M.; Elfeki, A. Growth performance, oxidative stress indices and hepatic carbohydrate metabolic enzymes activities of juvenile Oreochromis niloticus (L.), in response to dietary carbohydrate to protein ratio. Aquac. Res. 2015, 46, 14–27. [Google Scholar] [CrossRef]
- Adamidou, I.; Nengas, M.; Henry, N.; Ioakei, M.; Rigos, G.; Bell, G.J.; Jauncey, K. Effects of dietary inclusion of peas, chickpeas and faba beans on growth, feed utilization and health of gilthead seabream (Sparus aurata). Aquac. Nutr. 2011, 17, 288–296. [Google Scholar] [CrossRef]
- FAO’s Fisheries and Aquaculture Department. Statistical Collections Capture Production and Aquaculture Production Datasets 1950–2019 (Release Date: March 2020). Available online: http://www.fao.org/fishery/topic/16073 (accessed on 25 March 2020).
- Azaza, M.S.; Mensi, F.; Ksouri, J.; Dhraïef, M.N.; Abdelmouleh, A.; Brini, B.; Kraïem, M.M. Growth of Nile tilapia (Oreochromis niloticus L.) fed with diets containing graded levels of green algae (Ulva rigida) reared in geothermal waters in southern Tunisia. J. Appl. Ichthyol. 2008, 24, 202–207. [Google Scholar] [CrossRef]
- Saidi, S.A.; Azaza, M.S.; Abdelmouleh, A.; Pelt, J.V.; Kraïem, M.M.; El-feki, A. The use of tuna by-product meal in the practical diets of juvenile Nile tilapia (Oreochromis niloticus, L.): Effects on growth performance, nutrient digestibility and oxidative status. Aquac. Res. 2010, 41, 1875–1886. [Google Scholar] [CrossRef]
- Norhan, E.S. Assessment of sesame meal as a soybean meal replacement in European sea bass (Dicentrarchus labrax) diets based on aspects of growth, amino acid profiles, haematology, intestinal and hepatic integrity and macroelement contents. Fish Physiol. Biochem. 2020, 46, 861–879. [Google Scholar] [CrossRef]
- Wenji, X.; Junyan, J.; Dong, H.; Haokun, L. Physiological and transcriptomic responses to fishmeal-based diet and rapeseed meal-based diet in two strains of gibel carp (Carassius gibelio). Fish Physiol. Biochem. 2019, 45, 267–286. [Google Scholar] [CrossRef]
- Sakhawat, H.; Shunsuke, K. Dietary substitution of fishmeal by alternative protein with guanosine monophosphate supplementation influences growth, digestibility, blood chemistry profile, immunity, and stress resistance of red sea bream, Pagrus major. Fish Physiol. Biochem. 2017, 43, 1629–1644. [Google Scholar] [CrossRef]
- Li, X.-F.; Liu, W.-B.; Lu, K.-L.; Xu, W.-N.; Wang, Y. Dietary carbohydrate/lipid ratios affect stress, oxidative status and non-specific immune responses of fingerling blunt snout bream, Megalobrama amblycephala. Fish Shellfish Immun. 2012, 33, 316–323. [Google Scholar] [CrossRef]
- Yang, S.-D.; Liu, F.-G.; Liou, C.-H. Effects of dietary L-carnitine, plant proteins and lipid levels on growth performance, body composition, blood traits and muscular carnitine status in Juvenile Silver Perch (Bidyanus bidyanus). Aquaculture 2012, 342, 48–55. [Google Scholar] [CrossRef]
- Zuo, R.; Ai, Q.; Mai, K.; Xu, W.; Wang, J.; Xu, H.; Liufu, Z.; Zhang, Y. Effects of dietary docosahexaenoic to eicosapentaenoic acid ratio (DHA/EPA) on growth, nonspecific immunity, expression of some immune related genes and disease resistance of large yellow croaker (Larmichthys crocea) following natural infestation of parasites (Cryptocaryon irritans). Aquaculture 2012, 334, 101–109. [Google Scholar] [CrossRef]
- Carolina, C.; Amalia, P.-J.; Filipe, C. Nutritional history does not modulate hepatic oxidative status of European sea bass (Dicentrarchus labrax) submitted to handling stress. Fish Physiol. Biochem. 2018, 44, 911–918. [Google Scholar] [CrossRef]
- Naghmeh, J.; Bahram, F.; Mir, M.S. Growth performance and plasma metabolites in juvenile Siberian sturgeon Acipenser baerii (Brandt, 1869) subjected to various feeding strategies at different sizes. Fish Physiol. Biochem. 2018, 44, 1363–1374. [Google Scholar] [CrossRef]
- Hidalgo, M.C.; Urea, E.; Sanz, A. Comparative study of digestive enzymes in fish with different nutritional habits. Proteolytic and amylase activities. Aquaculture 1999, 170, 267–283. [Google Scholar] [CrossRef]
- Wilson, R.P.; Poe, W.E. Apparent inability of channel catfish to utilize dietary mono- and disaccharides as energy source. J. Nutr. 1987, 117, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Banos, N.; Baro, J.; Castejoon, C.; Navarro, I.; Gutierrez, J. Influence of high-carbohydrate enriched diets on plasma insulin levels and insulin and IGF-I receptors in trout. Regul. Pept. 1998, 77, 55–62. [Google Scholar] [CrossRef]
- Hemre, G.I.; Mommsen, T.P.; Krogdahl, A. Carbohydrates in fish nutrition: Effects on growth, glucose metabolism and hepatic enzymes. Aquac. Nutr. 2002, 8, 175–194. [Google Scholar] [CrossRef]
- Polakof, S.; Stéphane, P.; José, L.S.; Thomas, W.M. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Alexander, C.; Sahu, N.P.; Pal, A.K.; Akhtar, M.S. Haemato-immunological and stress responses of Labeo rohita (Hamilton) fingerlings: Effect of rearing temperature and dietary gelatinized carbohydrate. J. Anim. Physiol. Anim. Nutr. 2011, 95, 653–663. [Google Scholar] [CrossRef]
- Kumar, V.; Sahu, N.P.; Pal, A.K.; Kumar, S.; Gupta, S.K. Gelatinized to non-gelatinized starch ratio in the diet of Labeo rohita: Effect on digestive and metabolic response and on growth. J. Anim. Physiol. Anim. Nutr. 2008, 92, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.W. Glucose intolerance in teleost fish: Fact or fiction? Comp. Biochem. Physiol. B 2001, 129, 243–249. [Google Scholar] [CrossRef]
- Lin, J.H.; Shiau, S.Y. Hepatic enzyme adaptation of different dietary carbohydrate in juvenile tilapia (Oreochromis niloticus x O. aureus). Fish Physiol. Biochem. 1995, 14, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.K.; Sambo, A.B. Haematological assessment of the Nile tilapia Oreochromis niloticus exposed to sublethal concentrations of Portland cement powder in solution. Int. J. Zool. Res. 2007, 4, 48–52. [Google Scholar] [CrossRef]
- Javanmardi, S.; Rezaei, T.; Rosentrater, K.A. Effects of different levels of vitamin B6 in tank water on the Nile tilapia (Oreochromis niloticus): Growth performance, blood biochemical parameters, intestine and liver histology, and intestinal enzyme activity. Fish Physiol. Biochem. 2020. [Google Scholar] [CrossRef]
- Azaza, M.S.; Dhraïef, M.N.; Kraïem, M.M.; Baras, E. Influences of food particle size on growth, size heterogeneity, feed efficiency and gastric evacuation of juvenile Nile tilapia, Oreochromis niloticus Linnaeus, 1758. Aquaculture 2010, 309, 193–202. [Google Scholar] [CrossRef]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Azaza, M.S.; Dhraïef, M.N.; Kraïem, M.M. The effects of water temperature on growth and sex-ratio of juvenile Nile Tilapia Oreochromis niloticus (Linnaeus) reared in geothermal waters in southern Tunisia. J. Ther. Biol. 2008, 33, 98–105. [Google Scholar] [CrossRef]
- Popma, T.; Masser, M. Tilapia: Life History and Biology; Publication N° 283; Southern Regional Aquaculture Center (SRAC): Stoneville, MS, USA, 1999. [Google Scholar]
- Ross, L.G. Environmental physiology and energetics. In Tilapias: Biology and Exploitation; Beveridge, M.C.M., McAndrew, B.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 89–128. [Google Scholar]
- Jauncey, K.; Ross, B. A Guide to Tilapia Feeds and Feeding; Institute of Aquaculture: Stirling, UK, 1982; p. 289. [Google Scholar]
- El–Husseiny, O.M.; Galal, M.; Abdul-Aziz, A.; Goda, M.A.-S.; Ashraf, S. Effect of altering linoleic acid and linolenic acid dietary levels and ratios on the performance and tissue fatty acid profiles of Nile tilapia Oreochromis niloticus fry. Aquacult. Int. 2010, 18, 1105–1119. [Google Scholar] [CrossRef]
- Azaza, M.S.; Kammoun, W.; Abdelmouleh, A.; Kraïem, M.M. Growth performance, feed utilization and body composition of Nile tilapia, (Oreochromis niloticus L.) fed with differently heated soybean meal-based diets. Aquacult. Int. 2009, 17, 507–521. [Google Scholar] [CrossRef]
- Azaza, M.S.; Mensi, F.; Abdelmouleh, A.; Kraiem, M.M. Elaboration d’aliments composés secs pour le Tilapia du Nil Oreochromis niloticus (L., 1758) en élevage dans les eaux géothermales du sud tunisien. Bull. Inst. Natl. Des Sci. et Technol. de la Mer 2005, 32, 56–67. [Google Scholar]
- Khan, M.A.; Abidi, S.F. Dietary arginine requirement of Heteropneustes fossilis fry (Bloch) based on growth, nutrient retention and haematological parameters. Aquac. Nutr. 2011, 17, 418–428. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, P.; Li, D.; Shi, Z. Hematological and plasma biochemical responses of crucian carp (Carassius auratus) to intraperitoneal injection of extracted microcystins with the possible mechanisms of anaemia. Toxicon 2007, 49, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Anguiano, L.; Lascano, C.; Sotomayor, V.; Rosenbaum, E.; Venturino, A. Changes in the antioxidant metabolism in the embryonic development of the common South American toad Bufo arenarum: Differential responses to pesticide in early embryos and autonomous-feeding larvae. J. Biochem. Mol. Toxicol. 2008, 22, 259–267. [Google Scholar] [CrossRef]
- Habig, W.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferase: The first step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 1730–1739. [Google Scholar]
- Bell, J.G.; Cowey, C.B.; Adron, J.W.; Shanks, A.M. Some effects of vitamin E and selenium deprivation on tissue enzyme levels and indices of tissue peroxidation in rainbow trout (Salmo gairdneri). Br. J. Nutr. 1985, 53, 149–157. [Google Scholar] [CrossRef]
- Fernandez, F.; Miquel, A.G.; Cuplido, L.R.; Guinea, J.; Ross, E. Comparison of faecal collection methods for digestibility determinations in gilthead sea bream. J. Fish Biol. 1996, 49, 735–738. [Google Scholar] [CrossRef]
- Furukawa, A.; Tsukahara, H. On the acid digestion of chromic oxide as an index substance in the study of digestibility of fish feed. Bull. Jpn. Soc. Sci. Fish. 1966, 32, 502–506. [Google Scholar] [CrossRef]
- AOAC (Association of Analytical Chemists). Official Methods of Analysis of the Association of Official Analytical Chemists, 14th ed.; Association of Analytical Chemists: Rockville, MD, USA, 2000. [Google Scholar]
- New, M.B. Feed and Feeding of Fish and Shrimp. A Manuel on the Preparation of Compound Feeds for Shrimp and Fish in Aquaculture; FAO: Rome, Italy, 1987. [Google Scholar]
- Morais, S.; Gordon, J.; Derek, B.; Robertson, A.; William, J.; Paul, R.; Morris, C. Protein/lipid ratios in extruded diets for Atlantic cod (Gadus morhua L.): Effects on growth, feed utilisation, muscle composition and liver histology. Aquaculture 2001, 203, 101–119. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall: Upper Saddle River, NJ, USA, 1999; 663p. [Google Scholar]
- Ott, R.L.; Longnecker, M. An Introduction to Statistical Methods and Data Analysis, 5th ed.; Duxbury Thomson Learning: Pacific Grove, CA, USA, 2001; p. 1152. [Google Scholar]
- Amjad, S.; Balange, K.; Ghughuskar, M.M. Protein sparing effect of carbohydrates in the diet of Cirrhinus mrigala (Hamilton, 1822) fry. Aquaculture 2006, 258, 680–684. [Google Scholar] [CrossRef]
- Fernandez, A.; Anna, G.; Miquel, G.; Cordobaa, M.; Manuel, V.; Isidoro, M.; Anna, C.; Isabel, V.B. Effects of diets with distinct protein-to-carbohydrate ratios on nutrient digestibility, growth performance, body composition and liver intermediary enzyme activities in gilthead sea bream (Sparus aurata, L.) fingerlings. J. Exp. Mar. Biol. Ecol. 2007, 343, 1–10. [Google Scholar] [CrossRef]
- Wilson, R.P. Utilization of dietary carbohydrate by fish. Aquaculture 1994, 124, 67–80. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, J.H. Effect of dietary glucose, dextrin and starch on growth and body composition of juvenile starry flounder, Platichthys stellatus. Fish. Sci. 2004, 70, 53–58. [Google Scholar] [CrossRef]
- Wu, X.Y.; Liu, Y.J.; Tian, L.X.; Mai, K.S.; Yang, H.J. Utilization of several different carbohydrate sources by juvenile yellow seabream (Sparus latus). J. Fish. China 2007, 31, 463–471. [Google Scholar]
- Cui, X.-J.; Zhou, Q.-C.; Liang, H.O.; Yang, J.; Zhao, L.-M. Effects of dietary carbohydrate sources on the growth performance and hepatic carbohydrate metabolic enzyme activities of juvenile cobia (Rachycentron canadum Linnaeus.). Aquac. Res. 2010, 42, 99–107. [Google Scholar] [CrossRef]
- Hung, S.S.O.; Fynn-Aikins, K.F.; Lutes, P.B.; Xu, R.P. Ability of juvenile white sturgeon (Acipenser transmontanus) to utilize different carbohydrate source. J. Nutr. 1989, 119, 727–733. [Google Scholar] [CrossRef]
- Enes, P.; Panserat, S.; Kaushik, S.; Oliva-Teles, A. Hepatic glucokinase and glucose-6-phosphatase responses to dietary glucose and starch in gilthead sea bream (Sparus aurata) juveniles reared at two temperatures. Comp. Biochem. Physiol. 2008, 149, 80–86. [Google Scholar] [CrossRef]
- Furuichi, M.; Yone, Y. Availability of carbohydrate in nutrition of carp and red sea bream. Nippon Suisan Gakkaishi 1982, 48, 945–948. [Google Scholar] [CrossRef]
- Shiau, S.Y.; Lin, Y.H. Carbohydrate utilization and its protein-sparing effect in diets for grouper (Epinephelus malabaricus). Anim. Sci. 2001, 73, 299–304. [Google Scholar] [CrossRef]
- Enes, P.; Panserat, S.; Kaushik, S.; Oliva-Teles, A. Nutritional regulation of hepatic glucose metabolism in fish. Fish Physiol. Biochem. 2009, 35, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Lupatsch, I.; Kissil, G.; Sklan, D.; Pfeffer, E. Effects of varying dietary protein and energy supply on growth, body composition and protein utilization in gilthead sea bream (Sparus aurata L.). Aquac. Nutr. 2001, 7, 71–80. [Google Scholar] [CrossRef]
- Kaushik, S.J.; Covès, D.; Dutto, G.; Blanc, D. Almost total replacement of fish meal by plant protein sources in the diet of a marine teleost, the European seabass, Dicentrarchus labrax. Aquaculture 2004, 230, 391–404. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.J.; Tian, L.X.; Du, Z.Y.; Wang, J.T.; Wang, S.; Xiao, W.P. Effects of dietary carbohydrate level on growth and body composition of juvenile tilapia, Oreochromis niloticus x O. aureus. Aquac. Res. 2005, 36, 1408–1413. [Google Scholar] [CrossRef]
- Rawles, S.D.; Smith, S.B.; Gatlin, D.M. Hepatic glucose utilization and lipogenesis of hybrid striped bass (Morone chrysops x M. saxatilis) in response to dietary carbohydrate level and complexity. Aquac. Nutr. 2008, 14, 40–50. [Google Scholar] [CrossRef]
- Lanari, D.; Poli, B.M.; Ballestrazzi, R.; Lupi, P.; D’Agaro, E.; Mecatti, M. The effects of dietary fat and NFE levels on growing European sea bass (Dicentrarchus labrax L.). Growth rate, body and fillet composition, carcass traits and nutrient retention efficiency. Aquaculture 1999, 179, 1–4. [Google Scholar] [CrossRef]
- Kim, J.D.; Kaushik, S.J. Contribution of digestible energy from carbohydrates and estimation of protein/energy requirements for growth of rainbow trout (Oncorhynchus mykiss). Aquaculture 1992, 106, 161–169. [Google Scholar] [CrossRef]
- Hemre, G.-I.; Lie, O.; Lied, E.; Lambertsen, G. Starch as an energy source in feed for cod (Gadus morhua): Digestibility and retention. Aquaculture 1989, 80, 261–270. [Google Scholar] [CrossRef]
- Mohanta, K.N.; Mohanty, S.N.; Jena, J.K. Protein-sparing effect of carbohydrate in silver barb, Puntius gonionotus fry. Aquac. Nutr. 2007, 10, 311–317. [Google Scholar] [CrossRef]
- Osuigwe, D.I.; Obiekezie, A.I.; Onuoha, G.C. Some haematological changes in hybrid catfish (Heterobranchus longifilis × Clarias gariepinus) fed different dietary levels of raw and boiled jack bean (Canavalia ensiformis) seed meal. Afric. J. Biotechnol. 2005, 4, 1017–1021. [Google Scholar]
- Paknejad, H.; Pezhman, S.; Shekarabi, H.; Mehdi, S.M.; Abdolmajid, H.; Zohre, K.; Soheila, R. Dietary peppermint (Mentha piperita) powder affects growth performance, hematological indices, skin mucosal immune parameters, and expression of growth and stress-related genes in Caspian roach (Rutilus caspicus). Fish Physiol. Biochem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.C.; Wu, Z.H.; Chi, S.Y.; Yang, Q.H. Dietary lysine requirement of juvenile cobia (Rachycentron canadum). Aquaculture 2007, 273, 634–640. [Google Scholar] [CrossRef]
- Hemre, G.I.; Sandnes, K.; Lie, Ø.; Torrissen, O.; Waagbø, R. Blood chemistry and organ nutrient composition in Atlantic salmon (Salmo salar) fed graded amounts of wheat starch. Aquac. Nutr. 1995, 1, 37–42. [Google Scholar] [CrossRef]
- Hilton, J.W. The interaction of vitamin, minerals and diet composition in the diet of fish. Aquaculture 1989, 79, 233–244. [Google Scholar] [CrossRef]
- Waagbø, R.; Glette, J.; Sandnes, K.; Hemre, G.I. Influence of dietary carbohydrate on blood chemistry, immunity and disease resistance in Atlantic salmon, Salmo salar L. J. Fish Dis. 1994, 17, 245–258. [Google Scholar] [CrossRef]
- Yildirim, M.; Lim, C.; Shelby, R.; Klesius, P.H. Increasing fish oil levels in commercial diets influences hematological and immunological responses of channel catfish, Ictalurus punctatus. J. World Aquacult. Soc. 2009, 40, 76–86. [Google Scholar] [CrossRef]
- Tocher, D.R.; Mourente, G.; Van Der Eecken, A.; Evjemo, J.O.; Diaz, E.; Bell, J.G. Effects of dietary vitamin E on antioxidant defense mechanisms of juvenile turbot (Scophthalmus maximus L.), halibut (Hippoglossus hippoglossus L.) and sea bream (Sparus aurata L.). Aquac. Nutr. 2002, 8, 195–203. [Google Scholar] [CrossRef]
- Sheikhzadeh, N.; Tayefi-Nasrabadi, H.; Oushani, A.K.; Enferadi, M.H.N. Effects of Haematococcus pluvialis supplementation on antioxidant system and metabolism in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2012, 38, 413–419. [Google Scholar] [CrossRef]
- Rueda-Jasso, R.; Conceição, L.E.C.; Dias, J.; De Coen, W.; Gomes, E.; Rees, J.F. Effect of dietary non-protein energy levels on condition and oxidative status of Senegalese sole (Solea senegalensis) juveniles. Aquaculture 2004, 231, 417–433. [Google Scholar] [CrossRef]
- Wolff, S.P.; Dean, R.T. Glucose autoxidation and protein modification. The potential role of autoxidative glycosylation in diabetes. Biochem. J. 1987, 245, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Moreau, Y.; Desseaux, V.; Koukiekolo, R.; Mouren, G.M.; Santimone, M. Starch digestion in tropical fishes: Isolation, structural studies and inhibition kinetics of a-amylase from two tilapias Oreochromis niloticus and Sarotherodon melanotheron. Comp. Biochem. Physiol. B 2001, 128, 543–552. [Google Scholar] [CrossRef]
- Haleng, X.J.; Pincemail, J.; Defraigne, J.O.; Charlier, C.; Chapelle, J.P. Le stress oxidant. Rev. Med. Liege 2007, 62, 628–638. [Google Scholar] [PubMed]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef]
| Components | Experimental Diets | |||
|---|---|---|---|---|
| GLU-Diet | MAL-Diet | DEX-Diet | CST-Diet | |
| Ingredients (% g kg−1) | ||||
| Fish meal | 170 | 170 | 170 | 170 |
| Soybean meal (DSBM) a | 270 | 270 | 270 | 270 |
| Maize meal | 250 | 250 | 250 | 250 |
| Glucose | 200 | |||
| Maltose | 200 | |||
| Dextrin | 200 | |||
| Corn starch | 200 | |||
| Soybean oil/cod liver oil (3:1) b | 75 | 75 | 75 | 75 |
| Vit-mineral mix c | 10 | 10 | 10 | 10 |
| CMC (binder) d | 10 | 10 | 10 | 10 |
| Monocalcium phosphate e | 5 | 5 | 5 | 5 |
| L-methionine | 5 | 5 | 5 | 5 |
| Chromic oxide f | 5 | 5 | 5 | 5 |
| Proximate analysis (% g kg−1) g | ||||
| Dry matter (in original matter) | 881.7 | 928.7 | 917.3 | 890.4 |
| Crude protein | 251.4 | 250.6 | 254.3 | 259.1 |
| Crude lipid | 111.9 | 106.0 | 115.1 | 124.3 |
| Crude fibre | 66.6 | 58.7 | 60.9 | 68.3 |
| Ash | 5.21 | 61.3 | 54.7 | 60.2 |
| Carbohydrate h | 518.0 | 523.4 | 515.0 | 488.1 |
| Gross Energy (MJ kg−1 DM) i | 18.91 | 18.76 | 19.05 | 19.04 |
| Digestible Energy (MJ kg−1 DM) j | 13.55 | 13.38 | 13.69 | 13.87 |
| Variables | Experimental Diets | |||
|---|---|---|---|---|
| GLU-Diet | MAL-Diet | DEX-Diet | CST-Diet | |
| IBM (g) | 2.28 ± 0.1 | 2.16 ± 0.09 | 2.11 ± 0.07 | 2.22 ± 0.08 |
| FBM (g) | 24.48 ± 1.46 a | 31.12 ± 1.33 b | 30.48 ± 1.69 b | 28.93 ± 1.82 b |
| SR (%) | 92.78 ± 0.67 | 91.64 ± 3.47 | 90.00 ± 1.16 | 93.33 ± 2.00 |
| SGR (% day−1) | 5.27 ± 0.08 a | 5.93 ± 0.18 b | 5.96 ± 0.09 b | 5.71± 0.16 b |
| FI (g day−1) | 51.44 ± 2.61 a | 48.68 ± 1.77 ab | 42.95 ± 2.49 b | 43.43 ± 2.89 b |
| FCR (g g−1) | 1.94 ± 0.19 a | 1.66 ± 0.20 b | 1.49 ± 0.12 c | 1.55 ± 0.15 b,c |
| PER (g g−1) | 2.01 ± 0.13 a | 2.27 ± 0.18 ab | 2.67 ± 0.15 c | 2.54 ± 0.14 c |
| ADC (%) | Experimental Diets | |||
|---|---|---|---|---|
| GLU-Diet | MAL-Diet | DEX-Diet | CST-Diet | |
| Dry mater | 73.55 ± 3.17 a | 71.08 ± 2.25 a | 78.71 ± 2.04 b | 77.67 ± 2.21 b |
| Protein | 82.12 ± 3.19 a | 89.33 ± 2.78 b | 85.67 ± 2.14 b | 88.03 ± 2.78 b |
| Fat | 90.43 ± 2.79 | 87.16 ± 2.65 | 90.72 ± 2.98 | 86.04 ± 3.16 |
| Carbohydrate | 79.73 ± 2.98 a | 75.44 ± 2.42 a | 77.86 ± 2.89 a | 74.97 ± 3.11 b |
| Energy | 81.83 ± 3.04 | 79.44 ± 2.67 | 82.67 ±1.97 | 80.38 ± 2.27 |
| Variables | Experimental Diets | |||
|---|---|---|---|---|
| GLU-Diet | MAL-Diet | DEX-Diet | CST-Diet | |
| HSI (% BM) 1 | 2.75 ± 0.16 a | 2.62 ± 0.14 a | 2.88 ± 0.15 a | 3.16 ± 0.12 b |
| VSI (% BM) 2 | 6.94 ± 0.52 a | 6.81 ± 0.94 a | 7.92 ± 0.48 b | 7.83 ± 0.71 b |
| IPFR (% BM) 3 | 1.63 ± 0.65 a | 1.45 ± 0.14 a | 1.92 ± 0.48 a | 2.43 ± 0.62 b |
| Parameters | Experimental Diets | |||
|---|---|---|---|---|
| GLU-Diet | MAL-Diet | DEX-Diet | CST-Diet | |
| Hematocrit (Hct %) | 23.15 ± 0.59 a | 24.08 ± 1.54 a | 25.71 ± 1.87 b | 28.67 ± 1.36 b |
| Hemoglobin (Hb, g/dL) | 6.02 ± 0.20 a | 5.93 ± 0.11 a | 9.67 ± 0.10 b | 10.93 ± 0.13 b |
| RBCs a (×106/mm3) | 32.13 ± 3.9 | 30.56 ± 4.5 | 29.42 ± 4.7 | 37.08 ± 3.3 |
| WBCs b (×103/mm3) | 41.63 ± 2.78 a | 45.63 ± 4.17 a | 43.86 ± 1.78 a | 52.91 ± 3.22 b |
| MCH (pg) c | 101.4 ± 3.63 | 107.19 ± 1.80 | 104.38 ± 2.77 | 110.91 ± 2.85 |
| MCHC (g/dL) d | 32.25 ± 4.02 | 26.82 ± 3.90 | 31.90 ± 3.34 | 35.63 ± 2.96 |
| MCV (fl) e | 729.51 ± 14.51 a | 797.95 ± 8.86 a | 918.88 ± 9.37 b | 907.27 ± 12.54 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azaza, M.S.; Saidi, S.A.; Dhraief, M.N.; EL-feki, A. Growth Performance, Nutrient Digestibility, Hematological Parameters, and Hepatic Oxidative Stress Response in Juvenile Nile Tilapia, Oreochromis niloticus, Fed Carbohydrates of Different Complexities. Animals 2020, 10, 1913. https://doi.org/10.3390/ani10101913
Azaza MS, Saidi SA, Dhraief MN, EL-feki A. Growth Performance, Nutrient Digestibility, Hematological Parameters, and Hepatic Oxidative Stress Response in Juvenile Nile Tilapia, Oreochromis niloticus, Fed Carbohydrates of Different Complexities. Animals. 2020; 10(10):1913. https://doi.org/10.3390/ani10101913
Chicago/Turabian StyleAzaza, Mohamed S., Saber A. Saidi, Mohamed N. Dhraief, and Abdelfattah EL-feki. 2020. "Growth Performance, Nutrient Digestibility, Hematological Parameters, and Hepatic Oxidative Stress Response in Juvenile Nile Tilapia, Oreochromis niloticus, Fed Carbohydrates of Different Complexities" Animals 10, no. 10: 1913. https://doi.org/10.3390/ani10101913
APA StyleAzaza, M. S., Saidi, S. A., Dhraief, M. N., & EL-feki, A. (2020). Growth Performance, Nutrient Digestibility, Hematological Parameters, and Hepatic Oxidative Stress Response in Juvenile Nile Tilapia, Oreochromis niloticus, Fed Carbohydrates of Different Complexities. Animals, 10(10), 1913. https://doi.org/10.3390/ani10101913





