In Vitro Culture of Chicken Circulating and Gonadal Primordial Germ Cells on a Somatic Feeder Layer of Avian Origin
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fertilized Eggs and Incubation
2.2. Preparation of Feeder Cells
2.3. Chick Embryo Extracts
2.4. Preparation of Rat Tail Collagen
2.5. Culture of PGCs
2.5.1. Culture Medium
2.5.2. Culture of gPGCs
2.5.3. Culture of cPGCs
2.6. Migratory Abilities of Cultured PGCs to the Recipient Gonads
3. Results
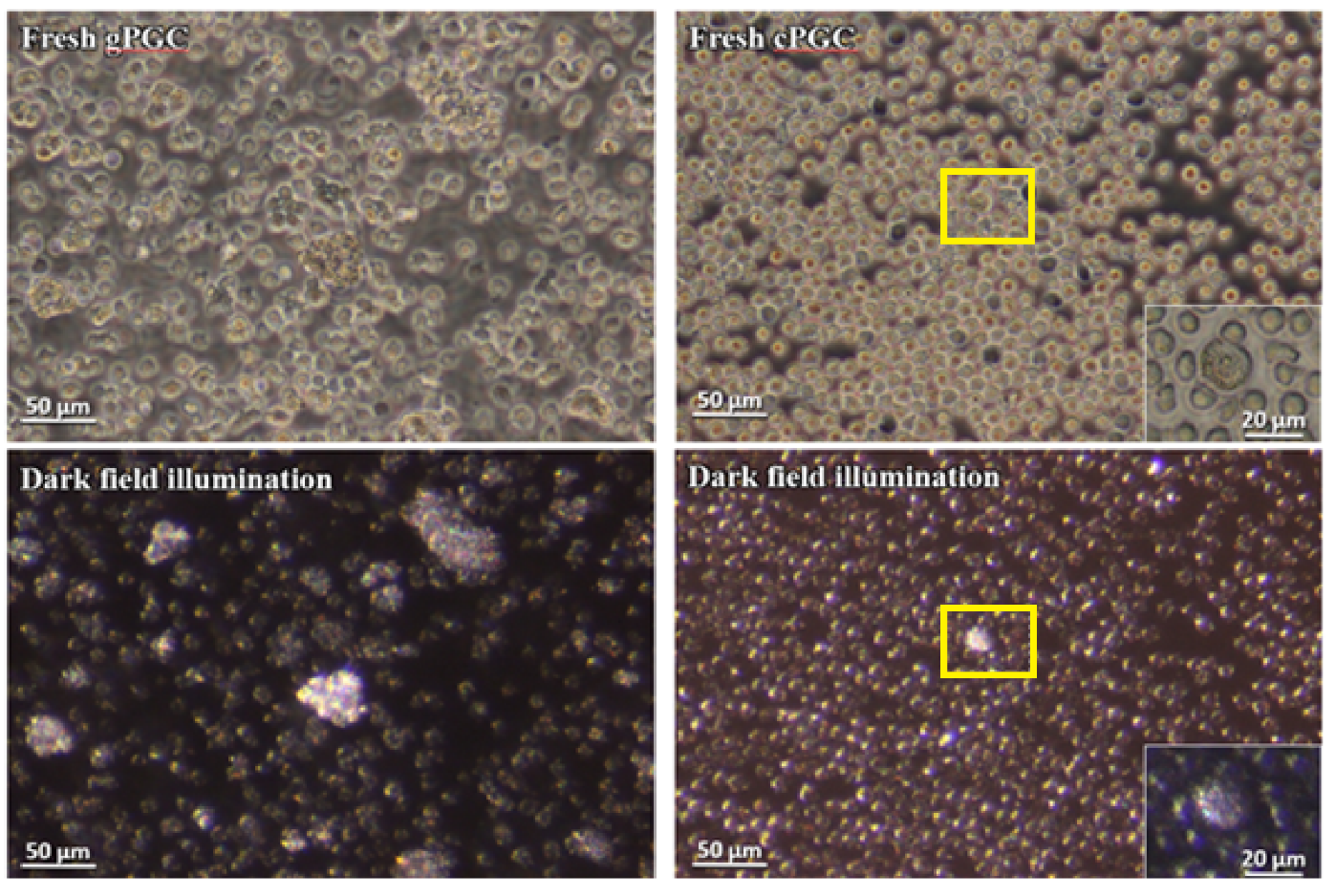
3.1. Identification of Fresh and Cultured PGCs
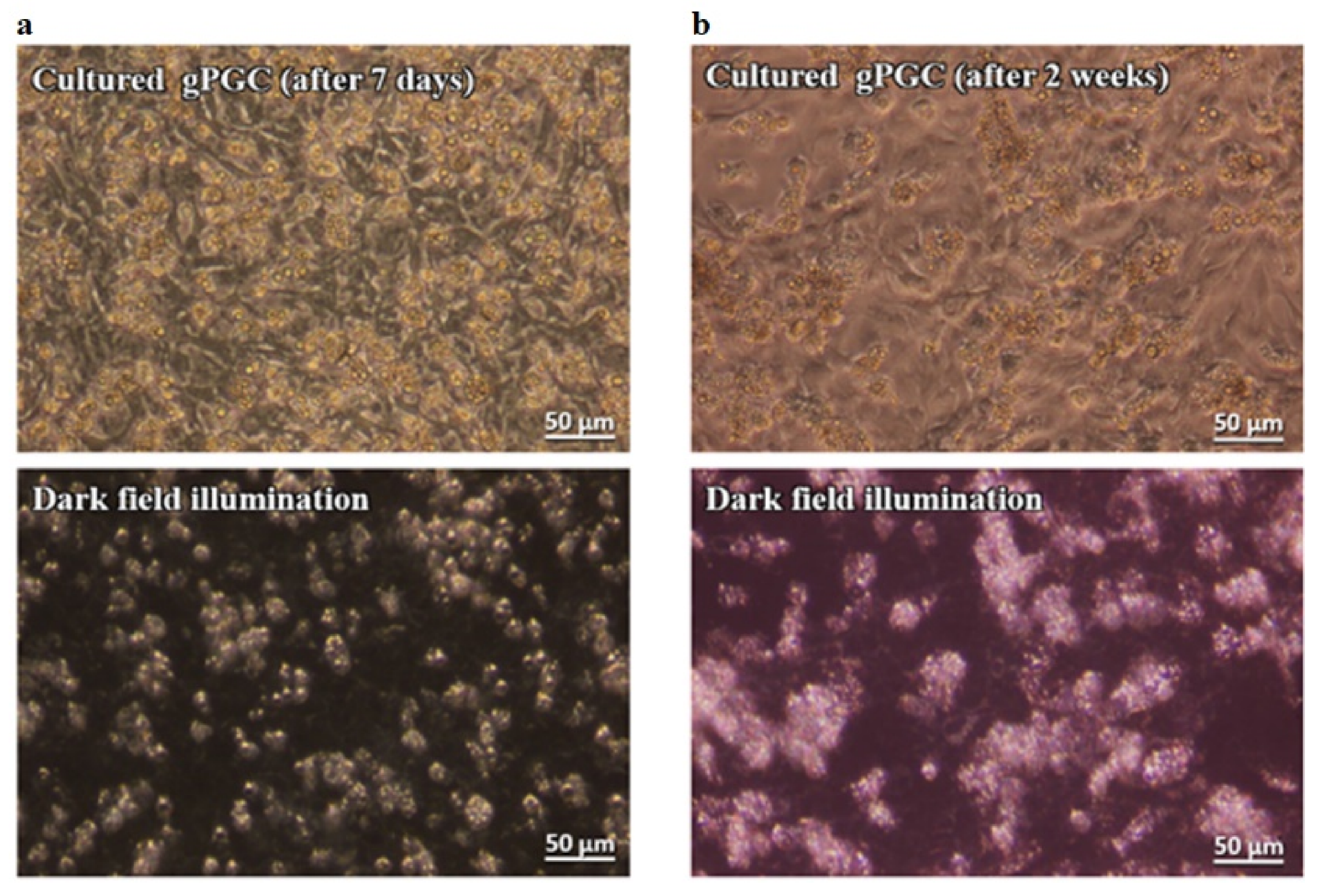
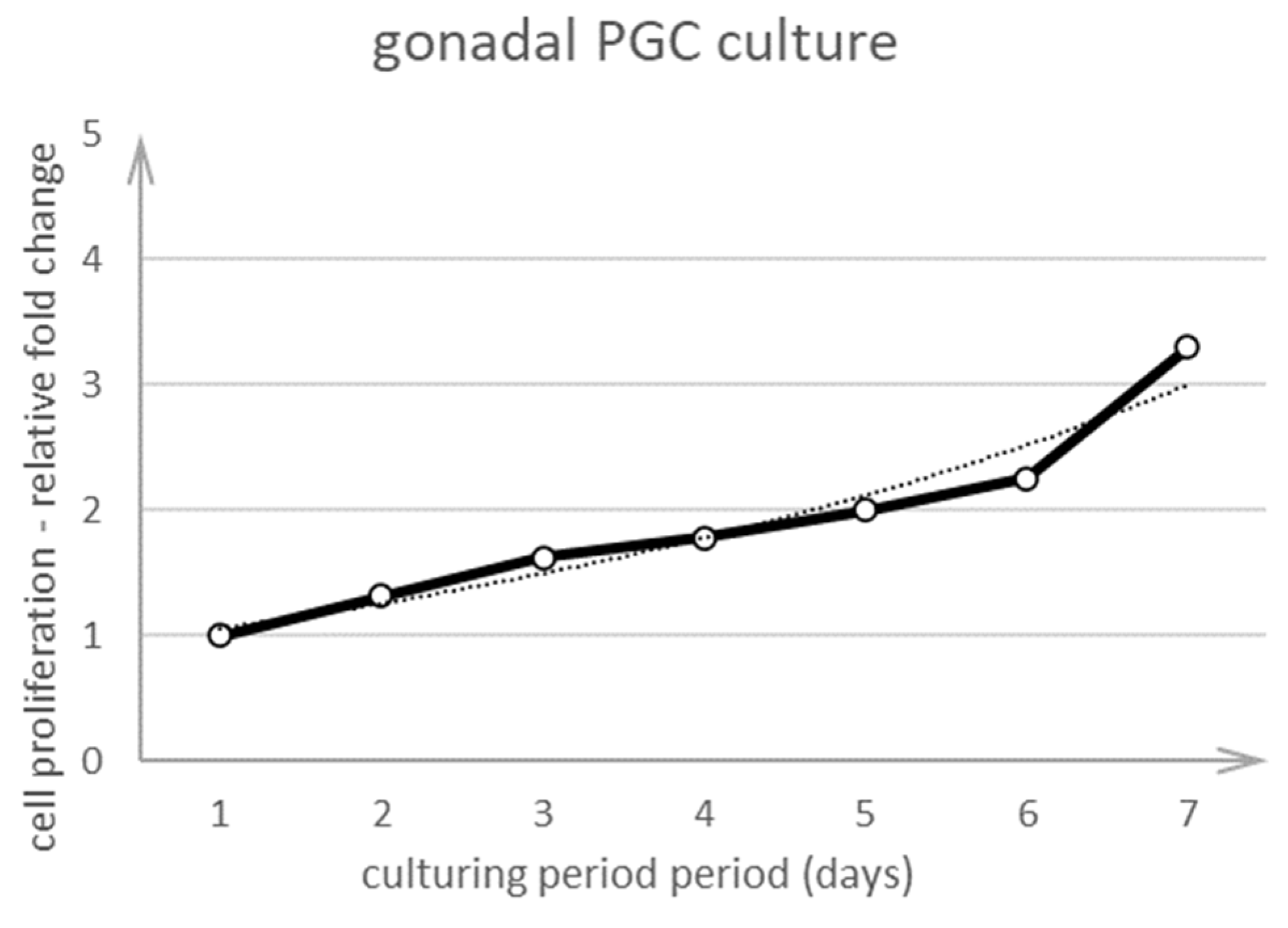
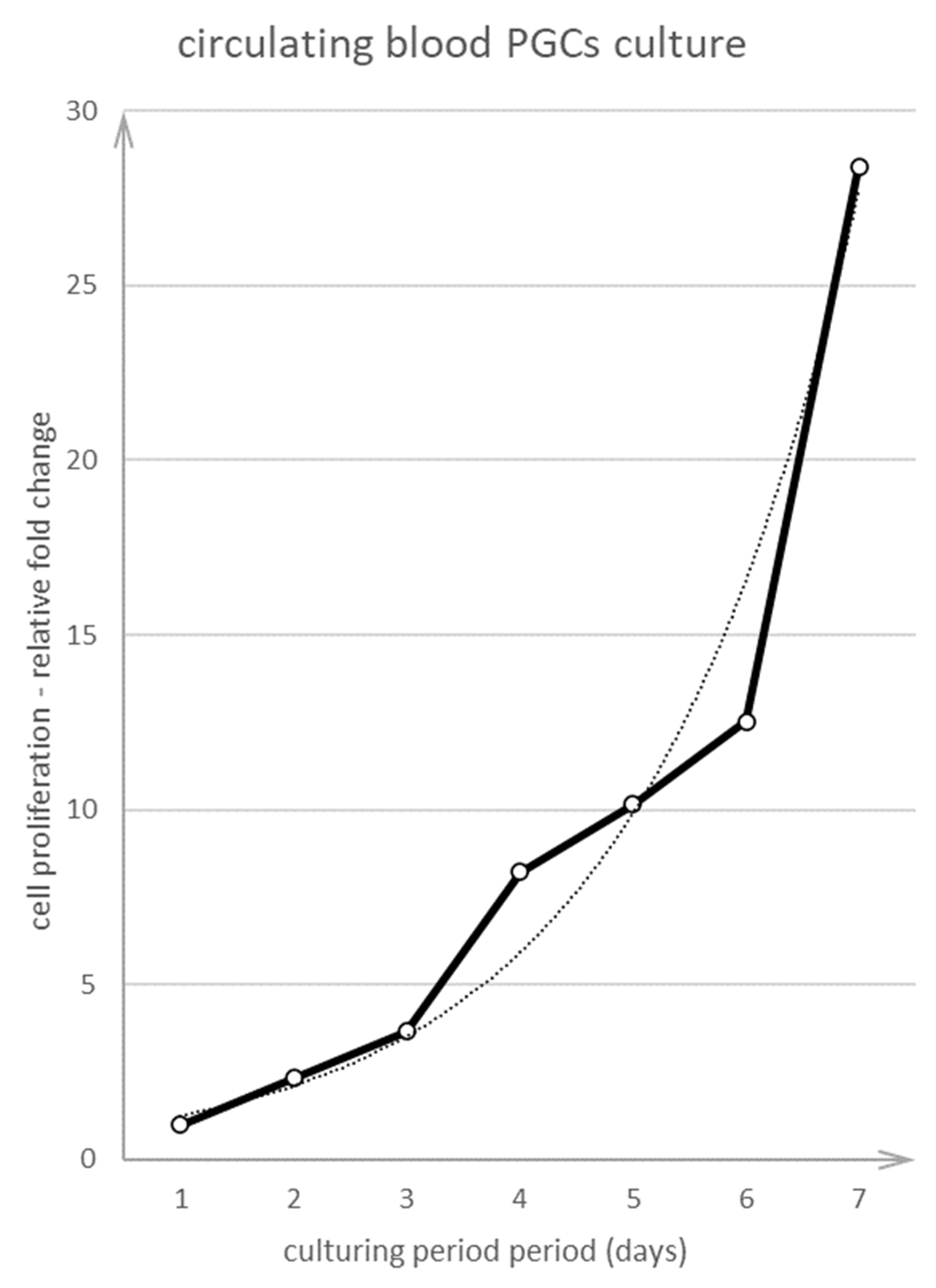
3.2. Culture of PGCs
3.2.1. Culture of PGCs Collected at Stage 18 H&H
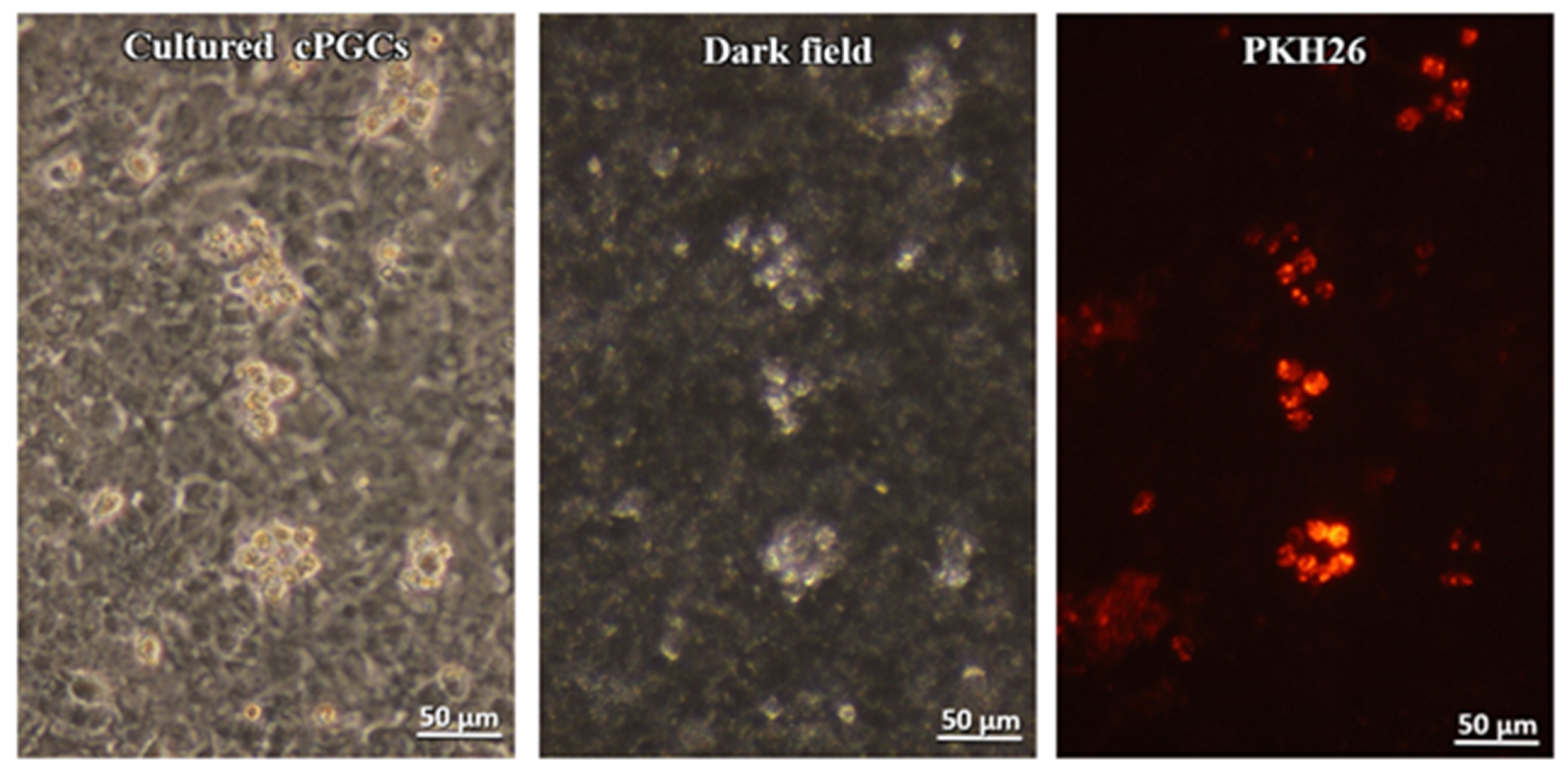
3.2.2. Culture of cPGCs at Stages 14 to 15 H&H
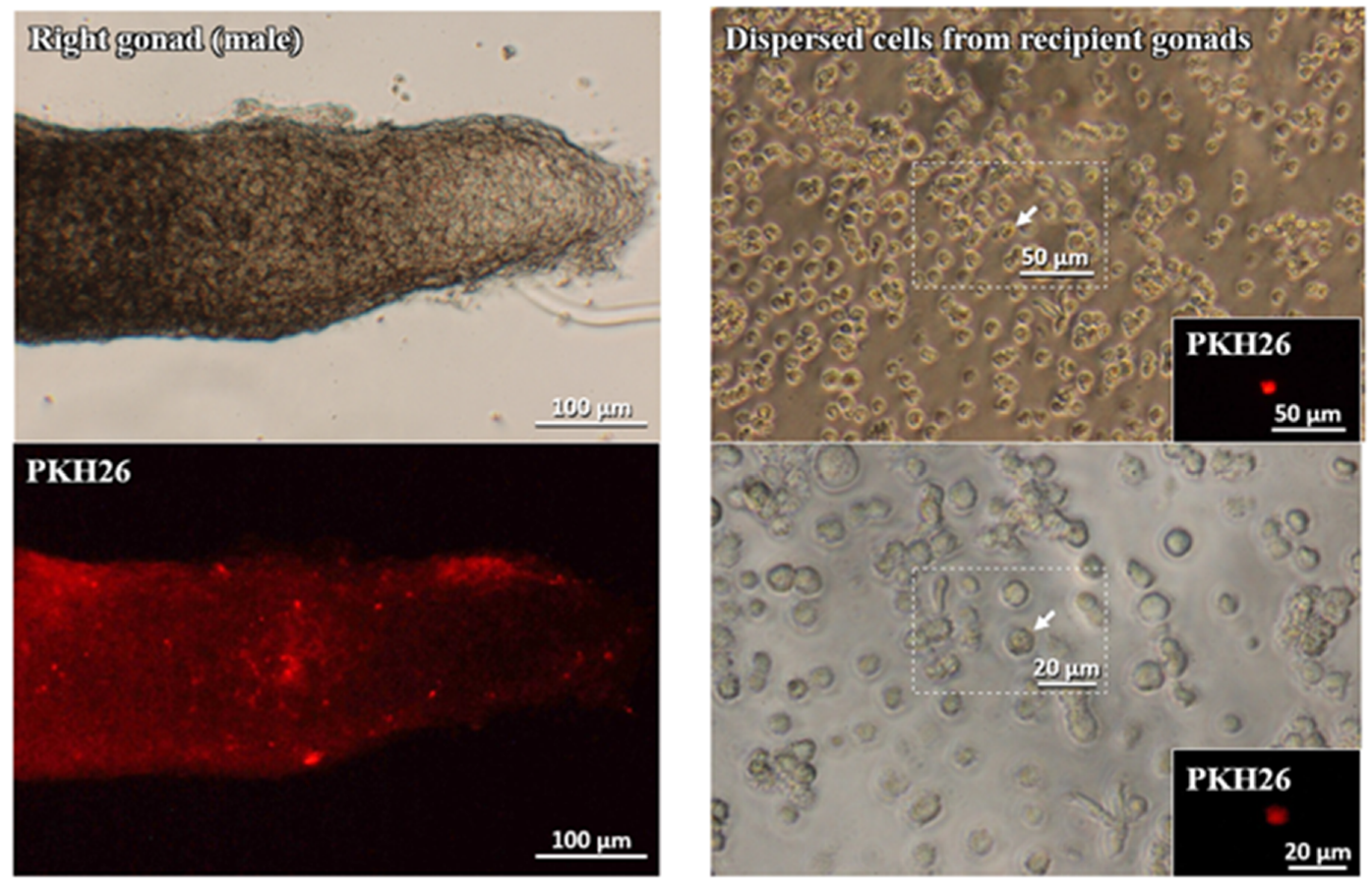
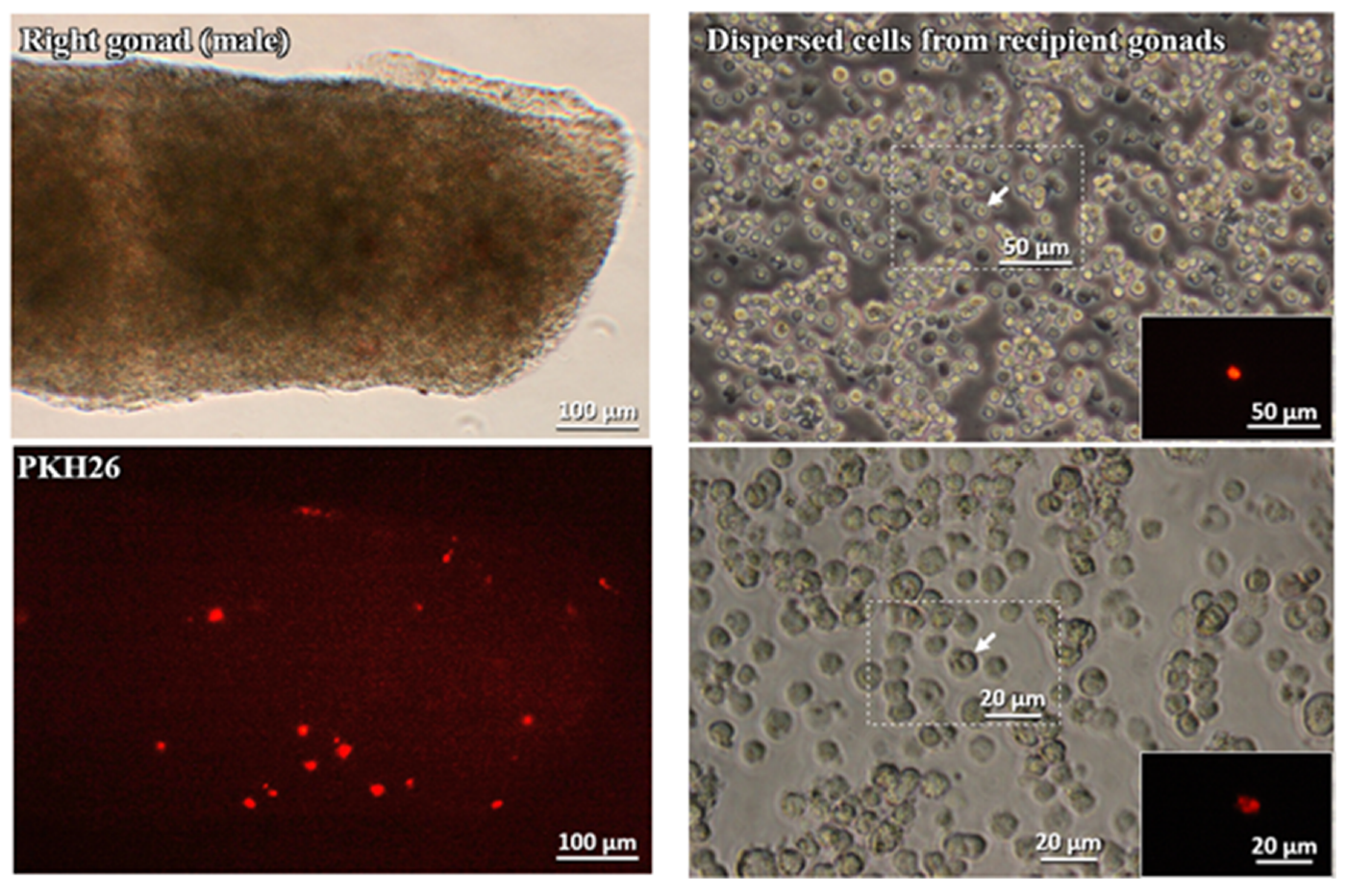
3.3. Migratory Ability of Cultured PGCs to the Recipient Gonads
3.3.1. Cultured gPGCs
3.3.2. Cultured cPGCs
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sawicka, D.; Chojnacka-Puchta, L.; Zielinski, M.; Plucienniczak, G.; Plucienniczak, A.; Bednarczyk, M. Flow cytometric analysis of apoptosis in cryoconserved chicken primordial germ cells. Cell. Mol. Biol. Lett. 2015, 20, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, M.E.; Gheyas, A.A.; Mason, A.S.; Nandi, S.; Taylor, L.; Sherman, A.; Smith, J.; Burt, D.W.; Hawken, R.; McGrew, M.J. Reviving rare chicken breeds using genetically engineered sterility in surrogate host birds. Proc. Natl. Acad. Sci. USA 2016, 116, 20930–20937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mucksová, J.; Reinišová, M.; Kalina, J.; Lejčková, B.; Hejnar, J.; Trefil, P. Conservation of chicken male germline by orthotopic transplantation of primordial germ cells from genetically distant donors. Biol. Reprod. 2019, 101, 200–207. [Google Scholar] [CrossRef]
- Molnár, M.; Lázár, B.; Sztán, N.; Végi, B.; Drobnyák, Á.; Tóth, R.; Liptói, K.; Marosán, M.; Gócza, E.; Nandi, S.; et al. Investigation of the Guinea fowl and domestic fowl hybrids as potential surrogate hosts for avian cryopreservation programmes. Sci. Rep. 2019, 9, 14284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chojnacka-Puchta, L.; Sawicka, D. CRISPR/Cas9 gene editing in a chicken model: Current approaches and applications. J. Appl. Genet. 2020, 61, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Bednarczyk, M.; Kozłowska, I.; Łakota, P.; Szczerba, A.; Stadnicka, K.; Kuwana, T. Generation of transgenic chickens by the non-viral, cell-based method: Effectivness of some elements of this strategy. J. Appl. Genet. 2018, 59, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Kuwana, T. Migration of Avian Primordial Germ Cells toward the Gonadal Anlage. Dev. Growth Differ. 1993, 35, 237–243. [Google Scholar] [CrossRef]
- Tajima, A.; Hayashi, H.; Kamizumi, A.; Ogura, J.; Kuwana, T.; Chikamune, T. Study on the concentration of circulating primordial germ cells (cPGCs) in early chick embryos. J. Exp. Zool. 1999, 284, 759–764. [Google Scholar] [CrossRef]
- Kuwana, T.; Rogulska, T. Migratory mechanisms of chick primordial germ cells toward gonadal anlage. Cell. Mol. Biol. 1999, 45, 725–736. [Google Scholar]
- Motono, M.; Ohashi, T.; Nishijima, K.I.; Iijima, S. Analysis of chicken primordial germ cells. Cytotechnology 2008, 57, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Raucci, F.; Fuet, A.; Pain, B. In vitro generation and characterization of chicken long-term germ cells from different embryonic origins. Theriogenology 2015, 84, 732–742.e2. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Han, J.Y. Derivation and characterization of pluripotent embryonic germ cells in chicken. Mol. Reprod. Dev. 2000, 56, 475–482. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Minematsu, T.; Harumi, T.; Kuwana, T. Preferential Migration of Transferred Primordial Germ Cells to Left Germinal Ridge of Recipient Embryos in Chickens. J. Poult. Sci. 2009, 46, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Van De Lavoir, M.C.; Diamond, J.H.; Leighton, P.A.; Mather-Love, C.; Heyer, B.S.; Bradshaw, R.; Kerchner, A.; Hooi, L.T.; Gessaro, T.M.; Swanberg, S.E.; et al. Germline transmission of genetically modified primordial germ cells. Nature 2006, 441, 766–769. [Google Scholar] [CrossRef]
- Macdonald, J.; Glover, J.D.; Taylor, L.; Sang, H.M.; McGrew, M.J. Characterisation and germline transmission of cultured avian primordial germ cells. PLoS ONE 2010, 5, e15518. [Google Scholar] [CrossRef] [Green Version]
- Tonus, C.; Cloquette, K.; Ectors, F.; Piret, J.; Gillet, L.; Antoine, N.; Desmecht, D.; Vanderplasschen, A.; Waroux, O.; Grobet, L. Long term-cultured and cryopreserved primordial germ cells from various chicken breeds retain high proliferative potential and gonadal colonisation competency. Reprod. Fertil. Dev. 2016, 28, 628–639. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kagami, H.; Tagami, T. Development, differentiation and manipulation of chicken germ cells. Dev. Growth Differ. 2013, 55, 20–40. [Google Scholar] [CrossRef]
- Kim, Y.M.; Han, J.Y. The early development of germ cells in chicken. Int. J. Dev. Biol. 2018, 62, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozdziak, P.E.; Angerman-Stewart, J.; Rushton, B.; Pardue, S.L.; Petitte, J.N. Isolation of chicken primordial germ cells using fluorescence-activated cell sorting. Poult. Sci. 2005, 84, 594–600. [Google Scholar] [CrossRef]
- Kuwana, T.; Hashimoto, K.; Nakanishi, A.; Yasuda, Y.; Tajima, A.; Naito, M. Long-term culture of avian embryonic cells in vitro. Int. J. Dev. Biol. 1996, 40, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Szczerba, A.; Kuwana, T.; Bednarczyk, M. The Developmental Changes in the Extra-Embryonic Vascular System in the Circulating Phase of Primordial Germ Cells in Aves. Folia Biol. 2019, 67, 79–83. [Google Scholar] [CrossRef]
- Oishi, I. Improvement of Transfection Efficiency in Cultured Chicken Primordial Germ Cells by Percoll Density Gradient Centrifugation. Biosci. Biotechnol. Biochem. 2010, 74, 2426–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyahara, D.; Mori, T.; Makino, R.; Nakamura, Y.; Oishi, I.; Ono, T.; Nirasawa, K.; Tagami, T.; Kagami, H. Culture conditions for maintain propagation, long-term survival and germline transmission of chicken primordial germ cell-like cells. J. Poult. Sci. 2014, 51, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Whyte, J.; Glover, J.D.; Woodcock, M.; Brzeszczynska, J.; Taylor, L.; Sherman, A.; Kaiser, P.; McGrew, M.J. FGF, Insulin, and SMAD Signaling Cooperate for Avian Primordial Germ Cell Self-Renewal. Stem Cell Rep. 2015, 5, 1171–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.W.; Kim, S.; Kim, T.M.; Kim, Y.M.; Seo, H.W.; Park, T.S.; Jeong, J.W.; Song, G.; Han, J.Y. Basic fibroblast growth factor activates MEK/ERK cell signaling pathway and stimulates the proliferation of chicken primordial germ cells. PLoS ONE 2010, 5, e12968. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Wang, Y.; Hou, L.; Chen, L.; Li, X.; Yue, W.; Ma, Y. Derivation and characteristics of pluripotent embryonic germ cells in duck. Poult. Sci. 2010, 89, 312–317. [Google Scholar] [CrossRef]
- Anand, M.; Lázár, B.; Tóth, R.; Páll, E.; Patakiné Várkonyi, E.; Liptói, K.; Homolya, L.; Hegyi, Z.; Hidas, A.; Gócza, E. Enhancement of chicken primordial germ cell in vitro maintenance using an automated cell image analyser. Acta Vet. Hung. 2018, 66, 518–529. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Lin, S.P.; Chang, Y.Y.; Chang, W.P.; Wei, L.Y.; Liu, H.C.; Huang, J.F.; Pain, B.; Wu, S.C. In vitro culture and characterization of duck primordial germ cells. Poult. Sci. 2019, 98, 1820–1832. [Google Scholar] [CrossRef]
- Naito, M.; Harumi, T.; Kuwana, T. Long-term culture of chicken primordial germ cells isolated from embryonic blood and production of germline chimaeric chickens. Anim. Reprod. Sci. 2015, 153, 50–61. [Google Scholar] [CrossRef]
- Kress, C.; Montillet, G.; Jean, C.; Fuet, A.; Pain, B. Chicken embryonic stem cells and primordial germ cells display different heterochromatic histone marks than their mammalian counterparts. Epigenet. Chromatin 2016, 9, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| No. of Recipient Embryo (Injected Stages) | Sex | Presence of Injected gPGCs in Gonads | |
|---|---|---|---|
| Right Gonad | Left Gonad | ||
| G-01 (stage 14) | Female | − | + |
| G-02 (stage 14) | Male | − | + |
| G-03 (stage 14) | Male | − | + |
| G-04 (stage 15) | Female | + | + |
| G-05 (stage15) | Female | − | + |
| G-06 (stage 15) | Male | + | + |
| G-07 (stage 16) | Female | ++ | + |
| G-08 (stage 15+) | Dead | ||
| G-09 (stage 16) | Female | + | + |
| G-10 (stage 15) | Female | + | + |
| G-11 (stage 15) | Male | + | ++ |
| G-12 (stage 16) | Dead | ||
| G-13 (stage 16) | Dead | ||
| G-14 (stage 16) | Dead | ||
| No. of Recipient Embryo (Injected Stages) | Sex | Presence of Injected gPGCs in Gonads | |
|---|---|---|---|
| Right Gonad | Left Gonad | ||
| C-01 (stage 14) | Dead | ||
| C-02 (stage 14) | Dead | ||
| C-03 (stage 15) | Dead | ||
| C-04 (stage 15) | Female | + | + |
| C-05 (stage 13) | Dead | ||
| C-06 (stage 14) | Male | + | ++ |
| C-07 (stage 15) | Dead | ||
| C-08 (stage 15) | Dead | ||
| C-09 (stage 14) | Dead | ||
| C-10 (stage 14) | Male | ++ | + |
| C-11 (stage 13+) | Female | ++ | + |
| C-12 (stage 13) | Male | − | − |
| C-13 (stage 16) | Male | − | − |
| C-14 (stage 15) | Female | ++ | − |
| C-15 (stage 14) | Male | + | + |
| C-16 (stage 16) | Male | ++ | + |
| C-17 (stage 16+) | Male | ++ | + |
| C-18 (stage 15+) | Dead | ||
| C-19 (stage 15) | Dead | ||
| C-20 (stage 16) | Female | + | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczerba, A.; Kuwana, T.; Paradowska, M.; Bednarczyk, M. In Vitro Culture of Chicken Circulating and Gonadal Primordial Germ Cells on a Somatic Feeder Layer of Avian Origin. Animals 2020, 10, 1769. https://doi.org/10.3390/ani10101769
Szczerba A, Kuwana T, Paradowska M, Bednarczyk M. In Vitro Culture of Chicken Circulating and Gonadal Primordial Germ Cells on a Somatic Feeder Layer of Avian Origin. Animals. 2020; 10(10):1769. https://doi.org/10.3390/ani10101769
Chicago/Turabian StyleSzczerba, Agata, Takashi Kuwana, Michelle Paradowska, and Marek Bednarczyk. 2020. "In Vitro Culture of Chicken Circulating and Gonadal Primordial Germ Cells on a Somatic Feeder Layer of Avian Origin" Animals 10, no. 10: 1769. https://doi.org/10.3390/ani10101769





