Expression of Phosphatonin-Related Genes in Sheep, Dog and Horse Kidneys Using Quantitative Reverse Transcriptase PCR
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
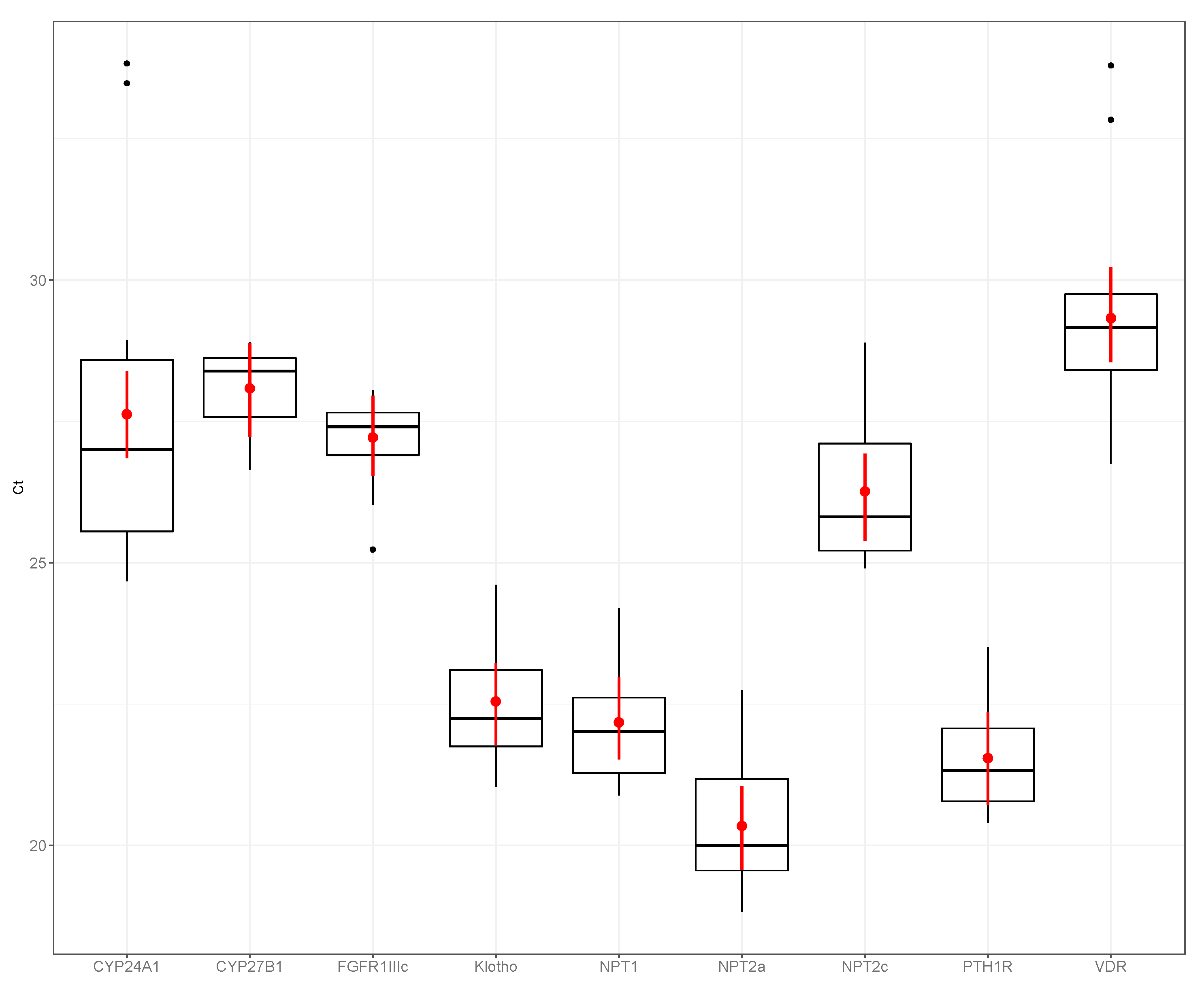
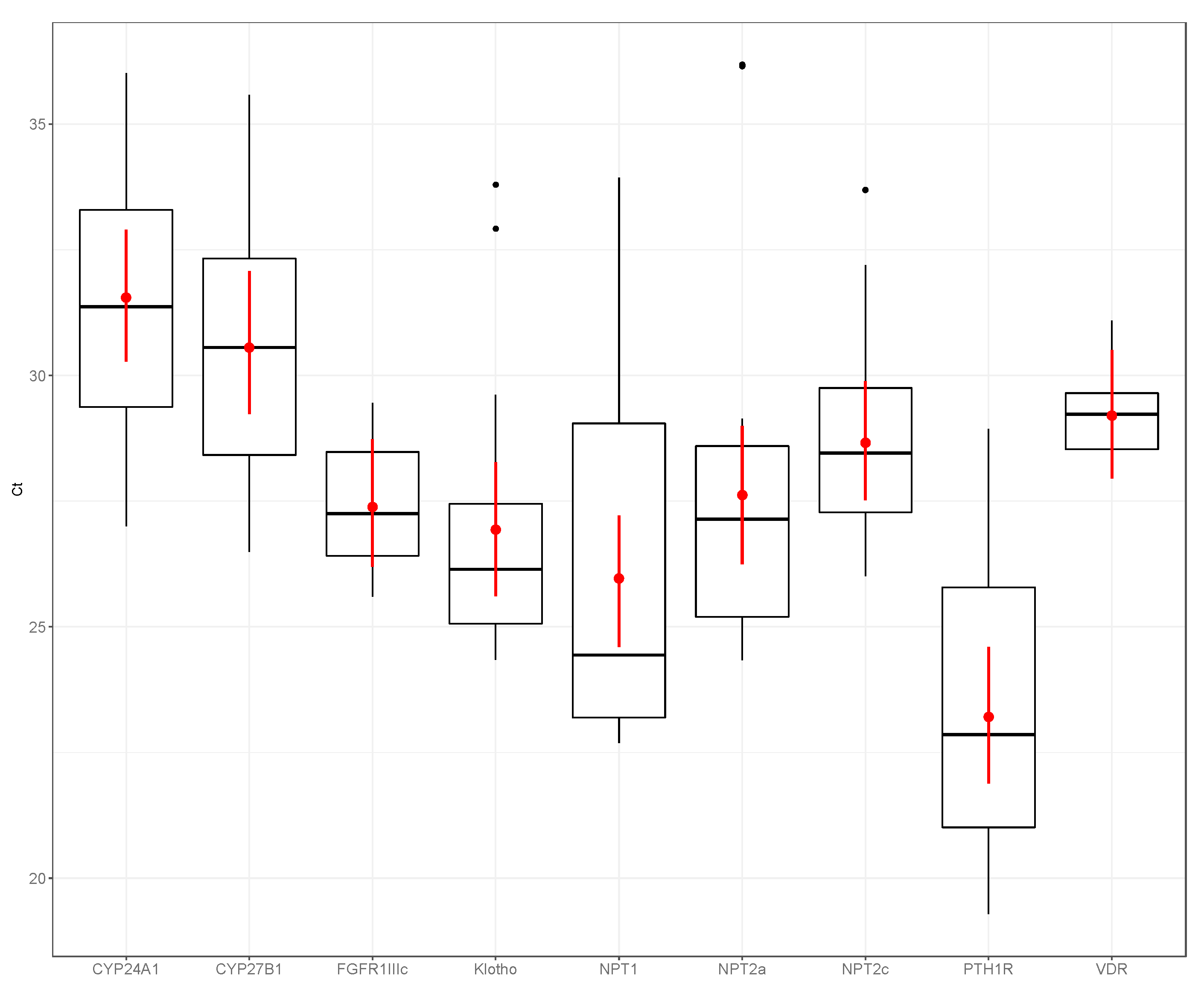
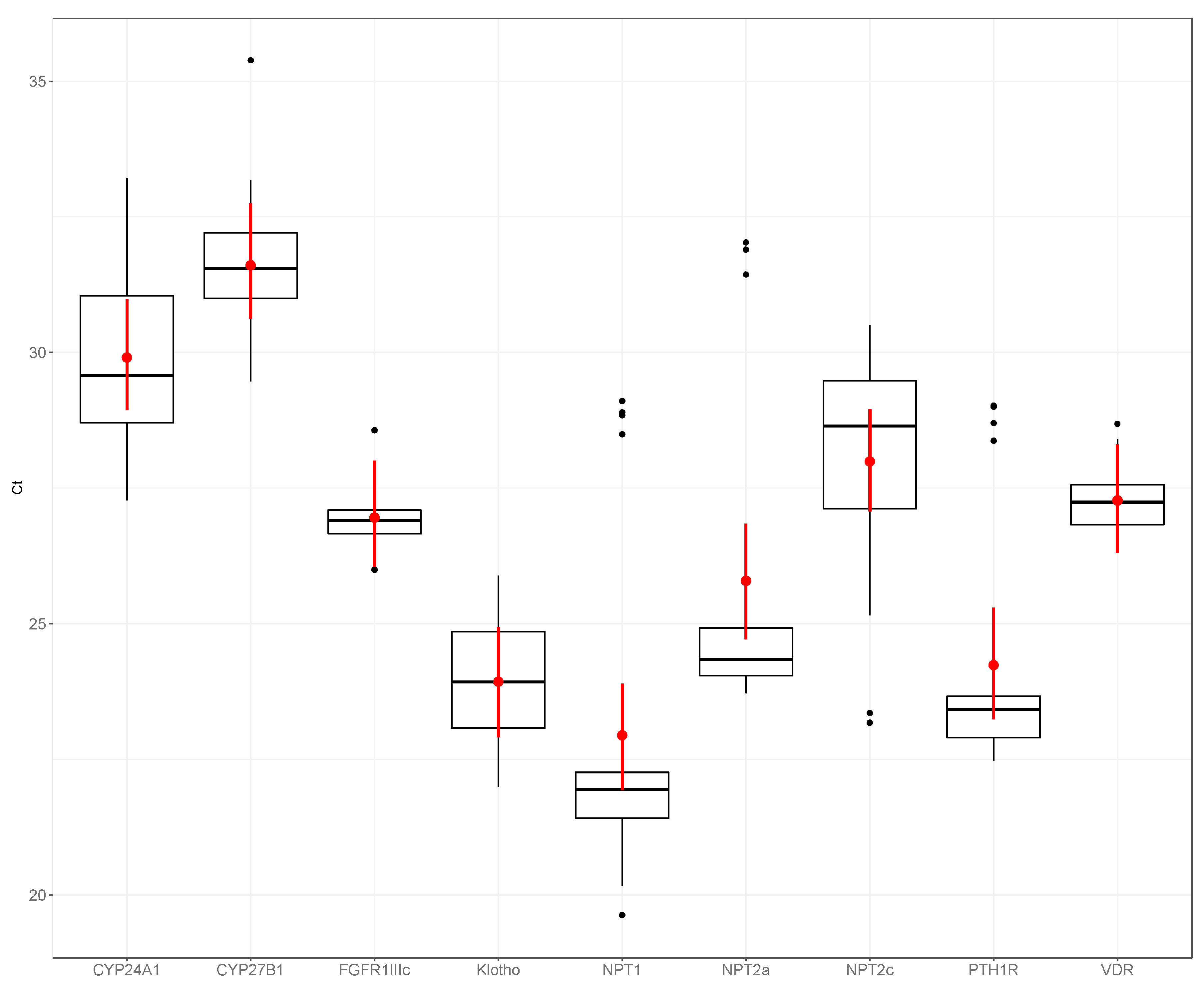
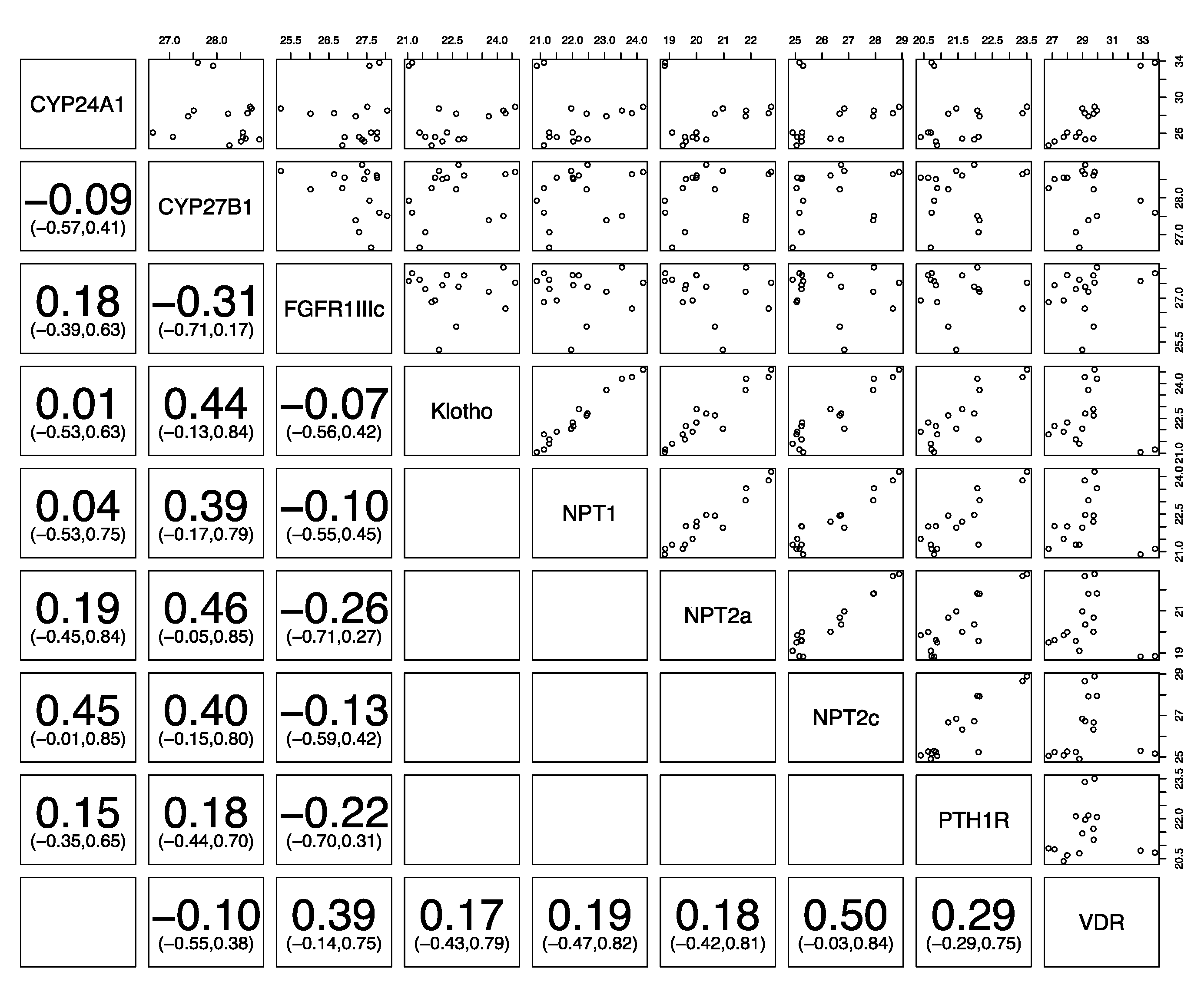
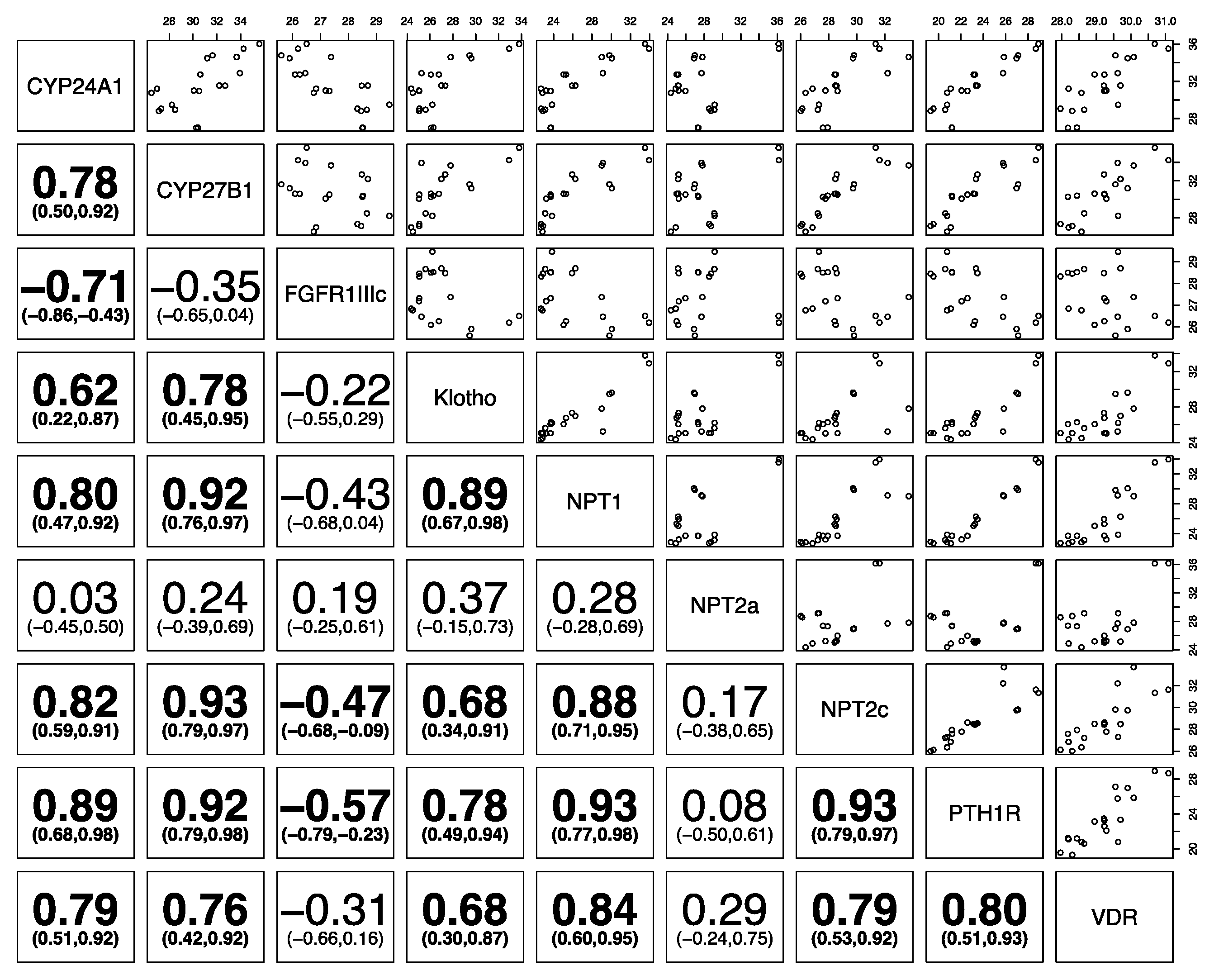
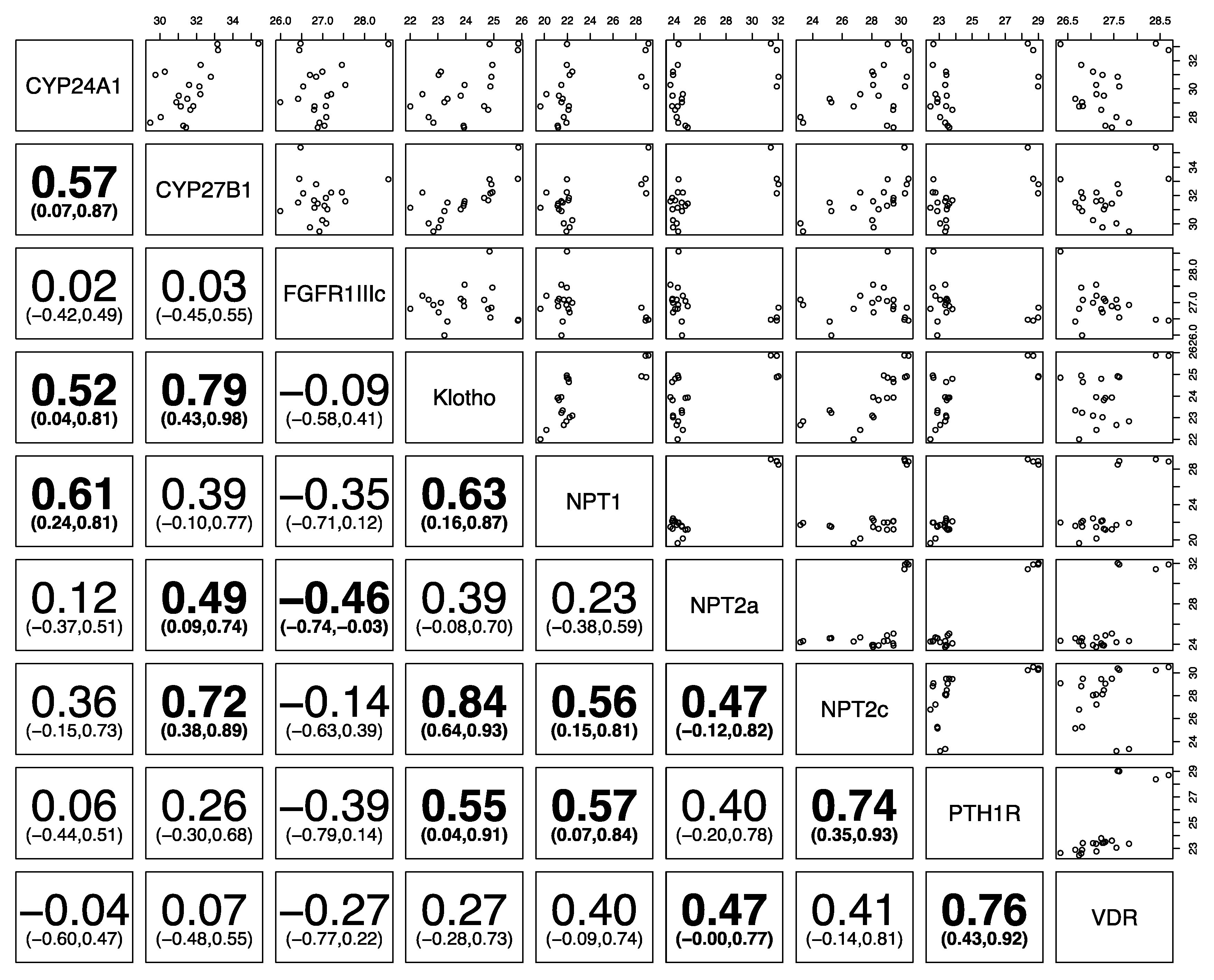
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hardcastle, M.R.; Dittmer, K.E. Fibroblast Growth Factor 23: A New Dimension to Diseases of Calcium-Phosphorus Metabolism. Vet. Pathol. 2015, 52, 770–784. [Google Scholar] [CrossRef]
- Bonewald, L.F.; Wacker, M.J. FGF23 production by osteocytes. Pediatr. Nephrol. 2013, 28, 563–568. [Google Scholar] [CrossRef]
- Erben, R.G.; Andrukhova, O. FGF23-Klotho signaling axis in the kidney. Bone 2017, 100, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Urakawa, I.; Yamazaki, Y.; Shimada, T.; Iijima, K.; Hasegawa, H.; Okawa, K.; Fujita, T.; Fukumoto, S.; Yamashita, T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006, 444, 770. [Google Scholar] [CrossRef] [PubMed]
- Tomoe, Y.; Segawa, H.; Shiozawa, K.; Kaneko, I.; Tominaga, R.; Hanabusa, E.; Aranami, F.; Furutani, J.; Kuwahara, S.; Tatsumi, S.; et al. Phosphaturic action of fibroblast growth factor 23 in Npt2 null mice. Am. J. Physiol.-Ren. Physiol. 2010, 298, F1341–F1350. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Kakitani, M.; Yamazaki, Y.; Hasegawa, H.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Tomizuka, K.; Yamashita, T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Investig. 2004, 113, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Wöhrle, S.; Bonny, O.; Beluch, N.; Gaulis, S.; Stamm, C.; Scheibler, M.; Müller, M.; Kinzel, B.; Thuery, A.; Brueggen, J.; et al. FGF receptors control vitamin D and phosphate homeostasis by mediating renal FGF-23 signaling and regulating FGF-23 expression in bone. J. Bone Miner. Res. 2011, 26, 2486–2497. [Google Scholar] [CrossRef]
- Silver, J.; Naveh-Many, T. FGF23 and the parathyroid. Adv. Exp. Med. Biol. 2012, 728, 92–99. [Google Scholar]
- Dittmer, K.E.; Perera, K.C.; Elder, P.A. Serum fibroblast growth factor 23 concentrations in dogs with chronic kidney disease. Res. Vet. Sci. 2017, 114, 348–350. [Google Scholar] [CrossRef]
- Geddes, R.F.; Finch, N.C.; Elliott, J.; Syme, H.M. Fibroblast growth factor 23 in feline chronic kidney disease. J. Vet. Intern. Med. 2013, 27, 234–241. [Google Scholar] [CrossRef]
- Fliser, D.; Kollerits, B.; Neyer, U.; Ankerst, D.P.; Lhotta, K.; Lingenhel, A.; Ritz, E.; Kronenberg, F.; Kuen, E.; Konig, P.; et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) Study. J. Am. Soc. Nephrol. 2007, 18, 2600–2608. [Google Scholar] [CrossRef]
- Finch, N.C.; Geddes, R.F.; Syme, H.M.; Elliott, J. Fibroblast growth factor 23 (FGF-23) concentrations in cats with early nonazotemic chronic kidney disease (CKD) and in healthy geriatric cats. J. Vet. Intern. Med. 2013, 27, 227–233. [Google Scholar] [CrossRef]
- Isakova, T.; Wahl, P.; Vargas, G.S.; Gutierrez, O.M.; Scialla, J.; Xie, H.; Appleby, D.; Nessel, L.; Bellovich, K.; Chen, J.; et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011, 79, 1370–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myakala, K.; Motta, S.; Murer, H.; Wagner, C.A.; Koesters, R.; Biber, J.; Hernando, N. Renal-specific and inducible depletion of NaPi-IIc/Slc34a3, the cotransporter mutated in HHRH, does not affect phosphate or calcium homeostasis in mice. Am. J. Physiol.-Ren. Physiol. 2014, 306, F833–F843. [Google Scholar] [CrossRef] [Green Version]
- Bergwitz, C.; Roslin, N.M.; Tieder, M.; Loredo-Osti, J.C.; Bastepe, M.; Abu-Zahra, H.; Frappier, D.; Burkett, K.; Carpenter, T.O.; Anderson, D.; et al. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am. J. Hum. Genet. 2006, 78, 179–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinour, D.; Davidovits, M.; Ganon, L.; Ruminska, J.; Forster, I.C.; Hernando, N.; Eyal, E.; Holtzman, E.J.; Wagner, C.A. Loss of function of NaPiIIa causes nephrocalcinosis and possibly kidney insufficiency. Pediatr. Nephrol. 2016, 31, 2289–2297. [Google Scholar] [CrossRef] [PubMed]
- Muscher-Banse, A.S.; Breves, G. Mechanisms and regulation of epithelial phosphate transport in ruminants: Approaches in comparative physiology. Pflügers Arch.-Eur. J. Physiol. 2019, 471, 185–191. [Google Scholar] [CrossRef]
- Firmenich, C.S.; Elfers, K.; Wilkens, M.R.; Breves, G.; Muscher-Banse, A.S. Modulation of renal calcium and phosphate transporting proteins by dietary nitrogen and/or calcium in young goats1. J. Anim. Sci. 2018, 96, 3208–3220. [Google Scholar] [CrossRef]
- Shirazi-Beechey, S.P.; Penny, J.I.; Dyer, J.; Wood, I.S.; Tarpey, P.S.; Scott, D.; Buchan, W. Epithelial phosphate transport in ruminants, mechanisms and regulation. Kidney Int. 1996, 49, 992–996. [Google Scholar] [CrossRef] [Green Version]
- Muscher, A.S.; Wilkens, M.R.; Mrochen, N.; Schröder, B.; Breves, G.; Huber, K. Ex vivo intestinal studies on calcium and phosphate transport in growing goats fed a reduced nitrogen diet. Br. J. Nutr. 2012, 108, 628–637. [Google Scholar] [CrossRef]
- Muscher-Banse, A.S.; Marholt, L.; Eigendorf, N.; Wilkens, M.R.; Schröder, B.; Breves, G.; Cehak, A. Segmental diversity of phosphate transport along the intestinal axis in horses1. J. Anim. Sci. 2017, 95, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Foote, A.P.; Lambert, B.D.; Brady, J.A.; Muir, J.P. Short communication: Phosphate transporter expression in Holstein cows. J. Dairy Sci. 2011, 94, 1913–1916. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.; Walter, C.; Schröder, B.; Breves, G. Phosphate transport in the duodenum and jejunum of goats and its adaptation by dietary phosphate and calcium. Am. J. Physiol. Integr. Comp. Physiol. 2002, 283, R296–R302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azarpeykan, S.; Dittmer, K.E.; Marshall, J.C.; Perera, K.C.; Gee, E.K.; Acke, E.; Thompson, K.G. Evaluation and Comparison of Vitamin D responsive gene expression in ovine, canine and equine kidney. PLoS ONE 2016, 11, e0162598. [Google Scholar] [CrossRef]
- Azarpeykan, S.; Dittmer, K.E. Evaluation of housekeeping genes for quantitative gene expression analysis in the equine kidney. J. Equine Sci. 2016, 27, 165–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, I.R.; Peeters, D.; Helps, C.R.; Day, M.J. Development and application of multiple internal reference (housekeeper) gene assays for accurate normalisation of canine gene expression studies. Vet. Immunol. Immunopathol. 2007, 117, 55–66. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Bates, D.; Machler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Simesen, M.G. Calcium, inorganic phosphorus, and magnesium metabolism in health and disease. In Clinical Biochemistry of Domestic Animals; Kaneko, J.J., Cornelius, C.E., Eds.; Academic Press, Inc.: New York, NY, USA; London, UK, 1970; pp. 313–375. [Google Scholar]
- Tenenhouse, H.S.; Roy, S.; Martel, J.; Gauthier, C. Differential expression, abundance, and regulation of Na+-phosphate cotransporter genes in murine kidney. Am. J. Physiol. 1998, 275, F527–F534. [Google Scholar] [CrossRef]
- Lederer, E.; Miyamoto, K. Clinical Consequences of Mutations in Sodium Phosphate Cotransporters. Clin. J. Am. Soc. Nephrol. 2012, 7, 1179–1187. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Tenenhouse, H.S. Npt2 gene disruption confers resistance to the inhibitory action of parathyroid hormone on renal sodium-phosphate cotransport. Endocrinology 2000, 141, 2159–2165. [Google Scholar] [CrossRef]
- Hoag, H.M.; Martel, J.; Gauthier, C.; Tenenhouse, H.S. Effects of Npt2 gene ablation and low-phosphate diet on renal Na(+)/phosphate cotransport and cotransporter gene expression. J. Clin. Investig. 1999, 104, 679–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasanen, L.A.; Wiitanen, P.A.; Lilius, E.M.; Hyyppa, S.; Poso, A.R. Accumulation of uric acid in plasma after repeated bouts of exercise in the horse. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 114, 139–144. [Google Scholar] [CrossRef]
- Castejon, F.; Trigo, P.; Munoz, A.; Riber, C. Uric acid responses to endurance racing and relationships with performance, plasma biochemistry and metabolic alterations. Equine Vet. J. 2006, 38, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Schröder, B.; Walter, C.; Breves, G.; Huber, K. Comparative studies on Na-dependent Pi transport in ovine, caprine and porcine renal cortex. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2000, 170, 387–393. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho and the Aging Process. Korean J. Intern. Med. 2011, 26, 113–122. [Google Scholar] [CrossRef]
- Cha, S.-K.; Ortega, B.; Kurosu, H.; Rosenblatt, K.P.; Kuro-O, M.; Huang, C.-L. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc. Natl. Acad. Sci. USA 2008, 105, 9805–9810. [Google Scholar] [CrossRef] [Green Version]
- Cha, S.-K.; Hu, M.-C.; Kurosu, H.; Kuro-o, M.; Moe, O.; Huang, C.-L. Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol. Pharmacol. 2009, 76, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of aging in mice by the hormone Klotho. Science (80-.) 2005, 309, 1829–1833. [Google Scholar] [CrossRef] [Green Version]
- Doi, S.; Zou, Y.; Togao, O.; Pastor, J.V.; John, G.B.; Wang, L.; Shiizaki, K.; Gotschall, R.; Schiavi, S.; Yorioka, N.; et al. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J. Biol. Chem. 2011, 286, 8655–8665. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.; David, V.; Quarles, L.D. Regulation and function of the FGF23/klotho endocrine pathways. Physiol. Rev. 2012, 92, 131–155. [Google Scholar] [CrossRef]
- Chanakul, A.; Zhang, M.Y.H.; Louw, A.; Armbrecht, H.J.; Miller, W.L.; Portale, A.A.; Perwad, F. FGF-23 Regulates CYP27B1 Transcription in the Kidney and in Extra-Renal Tissues. PLoS ONE 2013, 8, e72816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, B.; David, V.; Martin, A.; Huang, J.; Li, H.; Jiao, Y.; Gu, W.; Quarles, L.D. A Comparative Transcriptome Analysis Identifying FGF23 Regulated Genes in the Kidney of a Mouse CKD Model. PLoS ONE 2012, 7, e44161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kägi, L.; Bettoni, C.; Pastor-Arroyo, E.M.; Schnitzbauer, U.; Hernando, N.; Wagner, C.A. Regulation of vitamin D metabolizing enzymes in murine renal and extrarenal tissues by dietary phosphate, FGF23, and 1,25(OH)2D3. PLoS ONE 2018, 13, e0195427. [Google Scholar] [CrossRef] [PubMed]
- Mahon, M.J. The Parathyroid Hormone 1 Receptor Directly Binds to the FERM Domain of Ezrin, an Interaction that Supports Apical Receptor Localization and Signaling in LLC-PK1 Cells. Mol. Endocrinol. 2009, 23, 1691–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahon, M.J.; Donowitz, M.; Yun, C.C.; Segre, G. V Na(+)/H(+ ) exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature 2002, 417, 858–861. [Google Scholar] [CrossRef]
- Bastepe, M.; Turan, S.; He, Q. Heterotrimeric G proteins in the control of parathyroid hormone actions. J. Mol. Endocrinol. 2017, 58, R203–R224. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dittmer, K.E.; Heathcott, R.W.; Marshall, J.C.; Azarpeykan, S. Expression of Phosphatonin-Related Genes in Sheep, Dog and Horse Kidneys Using Quantitative Reverse Transcriptase PCR. Animals 2020, 10, 1806. https://doi.org/10.3390/ani10101806
Dittmer KE, Heathcott RW, Marshall JC, Azarpeykan S. Expression of Phosphatonin-Related Genes in Sheep, Dog and Horse Kidneys Using Quantitative Reverse Transcriptase PCR. Animals. 2020; 10(10):1806. https://doi.org/10.3390/ani10101806
Chicago/Turabian StyleDittmer, Keren E., Rosemary W. Heathcott, Jonathan C. Marshall, and Sara Azarpeykan. 2020. "Expression of Phosphatonin-Related Genes in Sheep, Dog and Horse Kidneys Using Quantitative Reverse Transcriptase PCR" Animals 10, no. 10: 1806. https://doi.org/10.3390/ani10101806
APA StyleDittmer, K. E., Heathcott, R. W., Marshall, J. C., & Azarpeykan, S. (2020). Expression of Phosphatonin-Related Genes in Sheep, Dog and Horse Kidneys Using Quantitative Reverse Transcriptase PCR. Animals, 10(10), 1806. https://doi.org/10.3390/ani10101806




