Environmental Investigations and Tissue Bioaccumulation of Heavy Metals in Grey Mullet from the Black Sea (Bulgaria) and the Ionian Sea (Italy)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Sampling
2.2. Sample Preparation, Tissue and Water Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Argamino, C.R.; Janairo, J.I.B. Qualitative assessment and management of microplastics in Asian green mussels (Perna viridis) cultured in Bacoor Bay, Cavite, Phillipines. Environ. Asia 2016, 9, 48–54. [Google Scholar]
- Bahnasawy, M.; Khidr, A.A.; Dheina, N. Seasonal variations of heavy metals concentrations in mullet, Mugil cephalus and Liza ramada (Mugilidae) from Lake Manzala, Egypt. Egypt. J. Aquat. Boil. Fish. 2009, 13, 81–100. [Google Scholar] [CrossRef] [Green Version]
- Bat, L.; Sezgin, M.; Üstün, F.; Şahin, F. Heavy metal concentrations in ten species of fishes caught in Sinop coastal waters of the Black Sea, Turkey. Turk. J. Fish. Aquat. Sci. 2012, 12, 371–376. [Google Scholar] [CrossRef]
- Marcovecchio, J.; Marcovecchio, J.E. The use of Micropogonias furnieri and Mugil liza as bioindicators of heavy metals pollution in La Plata river estuary, Argentina. Sci. Total. Environ. 2004, 323, 219–226. [Google Scholar] [CrossRef]
- Phillips, D.J.; Segar, D.A. Use of bio-indicators in monitoring conservative contaminants: Programme design imperatives. Mar. Pollut. Bull. 1986, 17, 10–17. [Google Scholar] [CrossRef]
- Atta, A.; Voegborlo, R.B.; Agorku, E.S. Total mercury distribution in different tissues of six species of freshwater fish from the Kpong hydroelectric reservoir in Ghana. Environ. Monit. Assess. 2011, 184, 3259–3265. [Google Scholar] [CrossRef]
- Canli, M.; Atli, G. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ. Pollut. 2003, 121, 129–136. [Google Scholar] [CrossRef]
- Muñoz-Olivas, R.; Cámara, C. Speciation related to human health. Trace Elem. Speciat. Environ. Food Health 2007, 331–353. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Govind, P. Heavy Metals Causing Toxicity in Animals and Fishes. Res. J. Anim. Res. J. Anim. Vet. Fish. Sci. Int. Sci. Congr. Assoc. 2014, 2, 17–23. [Google Scholar]
- Sfakianakis, D.G.; Renieri, E.; Kentouri, M.; Tsatsakis, A. Effect of heavy metals on fish larvae deformities: A review. Environ. Res. 2015, 137, 246–255. [Google Scholar] [CrossRef]
- Montalbano, G.; Capillo, G.; Laura, R.; Abbate, F.; Levanti, M.; Guerrera, M.; Ciriaco, E.; Germana, A. Neuromast hair cells retain the capacity of regeneration during heavy metal exposure. Ann. Anat. Anat. Anz. 2018, 218, 183–189. [Google Scholar] [CrossRef]
- Costa, R.; Albergamo, A.; Piparo, M.; Zaccone, G.; Capillo, G.; Manganaro, A.; Dugo, P.; Mondello, L. Multidimensional gas chromatographic techniques applied to the analysis of lipids from wild-caught and farmed marine species. Eur. J. Lipid Sci. Technol. 2016, 119, 1600043. [Google Scholar] [CrossRef]
- Fazio, F.; Saoca, C.; Sanfilippo, M.; Capillo, G.; Spanò, N.; Piccione, G. Response of vanadium bioaccumulation in tissues of Mugil cephalus (Linnaeus 1758). Sci. Total. Environ. 2019, 689, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Çelik, U.; Oehlenschläger, J. Determination of zinc and copper in fish samples collected from Northeast Atlantic by DPSAV. Food Chem. 2004, 87, 343–347. [Google Scholar] [CrossRef]
- Schroeder, H.A. The trace elements and man; Dodd Mead: New York, NY, USA, 1973; pp. 171. [Google Scholar]
- Ihunwo, O.C.; Dibofori-Orji, A.N.; Olowo, C.; Ibezim-Ezeani, M.U. Distribution and risk assessment of some heavy metals in surface water, sediment and grey mullet (Mugil cephalus) from contaminated creek in Woji, southern Nigeria. Mar. Pollut. Bull. 2020, 154, 111042. [Google Scholar] [CrossRef] [PubMed]
- Makedonski, L.; Peycheva, K.; Stancheva, M. Determination of heavy metals in selected black sea fish species. Food Control. 2017, 72, 313–318. [Google Scholar] [CrossRef]
- Peycheva, K.; Panayotova, V.; Merdzhanova, A.; Stancheva, R. Estimation of THQ and potential health risk for metals by comsumption of some black sea marine fishes and mussels in Bulgaria. Bulg. Chem. Commun. 2019, 51, 241–246. [Google Scholar]
- Soyinka, O.O. The feeding ecology of Mugil cephalus (Linnaeus) from a high brackish tropical lagoon in South-west, Nigeria. Afr. J. Biotechnol. 2008, 7, 4192–4198. [Google Scholar]
- Turkmen, M.; Turkmen, A.; Tepe, Y. Metal contaminations in five fish species from black, marmara, aegean and mediterranean seas, turkey. J. Chil. Chem. Soc. 2008, 53, 1424–1428. [Google Scholar] [CrossRef] [Green Version]
- Stoichev, T.; Makedonski, L.; Trifonova, T.; Stancheva, M.; Ribarova, F. DDT in fish from the Bulgarian region of the Black Sea. Chem. Ecol. 2007, 23, 191–200. [Google Scholar] [CrossRef]
- Brandt, P.; Rubino, A.; Quadfasel, D.; Alpers, W.; Sellschopp, J.; Fiekas, H.V. Evidence for the influence of Atlantic-Ionian Stream fluctuations on the tidally induced internal dynamics in the Strait of Messina. J. Phys. Oceanogr. 1999, 29, 1071–1080. [Google Scholar] [CrossRef]
- Grandjacquet, C.; Mascle, G. The Structure of the Ionian Sea, Sicily, and Calabria-Lucania. In The Ocean Basins and Margins; Nairn, A.E.M., Kanes, W.H., Stehli, F.G., Eds.; Springer US: Boston, MA, USA, 1978; pp. 257–329. [Google Scholar]
- Bottari, A.; Carveni, P.; Giacobbe, S.; Spanò, N.; Bottari, C. Genesis and geomorphologic and ecological evolution of the Ganzirri salt marsh (Messina, Italy). Quat. Int. 2005, 150–158. [Google Scholar] [CrossRef]
- Capillo, G.; Savoca, S.; Costa, R.; Sanfilippo, M.; Rizzo, C.; Giudice, A.L.; Albergamo, A.; Rando, R.; Bartolomeo, G.; Spanò, N.; et al. New Insights into the Culture Method and Antibacterial Potential of Gracilaria gracilis. Mar. Drugs 2018, 16, 492. [Google Scholar] [CrossRef] [Green Version]
- Manganaro, A.; Pulicanò, G.; Sanfilippo, M. Temporal evolution of the area of Capo Peloro (Sicily, Italy) from pristine site into urbanized area. Transit. Waters Bull. 2011, 5, 23–31. [Google Scholar]
- Lermusiaux, P.; Robinson, A. Features of dominant mesoscale variability, circulation patterns and dynamics in the Strait of Sicily. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2001, 48, 1953–1997. [Google Scholar] [CrossRef]
- Iaria, C.; Saoca, C.; Guerrera, M.C.; Ciulli, S.; Brundo, M.V.; Piccione, G.; Lanteri, G. Occurrence of diseases in fish used for experimental research. Lab. Anim. 2019, 53, 619–629. [Google Scholar] [CrossRef] [Green Version]
- European Parliament and Council of the European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; European Parliament and Council of the European Union: Brussels, Belgium, 2013. [Google Scholar]
- Kojadinovic, J.; Potier, M.; Le Corre, M.; Cosson, R.P.; Bustamante, P. Bioaccumulation of trace elements in pelagic fish from the Western Indian Ocean. Environ. Pollut. 2007, 146, 548–566. [Google Scholar] [CrossRef] [Green Version]
- Dang, F.; Wang, W.-X. Assessment of tissue-specific accumulation and effects of cadmium in a marine fish fed contaminated commercially produced diet. Aquat. Toxicol. 2009, 95, 248–255. [Google Scholar] [CrossRef]
- Farkas, A.; Salánki, J.; Specziár, A. Age- and size-specific patterns of heavy metals in the organs of freshwater fish Abramis brama L. populating a low-contaminated site. Water Res. 2003, 37, 959–964. [Google Scholar] [CrossRef]
- Doraghi, A.; Monikh, F.A.; Safahieh, A.; Savari, A. Heavy Metals Concentration in Mullet Fish, from Petrochemical Waste Receiving Creeks, Musa Estuary (Persian Gulf). J. Environ. Prot. 2011, 2, 1218–1226. [Google Scholar] [CrossRef] [Green Version]
- Türkmen, M.; Ciminli, C. Determination of metals in fish and mussel species by inductively coupled plasma-atomic emission spectrometry. Food Chem. 2007, 103, 670–675. [Google Scholar] [CrossRef]
- Sidoumou, Z.; Gnassia-Barelli, M.; Siau, Y.; Morton, V.; Roméo, M. Distribution and Concentration of Trace Metals in Tissues of Different Fish Species from the Atlantic Coast of Western Africa. Bull. Environ. Contam. Toxicol. 2005, 74, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Uysal, K. Heavy metal in edible portions (muscle and skin) and other organs (gill, liver and intestine) of selected freshwater fish species. Int. J. Food Prop. 2011, 14, 280–286. [Google Scholar] [CrossRef]
- Eken, M.; Göksu, M.Z.L.; Özak, A.A. Investigation of heavy metal levels in economically important fish species captured from the Tuzla lagoon. Food Chem. 2007, 102, 415–421. [Google Scholar] [CrossRef]
- Company, R.; Felícia, H.; Serafim, M.A.P.; Almeida, A.; Biscoito, M.J.; Bebianno, M.J. Metal concentrations and metallothionein-like protein levels in deep-sea fishes captured near hydrothermal vents in the Mid-Atlantic Ridge off Azores. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2010, 57, 893–908. [Google Scholar] [CrossRef]
- Kraemer, L.D.; Campbell, P.G.C.; Hare, L. Seasonal variations in hepatic Cd and Cu concentrations and in the sub-cellular distribution of these metals in juvenile yellow perch (Perca flavescens). Environ. Pollut. 2006, 142, 313–325. [Google Scholar] [CrossRef]
- Jezierska, B.; Witeska, M. The Metal Uptake and Accumulation in Fish Living in Polluted Waters. In Soil and Water Pollution Monitoring, Protection and Remediation; Springer: Dordrecht, The Netherlands, 2007; pp. 107–114. [Google Scholar]
- Karadede, H.; Oymak, S.A.; Ünlü, E. Heavy metals in mullet, Liza abu, and catfish, Silurus triostegus, from the Atatürk Dam Lake (Euphrates), Turkey. Environ. Int. 2004, 30, 183–188. [Google Scholar] [CrossRef]
- Al-Yousuf, M.; El-Shahawi, M.; Al-Ghais, S. Trace metals in liver, skin and muscle of Lethrinus lentjan fish species in relation to body length and sex. Sci. Total. Environ. 2000, 256, 87–94. [Google Scholar] [CrossRef]
- Lima, R.G.D.S.; Araújo, F.G.; Maia, M.F.; Pinto, A.S.D.S.B.; Junior, R.G.D.S.L. Evaluation of Heavy Metals in Fish of the Sepetiba and Ilha Grande Bays, Rio de Janeiro, Brazil. Environ. Res. 2002, 89, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Usero, J.; Izquierdo, C.; Morillo, J.; Gracia, I. Heavy metals in fish (Solea vulgaris, Anguilla anguilla and Liza aurata) from salt marshes on the southern Atlantic coast of Spain. Environ. Int. 2004, 29, 949–956. [Google Scholar] [CrossRef]
- Allen-Gil, S.; Martynov, V. Heavy metal burdens in nine species of freshwater and anadromous fish from the Pechora River, northern Russia. Sci. Total. Environ. 1995, 653–659. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Kraepiel, A.M.L.; Amyot, M. The chemical cycle and bioaccumulation of mercury. Annu. Rev. Ecol. Syst. 1998, 29, 543–566. [Google Scholar] [CrossRef] [Green Version]
- Boran, M.; Altinok, I. A review of heavy metals in water, sediment and living organisms in the black sea. Turk. J. Fish. Aquat. Sci. 2010, 10, 565–572. [Google Scholar]
- Wang, W.-X. Bioaccumulation and Biomonitoring. In Marine Ecotoxicology; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 99–119. [Google Scholar]
- Savoca, S.; Grifó, G.; Panarello, G.; Albano, M.; Giacobbe, S.; Capillo, G.; Spanó, N.; Consolo, G. Modelling prey-predator interactions in Messina beachrock pools. Ecol. Model. 2020, 434, 109206. [Google Scholar] [CrossRef]
- Costa, R.; Capillo, G.; Albergamo, A.; Volsi, R.L.; Bartolomeo, G.; Bua, G.; Ferracane, A.; Savoca, S.; Gervasi, T.; Rando, R.; et al. A Multi-screening Evaluation of the Nutritional and Nutraceutical Potential of the Mediterranean Jellyfish Pelagia noctiluca. Mar. Drugs 2019, 17, 172. [Google Scholar] [CrossRef] [Green Version]
- Spinelli, A.; Capillo, G.; Faggio, C.; Vitale, D.; Spanò, N. Returning of Hippocampus hippocampus (Linnaeus, 1758) (Syngnathidae) in the Faro Lake–oriented Natural Reserve of Capo Peloro, Italy. Nat. Prod. Res. 2018, 34, 595–598. [Google Scholar] [CrossRef]
- Capillo, G.; Panarello, G.; Savoca, S.; Sanfilippo, M.; Albano, M.; Volsi, R.L.; Consolo, G.; Spanò, N. Intertidal ponds of messina’s beachrock faunal assemblage, evaluation of ecosystem dynamics and communities’ interactions. AAPP Atti Accad. Peloritana Pericolanticl. Sci. Fis. Mat. Nat. 2018, 96, A41–A416. [Google Scholar]
- Pan, K.; Wang, W.-X. Validation of Biokinetic Model of Metals in the Scallop Chlamys nobilis in Complex Field Environments. Environ. Sci. Technol. 2008, 42, 6285–6290. [Google Scholar] [CrossRef]
- Agamy, N.; Gomaa, A. Heavy Metals and Chemical Composition of Mullet Fish and Water Quality of Its Farms. J. High Inst. Public Health 2012, 42, 63–81. [Google Scholar] [CrossRef] [Green Version]
- Fazio, F.; Piccione, G.; Tribulato, K.; Ferrantelli, V.; Giangrosso, G.; Arfuso, F.; Faggio, C. Bioaccumulation of Heavy Metals in Blood and Tissue of Striped Mullet in Two Italian Lakes. J. Aquat. Anim. Health 2014, 26, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Mendis, B.R.C.; Najim, M.; Kithsiri, H.M.P.; Azmy, S.A.M. Bioaccumulation of Heavy Metals in the Selected Commercially Important Edible Fish Species Gray Mullet (Mugil cephalus) from Negombo Estuary. J. Environ. Prof. Sri Lanka 2015, 4. [Google Scholar] [CrossRef]
- Priya, S.L.; Selvam, A.P.; Purvaja, R.; Senthilkumar, B.; Hariharan, G.; Ramesh, R. Bioaccumulation of heavy metals in mullet ( Mugil cephalus ) and oyster ( Crassostrea madrasensis ) from Pulicat lake, south east coast of India. Toxicol. Ind. Health 2010, 27, 117–126. [Google Scholar] [CrossRef]
- Stancheva, M.; Makedonski, L.; Petrova, E. Determination of heavy metals (Pb, Cd, As and Hg) in Black Sea grey mullet (Mugil cephalus). Bulg. J. Agric. Sci. 2013, 1, 30–34. [Google Scholar]
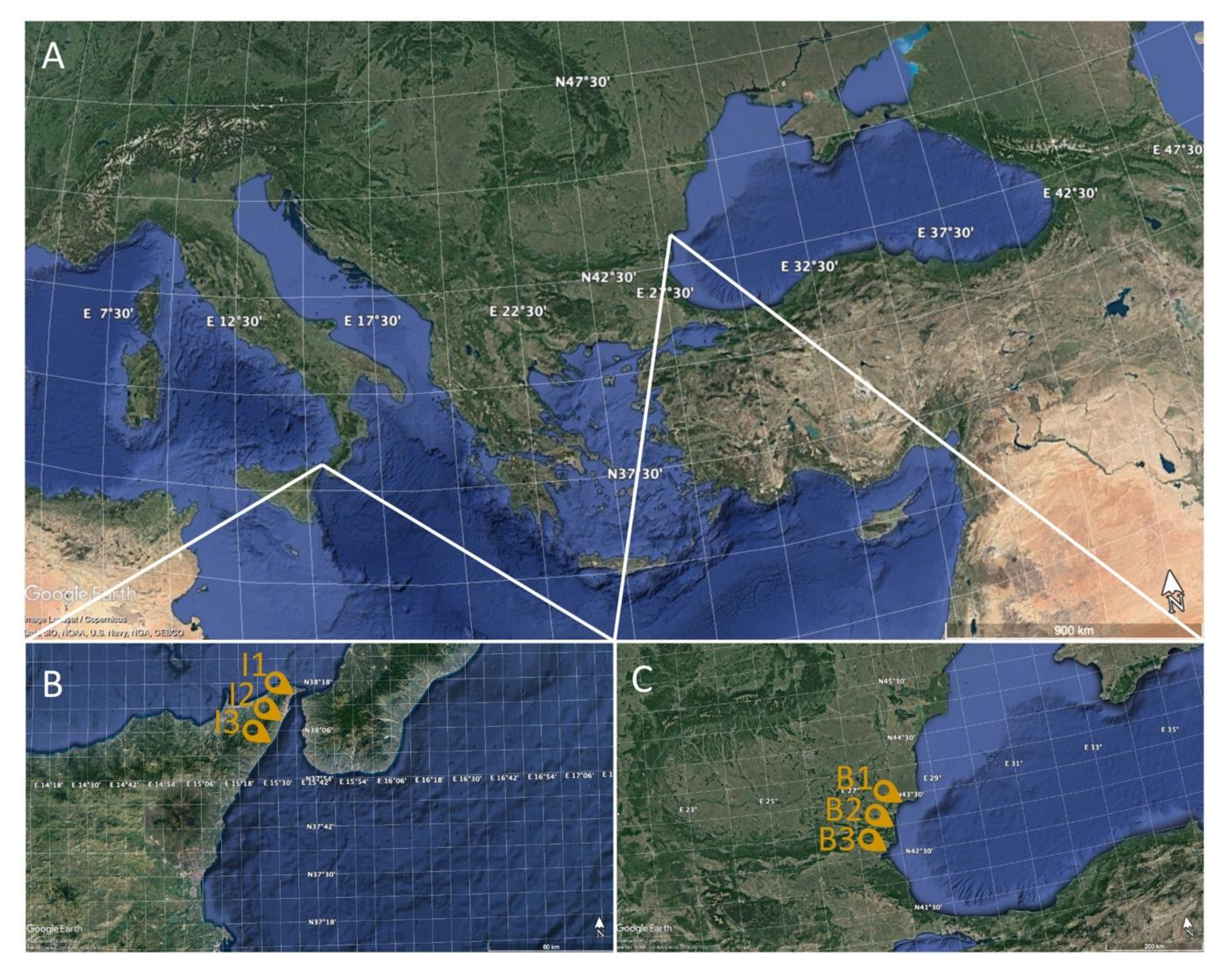
| Sample | Sampling Site | N | Sampling Stations | Weight (g) ± SD | Length (cm) ± SD |
|---|---|---|---|---|---|
| Gray mullet (Mugil cephalus) | Black Sea (Bulgaria) | 20 | B1, B2, B3 | 104.33 ± 13.80 | 23.17 ± 2.61 |
| Ionian Sea (Italy) | 20 | I1, I2, I3 | 97.12 ± 11.25 | 22.41 ± 2.38 |
| Trace Elements (μg/L) | Sampling Sites | |
|---|---|---|
| Black Sea (Bulgaria) | Ionian Sea (Italy) | |
| Cd | 0.0445 ± 0.0020 | 0.9289 ± 0.0095 * |
| Cr | 0.3751 ± 0.0044 | 0.9042 ± 0.0148 * |
| Pb | 0.3472 ± 0.0006 | 0.8060 ± 0.1095 * |
| Ni | 0.5286 ± 0.0113 | 0.8435 ± 0.0440 * |
| Al | 5.4634 ± 0.2208 | 0.9350 ± 0.0215 * |
| Cu | 0.5340 ± 0.0080 | 41.2700 ± 0.2720 * |
| Fe | 3.4591 ± 0.1834 | 0.8920 ± 0.0507 * |
| Mn | 0.5388 ± 0.0307 | 0.9385 ± 0.0048 * |
| Zn | 22.1829 ± 0.1049 | 0.8821 ± 0.0379 * |
| Heavy metals (μg/L) | Samples | Muscle | Gills | Liver |
|---|---|---|---|---|
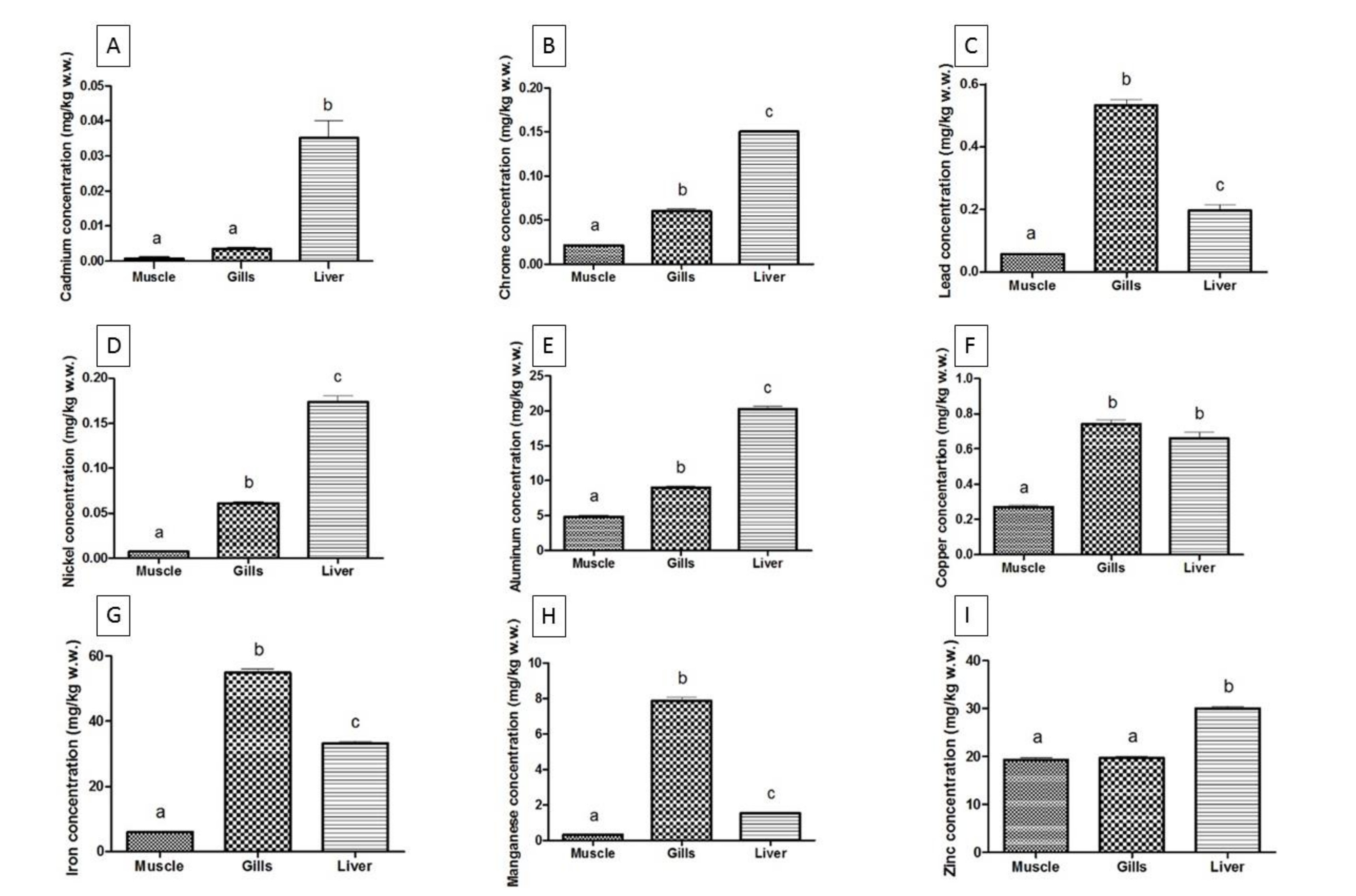
| Cd | Ionian Sea | 0.0008 ± 0.0010 a | 0.0034 ± 0.0021 a | 0.0351 ± 0.0217 a |
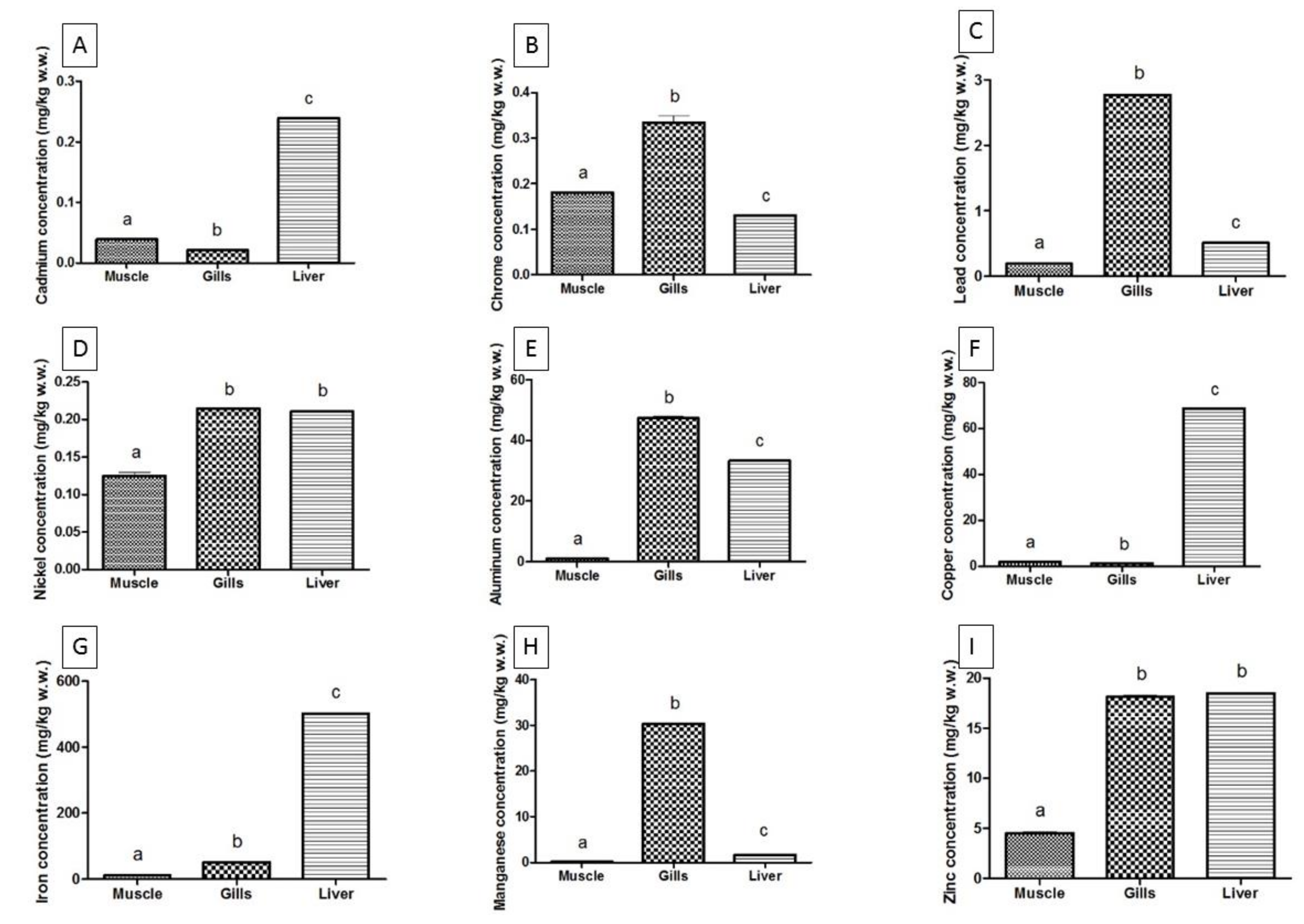
| Black Sea | 0.0391 ± 0.0043 b | 0.0214 ± 0.0058 b | 0.2391 ± 0.0034 b | |
| Cr | Ionian Sea | 0.0213 ± 0.0012 a | 0.0602 ± 0.0106 a | 0.1506± 0.0066 a |
| Black Sea | 0.1809 ± 0.0051 b | 0.3340 ± 0.0678 b | 0.1303 ± 0.0027 b | |
| Pb | Ionian Sea | 0.0572 ± 0.0104 a | 0.5320 ± 0.0844 a | 0.1970 ± 0.0771 a |
| Black Sea | 0.1914 ± 0.0046 b | 2.768 ± 0.0525 b | 0.5118 ± 0.0098 b | |
| Ni | Ionian Sea | 0.0071 ± 0.0011 a | 0.0610 ± 0.0079 a | 0.1735± 0.0315 a |
| Black Sea | 0.1249 ± 0.0211 b | 0.2144 ± 0.0033 b | 0.2111 ± 0.0062 b | |
| Al | Ionian Sea | 4.8500 ± 0.8082 a | 9.0230 ± 0.7341 a | 20.2900 ± 1.9230 a |
| Black Sea | 1.0210 ± 0.0061 b | 47.3800 ± 2.5700 b | 33.3100 ± 0.4651 b | |
| Cu | Ionian Sea | 0.2695 ± 0.0473 a | 0.7400 ± 0.1114 a | 0.6600 ± 0.1635 a |
| Black Sea | 1.8270 ± 0.0151 b | 1.1760 ± 0.0385 b | 68.6200 ± 0.3200 b | |
| Fe | Ionian Sea | 5.9250 ± 0.9792 a | 54.9100 ± 5.1440 a | 33.1500 ± 3.2970 a |
| Black Sea | 11.0600 ± 1.6310 b | 50.8900 ± 1.6730 b | 502.3000 ± 2.0410 b | |
| Mn | Ionian Sea | 0.3040 ± 0.0484 a | 7.8550 ± 0.9310 a | 1.5350 ± 0.2455 a |
| Black Sea | 0.1376 ± 0.0183 b | 30.3300 ± 0.7803 b | 1.6710 ± 0.0204 b | |
| Zn | Ionian Sea | 19.3000 ± 2.0800 a | 19.6000 ± 1.7890 a | 30.0000 ± 1.8920 a |
| Black Sea | 4.5330 ± 0.5551 b | 18.1100 ± 0.7027 b | 18.4800 ± 0.4849 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazio, F.; D’Iglio, C.; Capillo, G.; Saoca, C.; Peycheva, K.; Piccione, G.; Makedonski, L. Environmental Investigations and Tissue Bioaccumulation of Heavy Metals in Grey Mullet from the Black Sea (Bulgaria) and the Ionian Sea (Italy). Animals 2020, 10, 1739. https://doi.org/10.3390/ani10101739
Fazio F, D’Iglio C, Capillo G, Saoca C, Peycheva K, Piccione G, Makedonski L. Environmental Investigations and Tissue Bioaccumulation of Heavy Metals in Grey Mullet from the Black Sea (Bulgaria) and the Ionian Sea (Italy). Animals. 2020; 10(10):1739. https://doi.org/10.3390/ani10101739
Chicago/Turabian StyleFazio, Francesco, Claudio D’Iglio, Gioele Capillo, Concetta Saoca, Katya Peycheva, Giuseppe Piccione, and Lubomir Makedonski. 2020. "Environmental Investigations and Tissue Bioaccumulation of Heavy Metals in Grey Mullet from the Black Sea (Bulgaria) and the Ionian Sea (Italy)" Animals 10, no. 10: 1739. https://doi.org/10.3390/ani10101739
APA StyleFazio, F., D’Iglio, C., Capillo, G., Saoca, C., Peycheva, K., Piccione, G., & Makedonski, L. (2020). Environmental Investigations and Tissue Bioaccumulation of Heavy Metals in Grey Mullet from the Black Sea (Bulgaria) and the Ionian Sea (Italy). Animals, 10(10), 1739. https://doi.org/10.3390/ani10101739








