Effects of Long-Term Selection in the Border Collie Dog Breed: Inbreeding Purge of Canine Hip and Elbow Dysplasia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
- Individual identity number;
- Male parent;
- Female parent;
- Date of birth;
- Country of birth (i.e., country of origin).
- The minimum age of the dog for radiographic imaging is 1 year;
- The dog must be identified by a microchip;
- All dogs should be sufficiently sedated or anesthetized during the procedure to relax all muscles.
2.2. Data Analysis
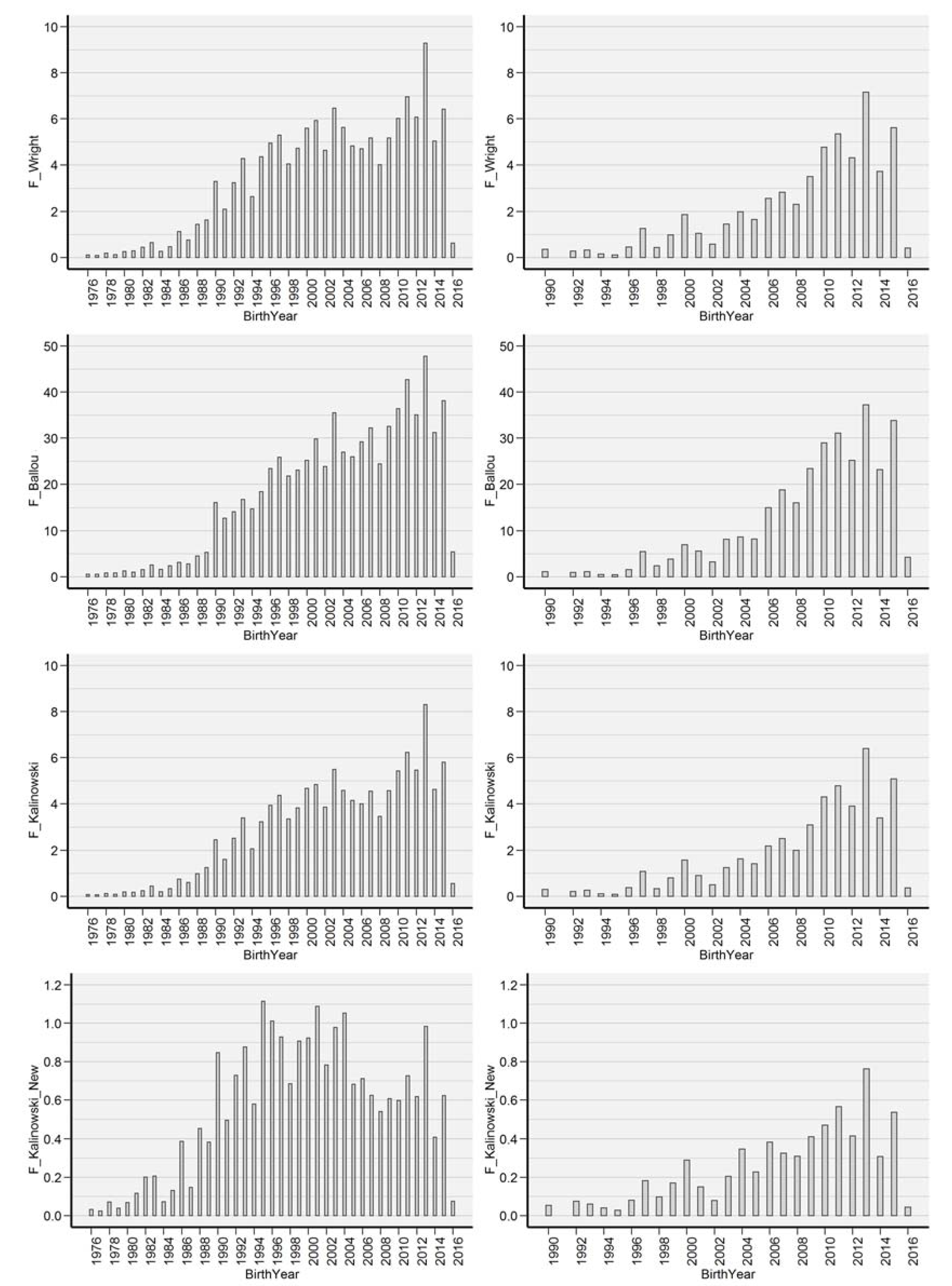
- F_W: Inbreeding coefficient described by [31].
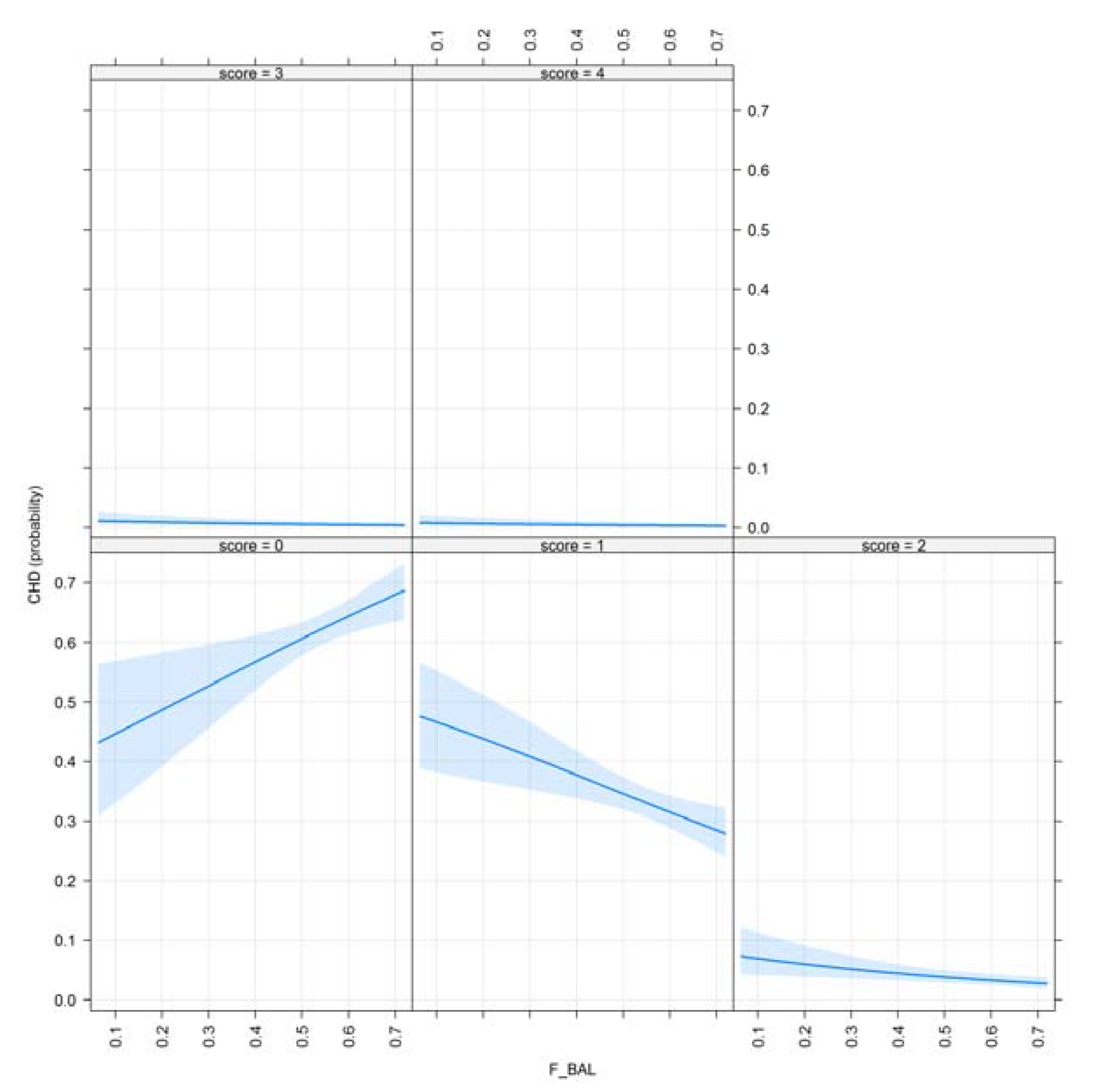
- F_BAL: Ancestral inbreeding coefficient, determined as the cumulative proportion of the genome exposed to inbreeding effects [26]. F_BAL was created to test the magnitude and effectiveness of inbreeding depression as the extent to which individual’s ancestors had been subjected to inbreeding.
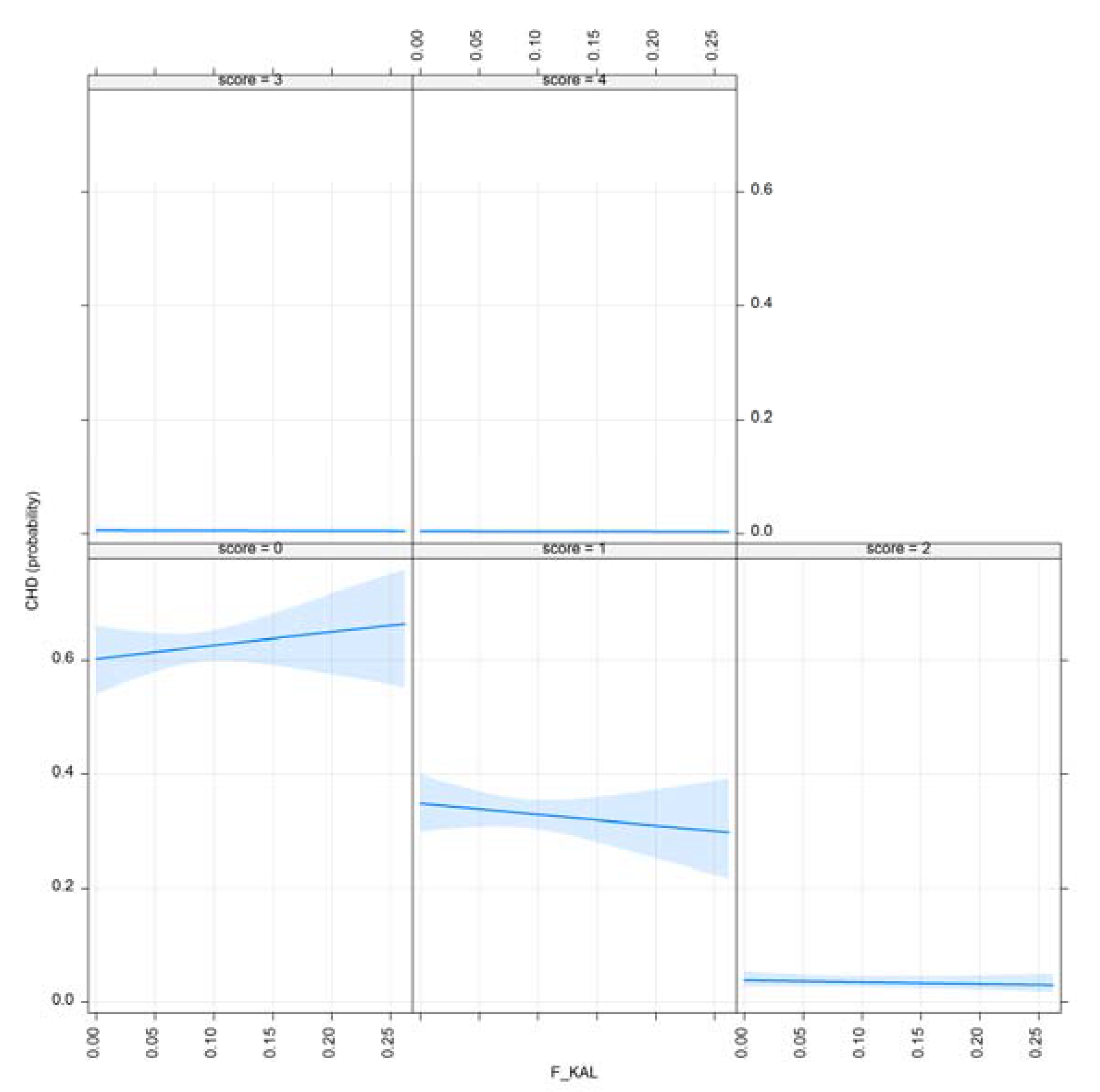
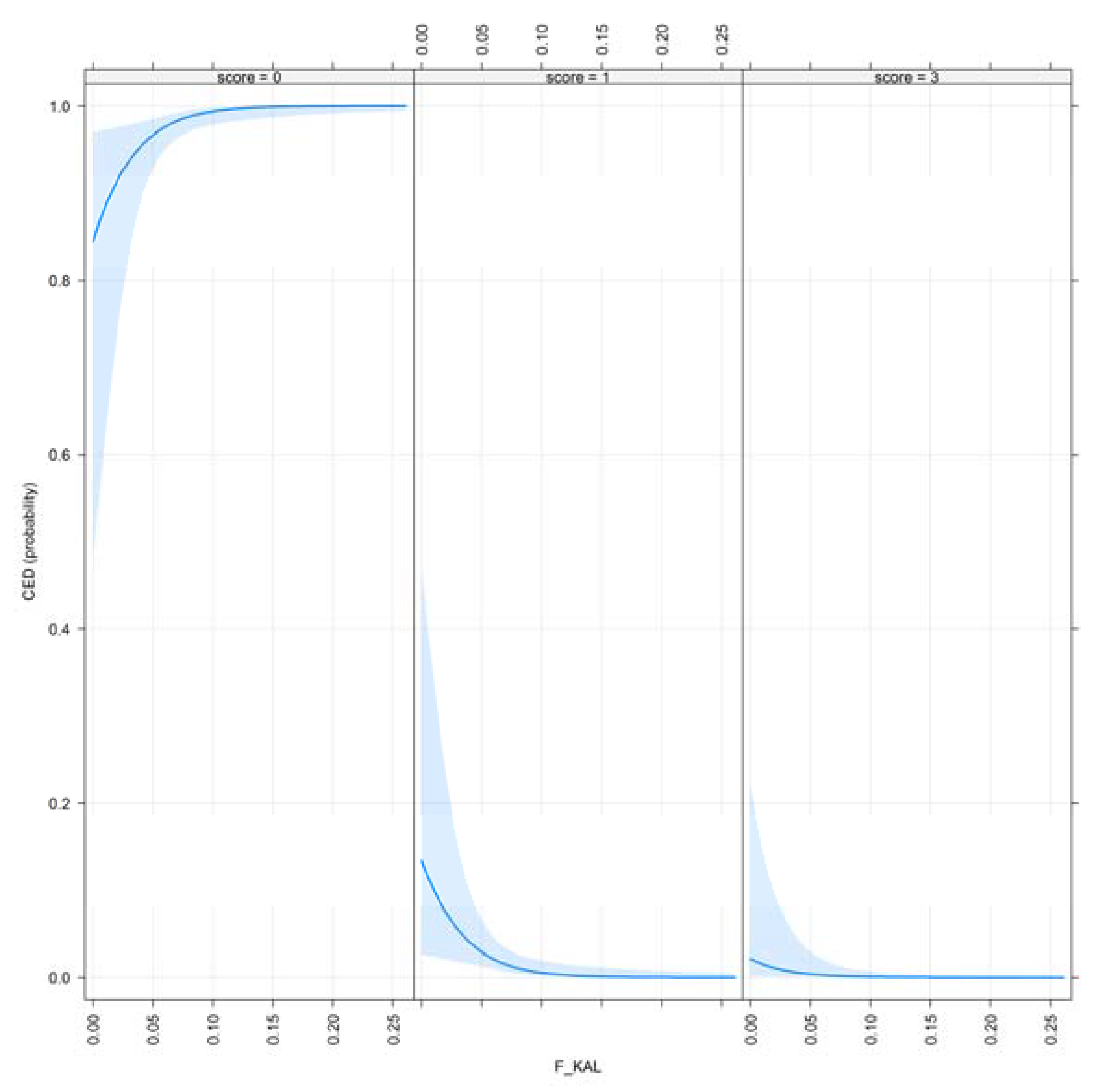
- F_KAL: Inbreeding coefficient defined by [17]. The probability that alleles had been autozygous (IBD) in the previous generation at least once, where the common ancestor was presented on both sides of the pedigree.
- F_KAL_NEW: Kalinowski new inbreeding coefficient, described as alleles IBD, was inbred for the first time [17].
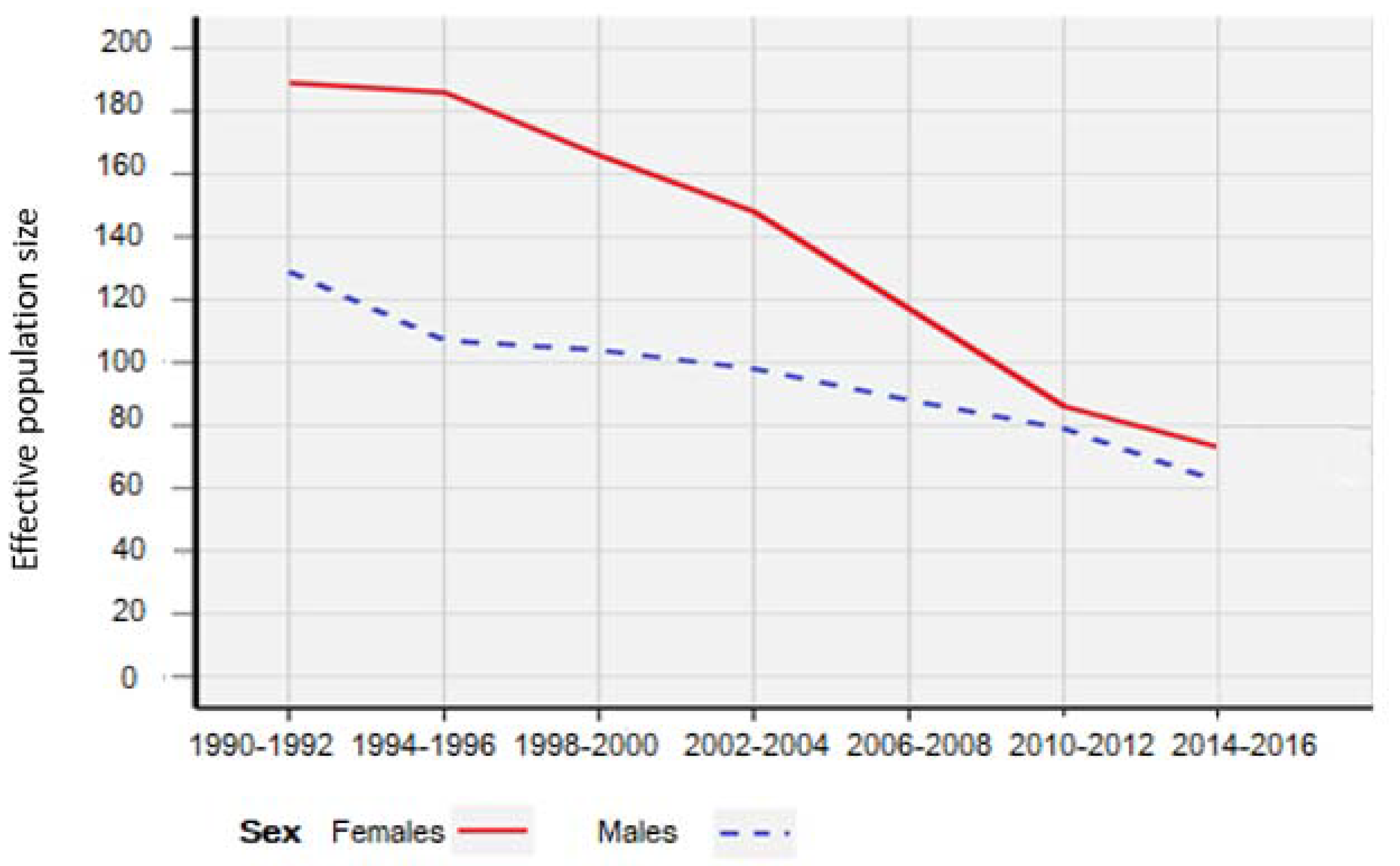
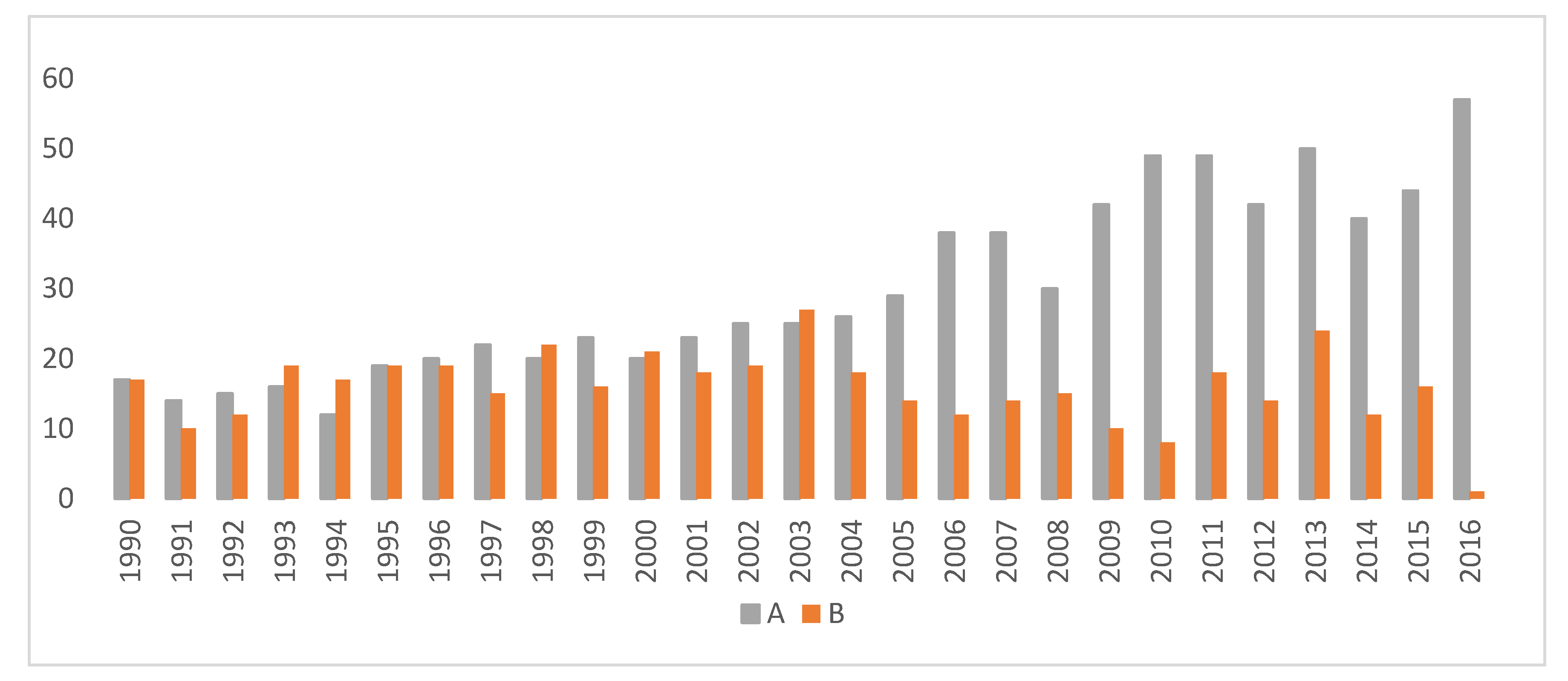
3. Results
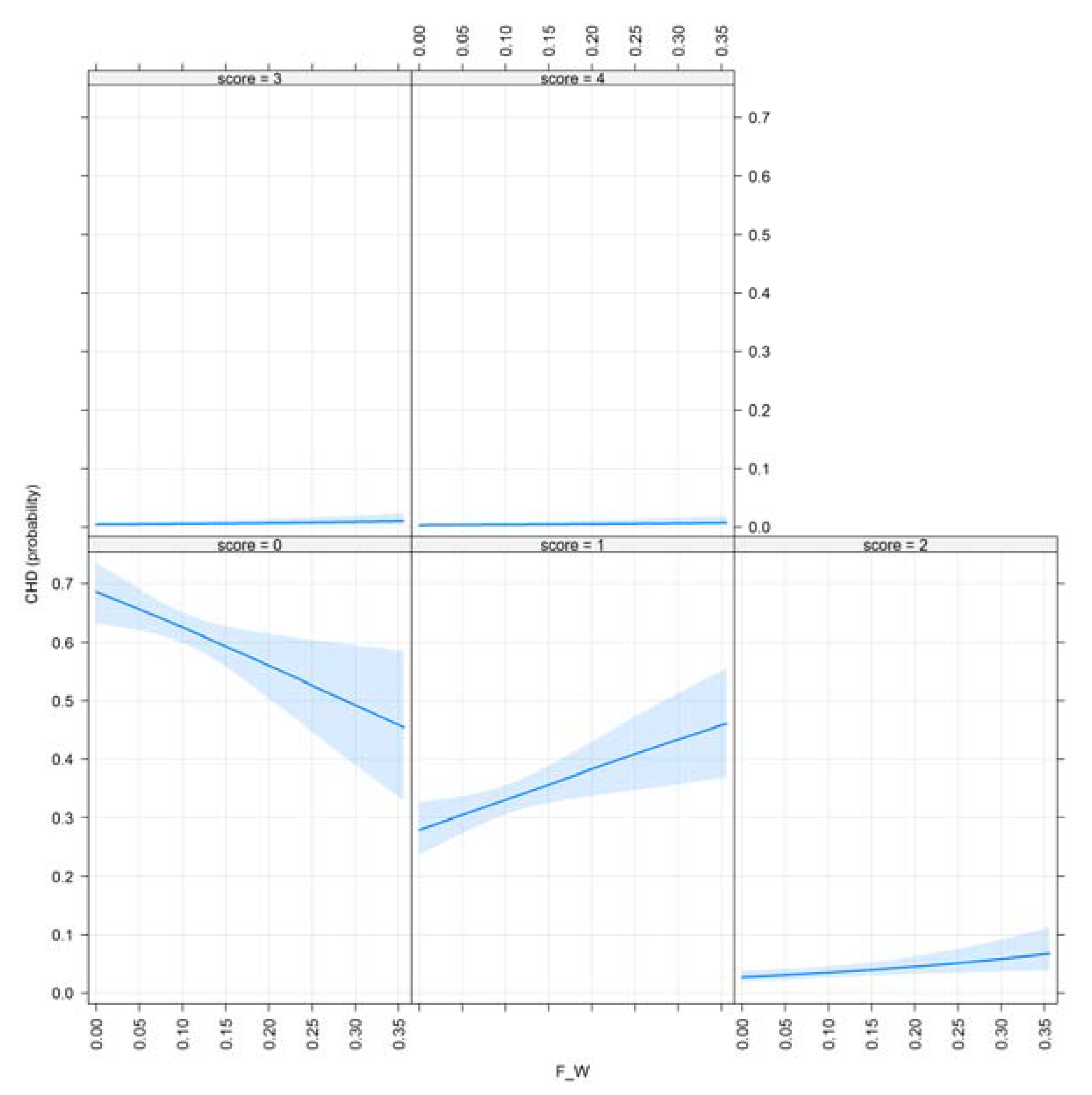
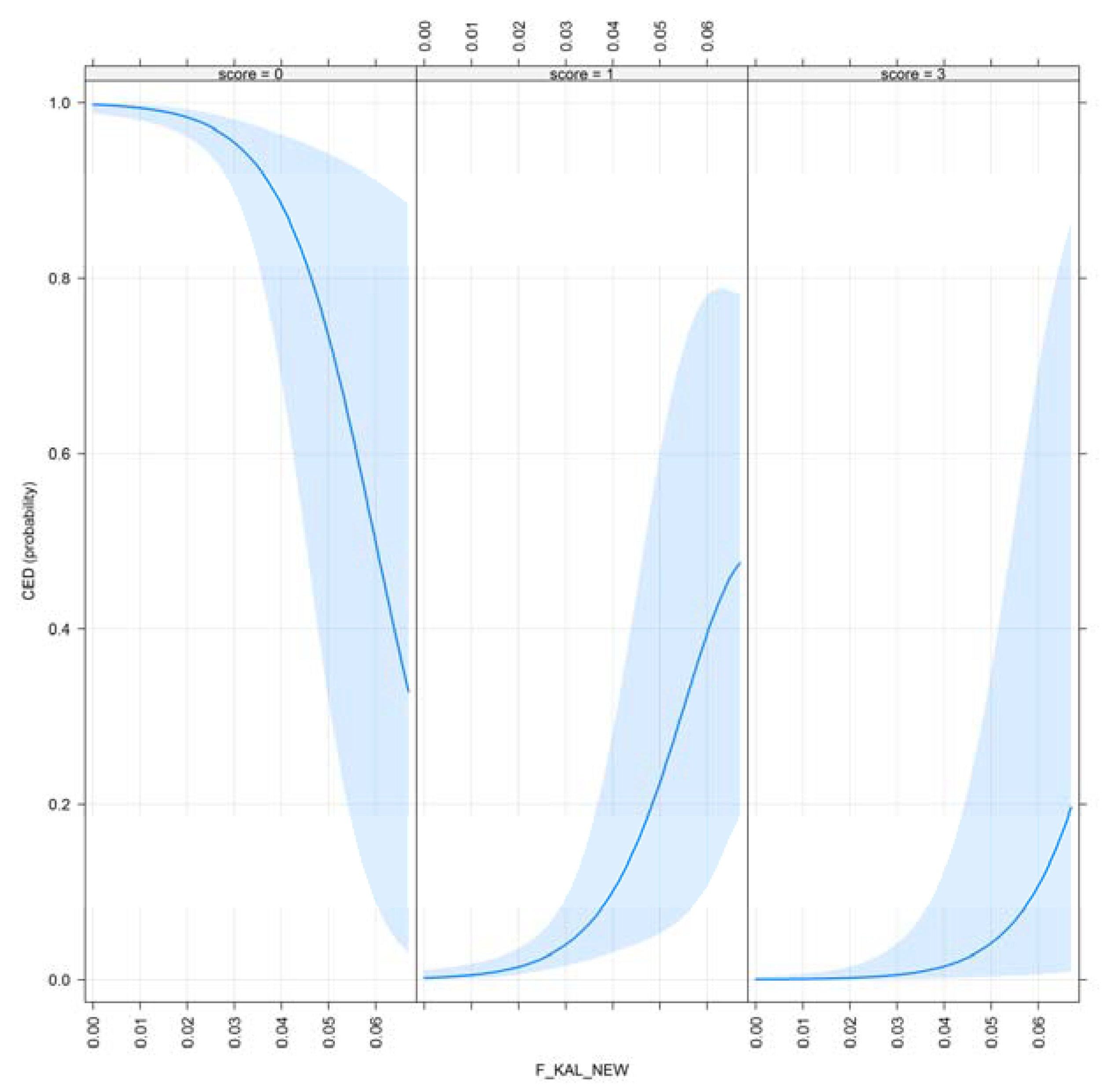
3.1. Results for Purging in Canine Hip Dysplasia
3.2. Results of Purging in Canine Elbow Dysplasia
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leroy, G. Genetic diversity, inbreeding and breeding practices in dogs: Results from pedigree analyses. Vet. J. 2011, 189, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Calboli, F.C.F.; Sampson, J.; Fretwell, N.; Balding, D.J. Population Structure and Inbreeding From Pedigree Analysis of Purebred Dogs. Genetics 2008, 179, 593–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroy, G.; Baumung, R. Mating practices and the dissemination of genetic disorders in domestic animals, based on the example of dog breeding. Anim. Genet. 2011, 42, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Mellersh, C. Give a dog a genome. Vet. J. 2008, 178, 46–52. [Google Scholar] [CrossRef]
- Malm, S.; Fikse, W.; Danell, B.; Strandberg, E.; Fikse, W.F. Genetic variation and genetic trends in hip and elbow dysplasia in Swedish Rottweiler and Bernese Mountain Dog. J. Anim. Breed. Genet. 2008, 125, 403–412. [Google Scholar] [CrossRef]
- Lewis, T.; Woolliams, J.A.; Blott, S.C. Genetic Evaluation of the Nine Component Features of Hip Score in UK Labrador Retrievers. PLoS ONE 2010, 5, e13610. [Google Scholar] [CrossRef] [Green Version]
- Peterson, C. Canine hip dysplasia: Pathogenesis, phenotypic scoring, and genetics. Dul. J. Undergr. Biol. 2017, 4, 19–27. [Google Scholar]
- Ginja, M.; Silvestre, A.; Gonzalo-Orden, J.; Ferreira, A.J.P. Diagnosis, genetic control and preventive management of canine hip dysplasia: A review. Vet. J. 2010, 184, 269–276. [Google Scholar] [CrossRef]
- Hazewinkel, H.; Nap, R. Elbow dysplasia, definition, and known aetiologies. In Proceedings of the 22nd Annual Meeting of the International Elbow Working Group, Münich, Germany, 8 September 2007. [Google Scholar]
- Hou, Y.; Wang, Y.; Lu, X.; Zhang, X.; Zhao, Q.; Todhunter, R.J.; Zhang, Z. Monitoring Hip and Elbow Dysplasia Achieved Modest Genetic Improvement of 74 Dog Breeds over 40 Years in USA. PLoS ONE 2013, 8, e76390. [Google Scholar] [CrossRef] [Green Version]
- Kaneene, J.B.; Mostosky, U.V.; Padgett, G.A. A retrospective cohort study of changes in hip joint phenotype of dogs in the United States. J. Am. Vet. Med. Ass. 1997, 211, 1542–1544. [Google Scholar]
- Kaneene, J.B.; Mostosky, U.V.; Miller, R. Update of a Retrospective Cohort Study of Changes in Hip Joint Phenotype of Dogs Evaluated by the OFA in the United States, 1989–2003. Vet. Surg. 2009, 38, 398–405. [Google Scholar] [CrossRef]
- Mäki, K.; Liinamo, A.E.; Ojala, M. Estimates of genetic parameters for hip and elbow dysplasia in Finnish Rottweilers. J. Anim. Sci. 2000, 78, 1141–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberbauer, A.M.; Keller, G.G.; Famula, T.R. Long-term genetic selection reduced prevalence of hip and elbow dysplasia in 60 dog breeds. PLoS ONE 2017, 12, e0172918. [Google Scholar] [CrossRef]
- Baers, G.; Keller, G.G.; Famula, T.R.; Oberbauer, A.M. Heritability of Unilateral Elbow Dysplasia in the Dog: A Retrospective Study of Sire and Dam Influence. Front. Vet. Sci. 2019, 6, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankham, R.; Bradshaw, C.J.; Brook, B.W. Genetics in conservation management: Revised recommendations for the 50/500 rules, Red List criteria and population viability analyses. Boil. Conserv. 2014, 170, 56–63. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Hedrick, P.W.; Miller, P.S. Inbreeding Depression in the Speke’s Gazelle Captive Breeding Program. Conserv. Boil. 2000, 14, 1375–1384. [Google Scholar] [CrossRef]
- Templeton, A.R.; Read, B. The elimination of inbreeding depression in a captive herd of Speke’s gazelle. In Genetics and Conservation: A Reference for Managing Wild Animal and Plant Populations; Schonewald-Cox, C.M., Chambers, S.M., MacBryde, B., Thomas, L., Eds.; Benjamin/Cummings: Menlo Park, CA, USA, 1983; pp. 241–261. [Google Scholar]
- Templeton, A.R.; Read, B. Factors eliminating inbreeding depression in a captive herd of speke’s gazelle (Gazella spekei). Zoo Boil. 1984, 3, 177–199. [Google Scholar] [CrossRef]
- Kristensen, T.N.; SøRensen, A.C. Inbreeding–Lessons from animal breeding, evolutionary biology and conservation genetics. Anim. Sci. 2005, 80, 121–133. [Google Scholar] [CrossRef]
- Hinrichs, D.; Bennewitz, J.; Wellmann, R.; Thaller, G. Estimation of ancestral inbreeding effects on stillbirth, calving ease and birthweight in German Holstein dairy cattle. J. Anim. Breed. Genet. 2014, 132, 59–67. [Google Scholar] [CrossRef]
- McParland, S.; Kearney, F.; Berry, D.P. Purging of inbreeding depression within the Irish Holstein-Firesian population. Genet. Sel. Evol. 2009, 41, 16. [Google Scholar]
- Boakes, E.; Wang, J.; Amos, W. An investigation of inbreeding depression and purging in captive pedigreed populations. Heredity 2007, 98, 172–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokor, Á. Equihun Pedigree Builder–MS Access application specifically developed for genealogy records process. Unpublished work. 2004. [Google Scholar]
- Ács, V.; Bokor, Á.; Nagy, I. Population Structure Analysis of the Border Collie Dog Breed in Hungary. Animals 2019, 9, 250. [Google Scholar] [CrossRef] [Green Version]
- Ballou, J.D. Ancestral inbreeding only minimally affects inbreeding depression in mammalian populations. J. Hered. 1997, 88, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, S.T. Evolutionary and statistical properties of three genetic distances. Mol. Ecol. 2002, 8, 1263–1273. [Google Scholar] [CrossRef] [Green Version]
- Doekes, H.P.; Curik, I.; Nagy, I.; Farkas, J.; Kövér, G.; Windig, J. Revised Calculation of Kalinowski’s Ancestral and New Inbreeding Coefficients. Diversity 2020, 12, 155. [Google Scholar] [CrossRef] [Green Version]
- FCI Workshop on Hip Dysplasia; Fédération Cynologique Internationale (FCI) Magazine: Dortmund, Germany, 14 June 1991.
- Boakes, E.H.; Wang, J. A simulation study on detecting purging of inbreeding depression in captive populations. Genet. Res. 2005, 86, 139–148. [Google Scholar] [CrossRef]
- Wright, S. Coefficients of inbreeding and relationship. Am. Nat. 1922, 56, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: http://tinyurl.com/carbook (accessed on 22 September 2020).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002. [Google Scholar]
- Gutiérrez, J.P.; Cervantes, I.; Molina, A.; Valera, M.; Goyache, F. Individual increase in inbreeding allowsestimating realised effective sizes from pedigrees. Genet. Sel. Evol. 2008, 40, 359–378. [Google Scholar]
- Suwanlee, S.; Baumung, R.; Sölkner, J.; Curik, I. Evaluation of ancestral inbreeding coefficients: Ballou’s formula versus gene dropping. Conserv. Genet. 2006, 8, 489–495. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Hedrick, P.W. An improved method for estimating inbreeding depression in pedigrees. Zoo Biol. 1998, 17, 481–497. [Google Scholar] [CrossRef]
- Irion, D.N.; Schaffer, A.L.; Famula, T.R.; Eggleston, M.L.; Hughes, S.S.; Pedersen, N.C. Analysis of genetic variation in 28 dog breed populations with 100 microsatellite markers. J. Hered. 2003, 94, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bataillon, T.; Kirkpatrick, M. Inbreeding depression due to mildly deleterious mutations in finite populations: size does matter. Genet. Res. 2000, 75, 75–81. [Google Scholar] [CrossRef]
- Urfer, S.R.; André, A.; Steiger, A.; Gaillard, C.; Creevy, K.E.; Kaeberlein, M.; Promislow, D. Pedigree Analysis of a Large Dog Population. In Proceedings of the Population, Evolutionary, and Quantitative Genetics Conference, Madison, WI, USA, 13–16 May 2018; p. 1. [Google Scholar]
- Wellmann, R.; Pfeiffer, I. Pedigree analysis for conservation of genetic diversity and purging. Genet. Res. 2009, 91, 209–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragab, M.; Sánchez, J.P.; Baselga, M. Effective population size and inbreeding depression on litter size in rabbits. A case study. J. Anim. Breed. Genet. 2014, 132, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Hazewinkel, H. The clinical diagnosis of elbow dysplasia. In Proceedings of the 13th Annual Meeting IEWG, Granada, Spain, 2 October 2002. [Google Scholar]
- Todd, E.T.; Ho, S.Y.W.; Thomson, P.C.; Ang, R.A.; Velie, B.D.; Hamilton, N.A. Founder-specific inbreeding depression affects racing performance in Thoroughbred horses. Sci. Rep. 2018, 8, 6167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, G.; Bouchard, G.; Fagin, B.; Lattimer, J.; Ellersieck, M. Influence of the estrous cycle on coxofemoral joint subluxations. Can. Pract. 1993, 18, 19–22. [Google Scholar]
- De La Riva, G.T.; Hart, B.L.; Farver, T.B.; Oberbauer, A.M.; Messam, L.L.M.; Willits, N.; Hart, L.A. Neutering Dogs: Effects on Joint Disorders and Cancers in Golden Retrievers. PLoS ONE 2013, 8, e55937. [Google Scholar] [CrossRef]
- Hart, B.L.; Hart, L.A.; Thigpen, A.P.; Willits, N.H. Long-Term Health Effects of Neutering Dogs: Comparison of Labrador Retrievers with Golden Retrievers. PLoS ONE 2014, 9, e102241. [Google Scholar] [CrossRef] [Green Version]
- Hart, B.L.; Hart, L.A.; Thigpen, A.P.; Willits, N.H. Neutering of German Shepherd Dogs: associated joint disorders, cancers and urinary incontinence. Vet. Med. Sci. 2016, 2, 191–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Cortegano, E.; Vilas, A.; Caballero, A.; García, N. Estimation of genetic purging under competitive conditions. Evolution 2016, 70, 1856–1870. [Google Scholar] [CrossRef] [PubMed]
- Bersabé, D.; García, N. On the genetic parameter determining the efficiency of purging: an estimate for Drosophila egg-to-pupae viability. J. Evol. Boil. 2012, 26, 375–385. [Google Scholar] [CrossRef] [PubMed]
| Hip Rating | Category Name | Hip Scores |
|---|---|---|
| A | Excellent | 0 |
| B | Borderline | 1 |
| C | Mild | 2 |
| D | Moderate | 3 |
| E | Severe | 4 |
| Elbow Rating | Category |
|---|---|
| 0 | Normal: No sign of arthrosis |
| 1 | Slight: Osteophytes, less than 2 mm |
| 2 | Medium: Osteophytes from 2 to 5 mm |
| 3 | Severe: Osteophytes, more than 5 mm |
| Model | Component Models | AIC | |
|---|---|---|---|
| CED | CHD | ||
| 1 | F_w + F_BAL | 95.56 | 2243.45 |
| 2 | F_KAL + F_KAL_New | 92.82 | 2250.16 |
| Variables | CED | CHD |
|---|---|---|
| Pr(>|z|) | Pr(>|z|) | |
| F_W | 0.802 | 0.009 ** |
| F_BAL | 0.425 | 0.003 ** |
| F_KAL | 0.001 *** | 0.003 ** |
| F_KAL_NEW | 0.011 * | 0.444 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ács, V.; Kövér, G.; Farkas, J.; Bokor, Á.; Nagy, I. Effects of Long-Term Selection in the Border Collie Dog Breed: Inbreeding Purge of Canine Hip and Elbow Dysplasia. Animals 2020, 10, 1743. https://doi.org/10.3390/ani10101743
Ács V, Kövér G, Farkas J, Bokor Á, Nagy I. Effects of Long-Term Selection in the Border Collie Dog Breed: Inbreeding Purge of Canine Hip and Elbow Dysplasia. Animals. 2020; 10(10):1743. https://doi.org/10.3390/ani10101743
Chicago/Turabian StyleÁcs, Virág, György Kövér, János Farkas, Árpád Bokor, and István Nagy. 2020. "Effects of Long-Term Selection in the Border Collie Dog Breed: Inbreeding Purge of Canine Hip and Elbow Dysplasia" Animals 10, no. 10: 1743. https://doi.org/10.3390/ani10101743
APA StyleÁcs, V., Kövér, G., Farkas, J., Bokor, Á., & Nagy, I. (2020). Effects of Long-Term Selection in the Border Collie Dog Breed: Inbreeding Purge of Canine Hip and Elbow Dysplasia. Animals, 10(10), 1743. https://doi.org/10.3390/ani10101743





