Phytate Degradation, Transcellular Mineral Transporters, and Mineral Utilization by Two Strains of Laying Hens as Affected by Dietary Phosphorus and Calcium
Abstract
:Simple Summary
Abstract
1. Introduction
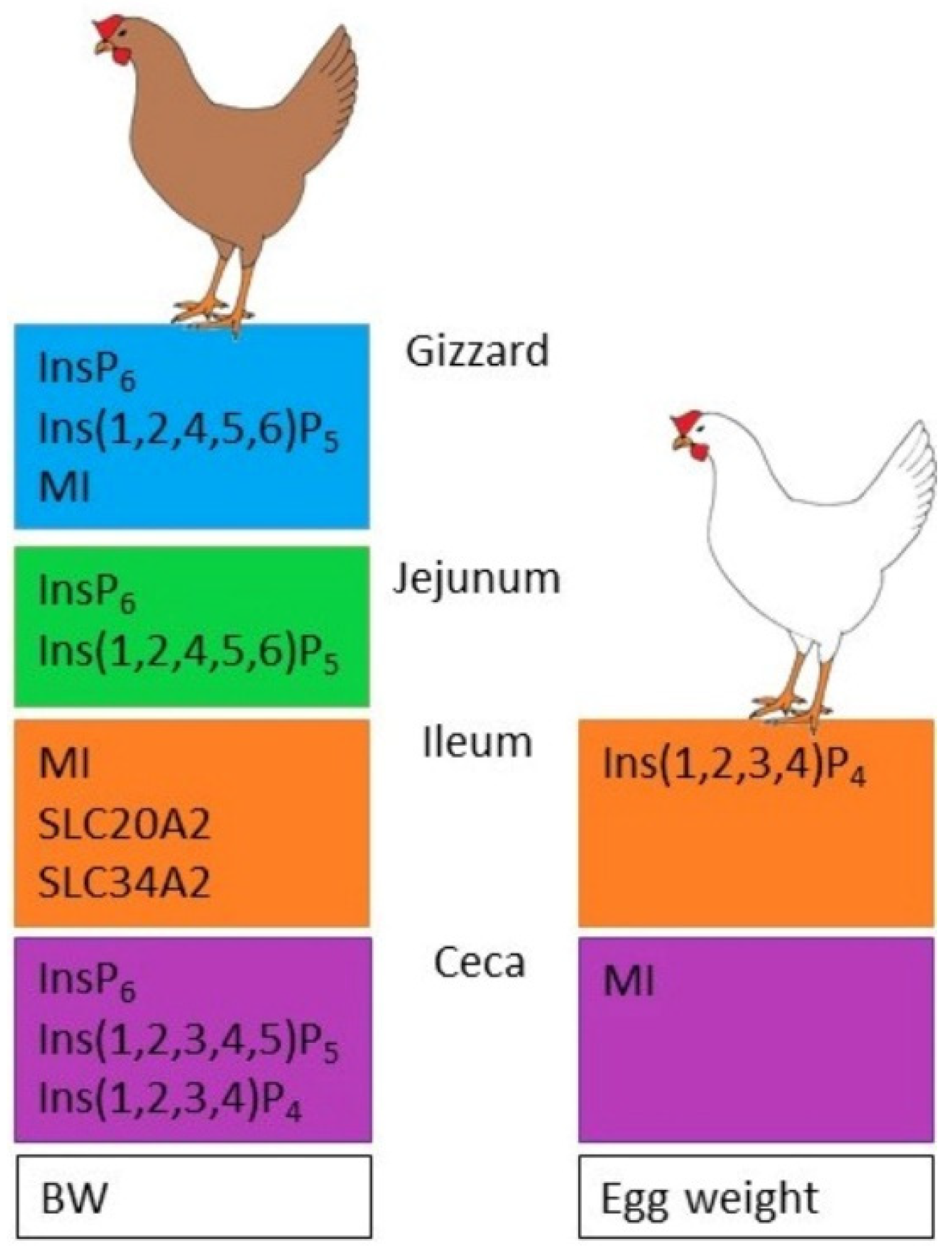
- Different P and Ca concentrations in the feed affect InsP6 degradation, InsPx pattern, MI concentration, and mineral transporter expression in the digestive tract and MI concentration in the blood and eggs of laying hens.
- The genetic background of the hens influences the assessed traits.
2. Materials and Methods
2.1. Birds and Housing
2.2. Diets
2.3. Experimental Procedures, Sampling, and Measurements
2.4. Sample Preparation and Chemical and Physical Analysis
2.5. Total RNA Isolation and cDNA Synthesis
2.6. Quantitative Real-Time PCR (qRT-PCR)
2.7. Statistical Analysis
3. Results
3.1. Performance and Blood Traits
3.2. InsP6, Inositol Phosphate Isomers, and MI in Gut Sections
3.2.1. Crop
3.2.2. Gizzard
3.2.3. Jejunum
3.2.4. Ileum
3.2.5. Ceca
3.3. Ca and P Intake, Concentration in the Small Intestine, and Utilization
3.4. Expression Pattern of Transcellular Mineral Transporters in the Ileum
3.5. MI in the Egg
4. Discussion
4.1. Strain Effects
4.2. Effects of Dietary Ca and P
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tamim, N.M.; Angel, R.; Christman, M. Influence of dietary calcium and phytase on phytate phosphorus hydrolysis in broiler chickens. Poult. Sci. 2004, 83, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Shastak, Y.; Zeller, E.; Witzig, M.; Schollenberger, M.; Rodehutscord, M. Effects of the composition of the basal diet on the evaluation of mineral phosphorus sources and interactions with phytate hydrolysis in broilers. Poult. Sci. 2014, 93, 2548–2559. [Google Scholar] [CrossRef] [PubMed]
- Zeller, E.; Schollenberger, M.; Kühn, I.; Rodehutscord, M. Hydrolysis of phytate and formation of inositol phosphate isomers without or with supplemented phytases in different segments of the digestive tract of broilers. J. Nutr. Sci. 2015, 4, e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeller, E.; Schollenberger, M.; Witzig, M.; Shastak, Y.; Kühn, I.; Hoelzle, L.E.; Rodehutscord, M. Interactions between supplemented mineral phosphorus and phytase on phytate hydrolysis and inositol phosphates in the small intestine of broilers. Poult. Sci. 2015, 94, 1018–1029. [Google Scholar] [CrossRef]
- Sommerfeld, V.; Schollenberger, M.; Kühn, I.; Rodehutscord, M. Interactive effects of phosphorus, calcium, and phytase supplements on products of phytate degradation in the digestive tract of broiler chickens. Poult. Sci. 2018, 97, 1177–1188. [Google Scholar] [CrossRef]
- Bar, A. Calcium transport in strongly calcifying laying birds: Mechanisms and regulation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 152, 447–469. [Google Scholar] [CrossRef]
- Proszkowiec-Weglarz, M.; Schreier, L.L.; Miska, K.B.; Angel, R.; Kahl, S.; Russell, B. Effect of early neonatal development and delayed feeding post-hatch on jejunal and ileal calcium and phosphorus transporter genes expression in broiler chickens. Poult. Sci. 2019, 98, 1861–1871. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J.; Guo, Y.; Sun, Q.; Hu, X. The influence of dietary calcium and phosphorus imbalance on intestinal NaPi-IIb and calbindin mRNA expression and tibia parameters of broilers. Asian-Australas. J. Anim. Sci. 2012, 25, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, H.; Rodehutscord, M. A meta-analysis of responses to dietary nonphytate phosphorus and phytase in laying hens. Poult. Sci. 2012, 91, 2072–2078. [Google Scholar] [CrossRef]
- Hughes, A.L.; Dahiya, J.P.; Wyatt, C.L.; Classen, H.L. Effect of Quantum phytase on nutrient digestibility and bone ash in White Leghorn laying hens fed corn-soybean meal-based diets. Poult. Sci. 2009, 88, 1191–1198. [Google Scholar] [CrossRef]
- Abudabos, A.M. Intestinal phytase activity in chickens (Gallus domesticus). Afr. J. Microbiol. Res. 2012, 6, 4932–4938. [Google Scholar]
- Habig, C.; Distl, O. Evaluation of bone strength, keel bone status, plumage condition and egg quality of two layer lines kept in small group housing systems. Br. Poult. Sci. 2013, 54, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, V.; Huber, K.; Bennewitz, J.; Camarinha-Silva, A.; Hasselmann, M.; Ponsuksili, S.; Seifert, J.; Stefanski, V.; Wimmers, K.; Rodehutscord, M. Phytate degradation, myo-inositol release, and utilization of phosphorus and calcium by two strains of laying hens in five production periods. Poult. Sci. 2020. [Google Scholar] [CrossRef]
- Gesellschaft für Ernährungsphysiologie. Empfehlungen zur Energie- und Nährstoffversorgung der Legehennen und Masthühner (Broiler), 1st ed.; DLG Verlag: Frankfurt am Main, Germany, 1999. [Google Scholar]
- Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten (VDLUFA). Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA–Methodenbuch), vol. III: Die Chemische Untersuchung von Futtermitteln, 1st ed.; VDLUFA: Darmstadt, Germany, 2007. [Google Scholar]
- Boguhn, J.; Baumgärtel, T.; Dieckmann, A.; Rodehutscord, M. Determination of titanium dioxide supplements in different matrices using two methods involving photometer and inductively coupled plasma optical emission spectrometer measurements. Arch. Anim. Nutr. 2009, 63, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Grubješić, G.; Titze, N.; Krieg, J.; Rodehutscord, M. Determination of in situ ruminal crude protein and starch degradation values of compound feeds from single feeds. Arch. Anim. Nutr. 2019, 73, 414–429. [Google Scholar] [CrossRef]
- Beck, P.; Piepho, H.-P.; Rodehutscord, M.; Bennewitz, J. Inferring relationships between phosphorus utilization, feed per gain, and bodyweight gain in an F2 cross of Japanese quail using recursive models. Poult. Sci. 2016, 95, 764–773. [Google Scholar] [CrossRef]
- de Verdal, H.; Narcy, A.; Bastianelli, D.; Chapuis, H.; Même, N.; Urvoix, S.; Le Bihan-Duval, E.; Mignon-Grasteau, S. Improving the efficiency of feed utilization in poultry by selection. 1. Genetic parameters of anatomy of the gastro-intestinal tract and digestive efficiency. BMC Genet. 2011, 12, 59. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Aggrey, S.E.; Pesti, G.M.; Edwards, H.M., Jr.; Bakalli, R.I. Genetics of phytate phosphorus bioavailability: Heritability and genetic correlations with growth and feed utilization traits in a randombred chicken population. Poult. Sci. 2003, 82, 1075–1079. [Google Scholar] [CrossRef]
- Shafey, T.M.; McDonald, M.W.; Dingle, J.G. Effects of dietary calcium and available phosphorus concentration on digesta pH and on the availability of calcium, iron, magnesium and zinc from the intestinal contents of meat chickens. Br. Poult. Sci. 1991, 32, 185–194. [Google Scholar] [CrossRef]
- Sabbagh, Y.; O’Brien, S.P.; Song, W.; Boulanger, J.H.; Stockmann, A.; Arbeeny, C.; Schiavi, S.C. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J. Am. Soc. Nephrol. 2009, 20, 2348–2358. [Google Scholar] [CrossRef]
- Xu, H.; Bai, L.; Collins, J.F.; Ghishan, F.K. Molecular cloning, functional characterization, tissue distribution, and chromosomal localization of a human, small intestinal sodium-phosphate (Na+-Pi) transporter (SLC34A2). Genomics 1999, 62, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Wubuli, A.; Reyer, H.; Muráni, E.; Ponsuksili, S.; Wolf, P.; Oster, M.; Wimmers, K. Tissue-wide gene expression analysis of sodium/phosphate co-transporters in pigs. Int. J. Mol. Sci. 2019, 20, 5576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gloux, A.; Le Roy, N.; Brionne, A.; Bonin, E.; Juanchich, A.; Benzoni, G.; Piketty, M.-L.; Prié, D.; Nys, Y.; Gautron, J.; et al. Candidate genes of the transcellular and paracellular calcium absorption pathways in the small intestine of laying hens. Poult. Sci. 2019, 98, 6005–6018. [Google Scholar] [CrossRef]
- Marounek, M.; Skřivan, M.; Rosero, O.; Rop, O. Intestinal and total tract phytate digestibility and phytase activity in the digestive tract of hens fed a wheat-maize-soyabean diet. J. Anim. Feed Sci. 2010, 19, 433–442. [Google Scholar] [CrossRef]
- Marounek, M.; Skřivan, M.; Dlouhá, G.; Břeňová, N. Availability of phytate phosphorus and endogenous phytase activity in the digestive tract of laying hens 20 and 47 weeks old. Anim. Feed Sci. Technol. 2008, 146, 353–359. [Google Scholar] [CrossRef]
- Classen, H.L.; Apajalahti, J.; Svihus, B.; Choct, M. The role of the crop in poultry production. Worlds Poult. Sci. J. 2016, 72, 459–472. [Google Scholar] [CrossRef] [Green Version]
- Svihus, B. Function of the digestive system. J. Appl. Poult. Res. 2014, 23, 306–314. [Google Scholar] [CrossRef]
- Peddie, J.; Dewar, W.A.; Gilbert, A.B.; Waddington, D. The use of titanium dioxide for determining apparent digestibility in mature domestic fowls (Gallus domesticus). J. Agric. Sci. 1982, 99, 233–236. [Google Scholar] [CrossRef]
- McCuaig, L.W.; Davies, M.I.; Motzok, I. Intestinal alkaline phosphatase and phytase of chicks: Effect of dietary magnesium, calcium, phosphorus and thyroactive casein. Poult. Sci. 1972, 51, 526–530. [Google Scholar] [CrossRef]
- Applegate, T.J.; Angel, R.; Classen, H.L. Effect of dietary calcium, 25-hydroxycholecalciferol, or bird strain on small intestinal phytase activity in broiler chickens. Poult. Sci. 2003, 82, 1140–1148. [Google Scholar] [CrossRef]
- Herwig, E.; Schwean-Lardner, K.V.; Walk, C.; van Kessel, A.G.; Bedford, M.R.; Classen, H.L. Effect of phytase and myo-inositol supplementation on the expression of inositol transporters in the small intestine of laying hens, and blood and yolk inositol concentrations. Poult. Sci. 2019, 98 (Suppl. S1), 194. [Google Scholar]
- Rodehutscord, M.; Sanver, F.; Timmler, R. Comparative study on the effect of variable phosphorus intake at two different calcium levels on P excretion and P flow at the terminal ileum of laying hens. Arch. Tierernahr. 2002, 56, 189–198. [Google Scholar] [CrossRef]
- Pelicia, K.; Garcia, E.A.; Faitarone, A.B.G.; Silva, A.P.; Berto, D.A.; Molino, A.B.; Vercese, F. Calcium and available phosphorus levels for laying hens in second production cycle. Braz. J. Poult. Sci. 2009, 11, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Ieda, T.; Saito, N.; Shimada, K. Effect of low calcium diet on messenger ribonucleic acid levels of Calbindin-D28K of intestine and shell gland in laying hens in relation to egg shell quality. Jpn. Poult. Sci. 1999, 36, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, D.; Bryden, W.L. Calcium and phosphorus metabolism and nutrition of poultry: Are current diets formulated in excess? Anim. Prod. Sci. 2017, 57, 2304–2310. [Google Scholar] [CrossRef]
- Boorman, K.N.; Gunaratne, S.P. Dietary phosphorus supply, egg-shell deposition and plasma inorganic phosphorus in laying hens. Br. Poult. Sci. 2001, 42, 81–91. [Google Scholar] [CrossRef]
- Jing, M.; Zhao, S.; Rogiewicz, A.; Slominski, B.A.; House, J.D. Assessment of the minimal available phosphorus needs of pullets during the pre-laying period. Poult. Sci. 2018, 97, 557–567. [Google Scholar] [CrossRef]
- Frost, T.J.; Roland, D.A. The effects of various dietary P levels on the circadian patterns of plasma 1,25-dihydroxycholecalciferol, total Ca, ionized Ca and P in laying hens. Poult. Sci. 1991, 70, 1564–1570. [Google Scholar] [CrossRef]
- Pongmanee, K.; Kühn, I.; Korver, D.R. Effects of phytase supplementation on eggshell and bone quality, and phosphorus and calcium digestibility in laying hens from 25 to 37 wk of age. Poult. Sci. 2020, 99, 2595–2607. [Google Scholar] [CrossRef]

| Ingredient, g/kg | P+Ca+ | P+Ca− | P−Ca+ | P−Ca− |
|---|---|---|---|---|
| Corn | 617.4 | 617.4 | 617.4 | 617.4 |
| Soybean meal | 265.0 | 265.0 | 265.0 | 265.0 |
| Soybean oil | 12.0 | 12.0 | 12.0 | 12.0 |
| DL-Methionine | 3.5 | 3.5 | 3.5 | 3.5 |
| Monocalcium phosphate | 5.3 | 5.3 | 3.0 | 3.0 |
| Sand | - | 12.8 | 1.3 | 14.1 |
| Limestone, fine 1 | 24.7 | 21.3 | 25.2 | 21.7 |
| Limestone, coarse 2 | 58.7 | 49.3 | 59.2 | 50.0 |
| Sodium chloride | 3.0 | 3.0 | 3.0 | 3.0 |
| Choline chloride | 1.0 | 1.0 | 1.0 | 1.0 |
| Sodium bicarbonate | 1.9 | 1.9 | 1.9 | 1.9 |
| Vitamin mix 3 | 2.0 | 2.0 | 2.0 | 2.0 |
| Mineral mix 4 | 0.5 | 0.5 | 0.5 | 0.5 |
| TiO2 | 5.0 | 5.0 | 5.0 | 5.0 |
| Calculated concentration | ||||
| Crude protein, g/kg DM | 189 | 189 | 189 | 189 |
| Total P, g/kg DM | 5.3 | 5.3 | 4.7 | 4.7 |
| Non-phytate P, g/kg DM | 2.9 | 2.9 | 2.3 | 2.3 |
| Ca, g/kg DM | 39.6 | 33.9 | 39.6 | 33.9 |
| Analyzed concentration5 | ||||
| Total P, g/kg DM | 5.3 | 5.3 | 4.7 | 4.7 |
| InsP6-P, g/kg DM | 2.3 | 2.2 | 2.4 | 2.4 |
| Ca, g/kg DM | 39.5 | 35.1 | 40.3 | 34.4 |
| Myo-inositol, µmol/g DM | 1.1 | 1.1 | 1.1 | 1.1 |
| Ins(1,2,3,4,5)P5, µmol/g DM | 0.5 | 0.5 | 0.5 | 0.5 |
| Ins(1,2,4,5,6)P5, µmol/g DM | 1.0 | 1.0 | 1.1 | 1.0 |
| InsP6, µmol/g DM | 12.6 | 12.0 | 12.8 | 12.7 |
| BW | ADFI | Average Egg Weight | Inorganic P | Calcium | myo-Inositol | |||
|---|---|---|---|---|---|---|---|---|
| Strain | Dietary P | Dietary Ca | g | g/d | g | mmol/L | mmol/L | mmol/L |
| LB 1 | P+ | Ca+ | 1784 bc | 104 | 57.2 | 1.8 | 7.5 | 0.15 |
| LB | P+ | Ca− | 1929 a | 114 | 58.2 | 1.7 | 7.3 | 0.14 |
| LB | P− | Ca+ | 1838 ab | 111 | 58.8 | 1.8 | 6.9 | 0.14 |
| LB | P− | Ca− | 1809 b | 110 | 59.6 | 1.8 | 7.1 | 0.16 |
| LSL 2 | P+ | Ca+ | 1648 d | 109 | 60.6 | 1.5 | 6.2 | 0.14 |
| LSL | P+ | Ca− | 1641 d | 116 | 59.5 | 1.8 | 7.3 | 0.15 |
| LSL | P− | Ca+ | 1599 d | 109 | 61.2 | 1.7 | 7.3 | 0.15 |
| LSL | P− | Ca− | 1683 cd | 118 | 62.6 | 1.6 | 6.9 | 0.15 |
| Pooled SEM | 41.0 | 3.3 | 1.15 | 0.11 | 0.47 | 0.010 | ||
| p-values | Strain | <0.001 | 0.165 | 0.002 | 0.133 | 0.425 | 0.847 | |
| P | 0.500 | 0.571 | 0.047 | 0.514 | 0.857 | 0.615 | ||
| Ca | 0.081 | 0.008 | 0.474 | 0.705 | 0.551 | 0.458 | ||
| Strain × P | 0.579 | 0.798 | 0.859 | 0.562 | 0.261 | 0.671 | ||
| Strain × Ca | 0.717 | 0.387 | 0.525 | 0.172 | 0.634 | 0.728 | ||
| P × Ca | 0.445 | 0.293 | 0.460 | 0.312 | 0.363 | 0.418 | ||
| Strain × P × Ca | 0.016 | 0.202 | 0.451 | 0.055 | 0.121 | 0.085 | ||
| Crop | Gizzard | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Dietary P | Dietary Ca | myo-Inositol | Ins(1,2,3,4,5)P5 | Ins(1,2,4,5,6)P5 | InsP6 | myo-Inositol | Ins(1,2,3,4,5)P5 | Ins(1,2,4,5,6)P5 | InsP6 |
| LB 1 | P+ | Ca+ | 0.85 | 0.3 | 0.8 | 10.6 | 0.65 | <LOQ 3 | 0.5 | 8.6 |
| LB | P+ | Ca− | 0.76 | 0.4 | 0.8 | 10.6 | 0.60 | n.d. 4 | 0.4 | 7.7 |
| LB | P− | Ca+ | 0.85 | 0.4 | 0.9 | 12.0 | 0.60 | 0.2 | 0.5 | 8.4 |
| LB | P− | Ca− | 0.77 | 0.4 | 0.9 | 11.6 | 0.65 | <LOQ | 0.4 | 7.0 |
| LSL 2 | P+ | Ca+ | 0.60 | 0.5 | 0.9 | 11.8 | 0.31 | n.d. | 0.3 | 5.8 |
| LSL | P+ | Ca− | 0.75 | 0.4 | 1.0 | 11.8 | 0.55 | n.d. | <LOQ | 5.2 |
| LSL | P− | Ca+ | 0.75 | 0.4 | 0.9 | 11.9 | 0.36 | n.d. | 0.3 | 5.7 |
| LSL | P− | Ca− | 0.80 | 0.4 | 0.9 | 11.8 | 0.55 | 0.2 | 0.3 | 6.7 |
| Pooled SEM | 0.090 | 0.03 | 0.06 | 0.67 | 0.059 | 0.04 | 0.06 | 0.85 | ||
| p-values | Strain | 0.235 | 0.024 | 0.019 | 0.188 | 0.001 | . | 0.005 | 0.002 | |
| P | 0.370 | 0.475 | 0.153 | 0.164 | 0.770 | . | 0.921 | 0.762 | ||
| Ca | 0.833 | 0.656 | 0.786 | 0.840 | 0.014 | . | 0.723 | 0.493 | ||
| Strain × P | 0.405 | 0.025 | 0.056 | 0.215 | 0.760 | . | 0.832 | 0.292 | ||
| Strain × Ca | 0.121 | 0.832 | 0.433 | 0.914 | 0.010 | . | 0.178 | 0.258 | ||
| P × Ca | 0.687 | 0.681 | 0.355 | 0.799 | 0.760 | . | 0.979 | 0.703 | ||
| Strain × P × Ca | 0.640 | 0.128 | 0.750 | 0.851 | 0.368 | . | . | 0.449 | ||
| Strain | Dietary P | Dietary Ca | myo-Inositol | Ins(1,2,5,6)P4 | Ins(1,2,3,4,6)P5 | Ins(1,2,3,4,5)P5 | Ins(1,2,4,5,6)P5 | InsP6 |
|---|---|---|---|---|---|---|---|---|
| LB 1 | P+ | Ca+ | 7.40 | 0.3 | 0.5 | 1.1 | 1.9 | 27.9 |
| LB | P+ | Ca− | 8.26 | 0.3 | 0.5 | 1.1 | 1.9 | 31.7 |
| LB | P− | Ca+ | 8.53 | 0.4 | 0.6 | 1.4 | 2.5 | 36.1 |
| LB | P− | Ca− | 8.82 | <LOQ 3 | 0.5 | 1.2 | 1.7 | 32.8 |
| LSL 2 | P+ | Ca+ | 8.71 | <LOQ | 0.5 | 1.1 | 1.7 | 28.4 |
| LSL | P+ | Ca− | 9.16 | 0.3 | <LOQ | 1.0 | 1.4 | 27.1 |
| LSL | P− | Ca+ | 8.55 | <LOQ | 0.5 | 1.0 | 1.6 | 26.3 |
| LSL | P− | Ca− | 9.54 | n.d. 4 | <LOQ | 0.9 | 1.3 | 27.8 |
| Pooled SEM | 0.532 | 0.06 | 0.06 | 0.12 | 0.24 | 2.33 | ||
| p-values | Strain | 0.110 | 0.704 | 0.342 | 0.049 | 0.021 | 0.019 | |
| P | 0.149 | 0.445 | 0.782 | 0.459 | 0.848 | 0.211 | ||
| Ca | 0.054 | 0.908 | 0.634 | 0.266 | 0.037 | 0.906 | ||
| Strain × P | 0.264 | . | 0.245 | 0.219 | 0.293 | 0.097 | ||
| Strain × Ca | 0.824 | . | . | 0.853 | 0.701 | 0.960 | ||
| P × Ca | 0.982 | . | 0.213 | 0.355 | 0.239 | 0.499 | ||
| Strain × P × Ca | 0.397 | . | . | 0.355 | 0.203 | 0.127 | ||
| Strain | Dietary P | Dietary Ca | myo-inositol | InsP3x | Ins(1,5,6)P3 | Ins(1,2,3,4)P4 | Ins(1,2,5,6)P4 | Ins(1,2,3,4,6)P5 | Ins(1,2,3,4,5)P5 | Ins(1,2,4,5,6)P5 | InsP6 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LB 1 | P+ | Ca+ | 1.93 | 0.4 | n.d. 3 | 0.2 | 0.3 | 0.8 | 1.7 | 2.2 | 40.0 |
| LB | P+ | Ca− | 3.76 | 0.4 | n.d. | 0.2 | <LOQ 4 | 0.7 | 1.6 | 2.4 | 43.5 |
| LB | P− | Ca+ | 2.36 | 0.4 | 0.4 | <LOQ | 0.4 | 0.8 | 1.7 | 2.6 | 46.3 |
| LB | P− | Ca− | 6.41 | 0.5 | <LOQ | 0.3 | <LOQ | 0.9 | 1.7 | 2.0 | 46.5 |
| LSL 2 | P+ | Ca+ | 1.83 | 0.5 | 0.3 | 0.4 | 0.5 | 1.0 | 2.1 | 2.9 | 45.6 |
| LSL | P+ | Ca− | 3.42 | 0.5 | n.d. | 0.3 | 0.4 | 0.6 | 1.4 | 2.1 | 35.6 |
| LSL | P− | Ca+ | 2.20 | 0.4 | <LOQ | 0.3 | 0.4 | 0.7 | 1.5 | 2.2 | 41.6 |
| LSL | P− | Ca− | 2.19 | 0.5 | <LOQ | <LOQ | <LOQ | 0.7 | 1.3 | 1.8 | 38.4 |
| Pooled SEM | 0.794 | 0.08 | 0.07 | 0.07 | 0.09 | 0.12 | 0.20 | 0.34 | 4.11 | ||
| p-values | Strain | 0.061 | 0.298 | . | 0.030 | 0.507 | 0.694 | 0.471 | 0.752 | 0.176 | |
| P | 0.766 | 0.488 | . | 0.605 | 0.884 | 0.730 | 0.352 | 0.328 | 0.471 | ||
| Ca | 0.001 | 0.433 | . | 0.449 | 0.748 | 0.170 | 0.076 | 0.113 | 0.405 | ||
| Strain × P | 0.126 | 0.429 | . | . | 0.459 | 0.119 | 0.247 | 0.355 | 0.350 | ||
| Strain × Ca | 0.449 | 0.763 | . | 0.628 | . | 0.221 | 0.144 | 0.352 | 0.138 | ||
| P × Ca | 0.710 | 0.493 | . | . | . | 0.134 | 0.368 | 0.655 | 0.752 | ||
| Strain × P × Ca | 0.140 | 0.968 | . | . | . | 0.355 | 0.362 | 0.229 | 0.366 | ||
| Strain | Dietary P | Dietary Ca | myo-Inositol | InsP3x | Ins(1,2,3,4)P4 | Ins(1,2,5,6)P4 | Ins(1,2,3,4,6)P5 | Ins(1,2,3,4,5)P5 | Ins(1,2,4,5,6)P5 | InsP6 |
|---|---|---|---|---|---|---|---|---|---|---|
| LB 1 | P+ | Ca+ | 0.24 c | 0.3 | 0.5 | 0.5 | 0.5 | 2.6 | 1.9 | 26.0 |
| LB | P+ | Ca− | 0.15 c | n.d. | 0.4 | 0.4 | n.d. | 2.3 | 2.0 | 25.4 |
| LB | P− | Ca+ | 0.32 c | n.d. | 0.6 | 0.4 | 0.4 | 3.3 | 1.9 | 25.6 |
| LB | P− | Ca− | 0.42 c | n.d. | 0.2 | n.d. | n.d. | 1.6 | 0.4 | 9.5 |
| LSL 2 | P+ | Ca+ | 0.64 bc | n.d. | 0.4 | n.d. | n.d. | 1.6 | n.d. | 2.4 |
| LSL | P+ | Ca− | 1.09 ab | n.d. | 0.3 | n.d. | n.d. | 0.9 | 0.2 | 6.1 |
| LSL | P− | Ca+ | 1.30 a | n.d. | 0.3 | n.d. | n.d. | 1.4 | n.d. | 3.0 |
| LSL | P− | Ca− | 0.94 ab | n.d. | n.d. | n.d. | n.d. | 1.5 | 0.5 | 5.1 |
| Pooled SEM | 0.189 | 0.08 | 0.09 | 0.09 | 0.12 | 0.48 | 0.50 | 5.79 | ||
| p-values | Strain | <0.001 | . | 0.024 | . | . | 0.019 | 0.119 | 0.001 | |
| P | 0.088 | . | 0.191 | 0.454 | 0.710 | 0.797 | 0.598 | 0.299 | ||
| Ca | 0.831 | . | 0.012 | 0.350 | . | 0.045 | 0.115 | 0.510 | ||
| Strain × P | 0.752 | . | 0.472 | . | . | 0.777 | 0.099 | 0.322 | ||
| Strain × Ca | 0.885 | . | 0.683 | . | . | 0.295 | . | 0.163 | ||
| P × Ca | 0.214 | . | 0.089 | . | . | 0.609 | 0.071 | 0.285 | ||
| Strain × P × Ca | 0.049 | . | . | . | 0.101 | . | 0.395 | |||
| Ca Intake | Ca Jejunum | Ca Ileum | Ca Excretion | Ca Utilization | P Intake | P Jejunum | P Ileum | P Excretion | P Utilization | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Dietary P | Dietary Ca | g/d | g/kg | g/kg | g/d | % | g/d | g/kg | g/kg | g/d | % |
| LB 1 | P+ | Ca+ | 3.91 | 27.9 | 53.2 | 1.33 | 66.1 | 0.53 | 8.7 | 11.0 | 0.39 | 24.9 |
| LB | P+ | Ca− | 3.86 | 22.4 | 32.2 | 1.08 | 72.7 | 0.58 | 9.2 | 11.6 | 0.41 | 31.0 |
| LB | P− | Ca+ | 4.14 | 27.3 | 37.2 | 1.56 | 63.3 | 0.49 | 10.6 | 12.5 | 0.36 | 26.0 |
| LB | P− | Ca− | 3.60 | 21.3 | 34.1 | 0.99 | 72.6 | 0.50 | 9.5 | 12.0 | 0.37 | 25.1 |
| LSL 2 | P+ | Ca+ | 4.09 | 28.3 | 44.1 | 1.49 | 63.3 | 0.55 | 9.1 | 12.4 | 0.43 | 21.9 |
| LSL | P+ | Ca− | 3.96 | 27.0 | 35.0 | 1.10 | 72.7 | 0.60 | 9.0 | 11.2 | 0.43 | 28.3 |
| LSL | P− | Ca+ | 4.06 | 26.4 | 47.2 | 1.32 | 67.5 | 0.48 | 9.3 | 11.4 | 0.36 | 23.3 |
| LSL | P− | Ca− | 3.84 | 21.6 | 37.3 | 1.05 | 72.9 | 0.53 | 8.1 | 10.7 | 0.38 | 27.9 |
| Pooled SEM | 0.114 | 3.49 | 7.12 | 0.117 | 2.47 | 0.015 | 0.58 | 1.04 | 0.017 | 2.45 | ||
| p-values | Strain | 0.187 | 0.485 | 0.273 | 0.990 | 0.848 | 0.155 | 0.222 | 0.590 | 0.128 | 0.373 | |
| P | 0.571 | 0.492 | 0.557 | 0.788 | 0.788 | <0.001 | 0.308 | 0.887 | <0.001 | 0.556 | ||
| Ca | 0.004 | 0.027 | 0.014 | <0.001 | <0.001 | <0.001 | 0.182 | 0.515 | 0.299 | 0.013 | ||
| Strain × P | 0.696 | 0.555 | 0.360 | 0.236 | 0.228 | 0.712 | 0.051 | 0.220 | 0.289 | 0.351 | ||
| Strain × Ca | 0.432 | 0.669 | 0.605 | 0.602 | 0.840 | 0.433 | 0.655 | 0.463 | 0.817 | 0.356 | ||
| P × Ca | 0.071 | 0.691 | 0.417 | 0.513 | 0.835 | 0.311 | 0.077 | 0.817 | 0.578 | 0.167 | ||
| Strain × P × Ca | 0.215 | 0.933 | 0.316 | 0.147 | 0.268 | 0.232 | 0.719 | 0.519 | 0.781 | 0.408 | ||
| Strain | Dietary P | Dietary Ca | ATP2B1 | CALB1 | NCX1 | SLC20A1 | SLC20A2 | SLC34A2 |
|---|---|---|---|---|---|---|---|---|
| LB 1 | P+ | Ca+ | 14.70 | 21.01 | 9.39 | 12.64 | 12.26 | 14.02 |
| LB | P+ | Ca− | 14.99 | 21.29 | 9.16 | 13.08 | 12.34 | 14.26 |
| LB | P− | Ca+ | 14.72 | 20.64 | 9.32 | 12.63 | 12.09 | 13.87 |
| LB | P− | Ca− | 14.80 | 21.05 | 9.06 | 13.18 | 12.23 | 14.16 |
| LSL 2 | P+ | Ca+ | 14.30 | 21.05 | 9.47 | 11.90 | 11.90 | 13.26 |
| LSL | P+ | Ca− | 14.56 | 21.39 | 9.64 | 12.05 | 12.01 | 13.83 |
| LSL | P− | Ca+ | 14.49 | 21.47 | 9.56 | 12.44 | 12.24 | 13.78 |
| LSL | P− | Ca− | 14.61 | 21.45 | 9.53 | 12.44 | 12.10 | 13.74 |
| Pooled SEM | 0.252 | 0.227 | 0.252 | 0.306 | 0.131 | 0.229 | ||
| p-values | Strain | 0.077 | 0.081 | 0.083 | <0.001 | 0.089 | 0.007 | |
| P | 0.898 | 0.827 | 0.779 | 0.189 | 0.596 | 0.760 | ||
| Ca | 0.221 | 0.090 | 0.605 | 0.156 | 0.531 | 0.090 | ||
| Strain × P | 0.488 | 0.067 | 0.834 | 0.278 | 0.027 | 0.270 | ||
| Strain × Ca | 0.993 | 0.526 | 0.343 | 0.277 | 0.407 | 0.986 | ||
| P × Ca | 0.568 | 0.670 | 0.740 | 0.954 | 0.553 | 0.364 | ||
| Strain × P × Ca | 0.914 | 0.392 | 0.797 | 0.741 | 0.309 | 0.295 | ||
| Albumen | Albumen | Yolk | Yolk | Egg | |||
|---|---|---|---|---|---|---|---|
| Strain | Dietary P | Dietary Ca | µmol/g | µmol | µmol/g | µmol | µmol |
| LB 1 | P+ | Ca+ | 0.54 | 18.8 | 0.79 b | 11.4 b | 30.2 |
| LB | P+ | Ca− | 0.53 | 19.0 | 0.87 ab | 13.3 a | 32.3 |
| LB | P− | Ca+ | 0.52 | 18.8 | 0.90 a | 13.6 a | 32.4 |
| LB | P− | Ca− | 0.51 | 19.2 | 0.87 ab | 12.9 ab | 32.0 |
| LSL 2 | P+ | Ca+ | 0.47 | 17.2 | 0.84 ab | 13.5 a | 30.7 |
| LSL | P+ | Ca− | 0.50 | 17.5 | 0.82 ab | 13.4 a | 30.9 |
| LSL | P− | Ca+ | 0.51 | 18.4 | 0.79 b | 13.1 a | 31.5 |
| LSL | P− | Ca− | 0.50 | 18.8 | 0.85 ab | 14.3 a | 33.1 |
| Pooled SEM | 0.030 | 1.21 | 0.032 | 0.67 | 1.46 | ||
| p-values | Strain | 0.324 | 0.433 | 0.341 | 0.185 | 0.909 | |
| P | 0.976 | 0.367 | 0.293 | 0.207 | 0.179 | ||
| Ca | 0.967 | 0.658 | 0.324 | 0.169 | 0.305 | ||
| Strain × P | 0.222 | 0.431 | 0.142 | 0.470 | 0.783 | ||
| Strain × Ca | 0.735 | 0.995 | 0.940 | 0.997 | 0.997 | ||
| P × Ca | 0.555 | 0.917 | 0.763 | 0.454 | 0.773 | ||
| Strain × P × Ca | 0.577 | 0.990 | 0.023 | 0.025 | 0.266 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommerfeld, V.; Omotoso, A.O.; Oster, M.; Reyer, H.; Camarinha-Silva, A.; Hasselmann, M.; Huber, K.; Ponsuksili, S.; Seifert, J.; Stefanski, V.; et al. Phytate Degradation, Transcellular Mineral Transporters, and Mineral Utilization by Two Strains of Laying Hens as Affected by Dietary Phosphorus and Calcium. Animals 2020, 10, 1736. https://doi.org/10.3390/ani10101736
Sommerfeld V, Omotoso AO, Oster M, Reyer H, Camarinha-Silva A, Hasselmann M, Huber K, Ponsuksili S, Seifert J, Stefanski V, et al. Phytate Degradation, Transcellular Mineral Transporters, and Mineral Utilization by Two Strains of Laying Hens as Affected by Dietary Phosphorus and Calcium. Animals. 2020; 10(10):1736. https://doi.org/10.3390/ani10101736
Chicago/Turabian StyleSommerfeld, Vera, Adewunmi Omolade Omotoso, Michael Oster, Henry Reyer, Amélia Camarinha-Silva, Martin Hasselmann, Korinna Huber, Siriluck Ponsuksili, Jana Seifert, Volker Stefanski, and et al. 2020. "Phytate Degradation, Transcellular Mineral Transporters, and Mineral Utilization by Two Strains of Laying Hens as Affected by Dietary Phosphorus and Calcium" Animals 10, no. 10: 1736. https://doi.org/10.3390/ani10101736







