Simple Summary
Understanding animal responses to stressful stimuli is a fundamental aspect to evaluating animal welfare. Stress-induced hyperthermia (SIH) is a term used to describe a short-term increase in body temperature that occurs in response to stressful stimuli. Recently there has been increasing interest in SIH as a physiological measure of psychological stress in livestock species. Previously, studies have suggested that cattle with more excitable temperaments exhibit an increased stress response. This study evaluated the influence of temperament on SIH, during a standardized handling procedure in Bos taurus cattle. In this study, body temperature increased, regardless of sex or temperament traits, characterizing SIH. Nevertheless, both flight speed (FS) and crush score (CS) were associated with an elevated rectal temperature (TREC) 30 min prior to the handling procedure, and this continued from the start of handling (T0) to 10 min post-handling (T10). The results from this study suggest that temperament may be related to variation in SIH in cattle during handling. Understanding the variation in behavioral and physiological response to stressful events may enable the development of new measures for genetic selection in cattle.
Abstract
Previous studies have indicated that cattle with more excitable temperaments exhibit an increased stress response. The objective of this study was to investigate the relationship between temperament traits, handling, and stress-induced hyperthermia (SIH) in beef cattle. Rectal temperatures (TREC, °C) of 60 purebred Angus cattle (30 heifers, 30 steers; 235.2 ± 5.11 kg) were recorded at 20 s intervals from 30 min prior to handling until two hours post handling. All cattle were exposed to a standardized handling procedure consisting of (i) being restrained in a weighing box for 30 s; (ii) being held within the crush for 30 s; and then (iii) being restrained in a head bail for 60 s. Cattle temperaments were evaluated via three traits: (1) agitometer score (AG); (2) crush score (CS); and (3) flight speed (FS) during the handling procedure. Agitometer scores and FS measures were used to describe an AG category (AGCAT) and an FS category (FSCAT) that were used to classify animals into three temperament categories: 1, calm; 2, intermediate; and 3, temperamental. Pearson’s correlation coefficients were used to evaluate the associations between (i) AG, CS, FS, and TREC 30 min prior to entry into the weighing box (T-30) and then at 1 min intervals between time of entry into the weighing box (T0) until 10 min post-weighing (T10); and (ii) the relationship between AG, CS, and FS. The relationship between TREC and temperament traits over the 2.5 h were modeled by using a first-order autoregressive repeated measures model. Flight speed had strong to moderate associations with TREC at T-30 (r ≥ 0.37; p ≤ 0.006) and between T0 and T10 (r ≥ 0.36; p ≤ 0.01). There were moderate associations amongst TREC between T0 and T10 and CS (r ≥ 0.31; p ≤ 0.01). A weak relationship existed with CS (r = 0.16; p = 0.16). There were no associations between AG and TREC at T-30 (r ≥ −0.15; p = 0.84) or between T0 and T10 (r ≤ 0.04; p ≥ 0.4). Rectal temperature, irrespective of sex and temperament traits, was influenced by time (p < 0.0001), and maximum TREC (39.3 ± 0.04 °C) occurred between 4 and 5.7 min after entry into the weighing box. In addition, CS (p = 0.007) influenced TREC in these cattle. There were also time × temperament trait × sex interactions with the CS (p = 0.0003) and FSCAT (p = 0.043) categories; however, time × temperament trait interactions were not statistically significant. Results from this study suggest that cattle with excitable temperaments, as evaluated by FS and CS, have a greater increase in TREC. In addition, these results suggest that a relationship exists between basal TREC and FS and CS. Together, these results highlight that temperament, as assessed by FS and CS, influences both basal TREC and the peak temperature recorded following handling but does not influence the magnitude of change in TREC post handling.
1. Introduction
In livestock production enterprises, temperament is an important consideration, as individuals that are classified to have more excitable temperaments have been associated with decreased average daily gain [1,2]; reduced carcass quality characteristics [2,3]; and reduced immune function [4,5]. Furthermore, previous studies have suggested that cattle with more excitable temperaments exhibit an increased stress response [6,7,8,9]. As cattle temperament becomes more excitable/temperamental/reactive, their reactivity to human contact and handling procedures can become more aggressive or fearful [10], which may result in a heightened stimulation of catecholamines and glucocorticoids [11,12]. The latter suggests that the functional characteristics of the hypothalamic-pituitary-adrenal axis may vary with animal temperament [8,9].
Cattle with a lower rectal temperature (TREC, °C) in tropical environments have been reported to have calmer temperaments, greater reproductive efficiency, better growth rate, and a greater resistance to ticks [13,14]. Temperament in cattle is considered to be the individual variation in behavioral responses to stressors or stressful events, including human handling [2,9,10]. Cattle temperament traits may influence how an animal responds to routine handling and husbandry procedures on-farm [10,15]. Furthermore, previous studies have established a relationship between flight speed (FS) and TREC [7,13], and FS has been described to have a moderate to high (≥0.3) heritability [13,16,17,18]. Rectal temperature has been described as moderately heritable in Bos indicus [13,14] and Bos taurus [19,20] cattle, when evaluated during hot environmental conditions.
Body temperature in cattle is tightly regulated typically within ±1 °C gradient [21]. Increases in body temperature have been associated with exercise [22], digestion [23], inflammation and activation of the immune system [6,24], hot environmental conditions [25], and psychosocial stressors [26]. Within minutes of exposure to acute physiological stressors, there can be a short-term transient increase in body temperature. This phenomenon is termed stress-induced hyperthermia (SIH) or emotional fever [26], and has been reported in numerous species, including rodents [26], humans [27], sheep [28,29], and, more recently, cattle [30].
Previous studies have established that bulls classified as temperamental prior to road transport or a lipopolysaccharide challenge have higher TREC when compared with bulls classified as calm [6,7], suggesting overall that routine handling procedures induce a stress or fear response in cattle [30,31,32]. Understanding how temperament relates to physiological stress responses may further enhance the ability to select animals that have a greater capacity to cope with production systems.
The objectives of this study were to evaluate the influence of temperament and handling on SIH in Bos taurus cattle during a standardized handling procedure. Additionally, within the current study indwelling intra-rectal loggers were used to evaluate the influence of temperament on baseline TREC prior to handling and during a standardized handling procedure in Bos taurus cattle.
2. Materials and Methods
This study was conducted with the approval of the CSIRO McMaster Laboratory Animal Ethics Committee (ARA18-04), in accordance with the guidelines of the Australian National Health and Medical Research Council [33]. Data were collected over a single day during a southern hemisphere autumn (May). The study was undertaken in the New England district of New South Wales, Australia (30.52° S, 151.67° E, 1050 m above mean sea level), at the FD McMaster Research Laboratory. Climatic conditions were obtained between 08:30 h and 14:30 h, to encompass climatic conditions 30 min prior to handling and 2 h post-handling. Climatic data were obtained at 30 min intervals from the Australian Bureau of Meteorology’s (www.bom.gov.au) weather station (ID056238), located at Armidale Airport (30.53° S, 151.62° E, 1079 m above mean sea level). During the handling procedure, average (±SEM) ambient temperature, dew point temperature, relative humidity, and wind speed were 12.3 ± 0.81 °C, 3.1 ± 0.81 °C, 57.2 ± 6.23%, and 2.4 ± 0.12 m/s, respectively.
2.1. Animals
Thirty purebred Angus heifers (226.2 ± 5.01 kg) and 30 purebred Angus steers (244.3 ± 8.59 kg), aged between 7 and 10 months, were sourced from the FD McMaster Research Laboratory Angus performance-recorded herd and used in the study. Prior to this study, the FD McMaster Research Laboratory Angus performance-recorded herd was managed as a single herd. Cattle used in this study had previously been yard-weaned over a seven-day period, nine weeks prior to the study. Weaning occurred in a different handling facility to the one used in the current study. During weaning, cattle were fed a mix of lucerne hay (Medicago sativa) and lucerne-based pelleted concentrate. After weaning, and throughout the study, cattle were group-housed as a contemporary cohort on grazing pastures (Phalaris aquatic, Dactylis glomerata, and Plantago lanceolata). During the study, cattle were relocated to a paddock adjacent to the cattle-handling facilities and were supplemented with approximately 1.5 kg/head of whole cotton seed (Gossypium spp.) daily.
2.2. Body Temperature
On day 1, cattle were relocated from a paddock adjacent to the handling facility and intra-rectal data loggers were placed into the rectal cavity of all animals (n = 60). The handling facility was the same facility used for the handling procedure (see below) on day 2. Intra-rectal loggers were prepared and placed in situ using a technique modified from Lea et al. [34]. Briefly, intra-rectal loggers consisted of an iButton (DS1922L, Thermochron iButton Device; Maxim Integrated, San Jose, CA, USA) attached to soft polyethylene piping (180 mm in length × 8 mm in diameter) and fixed in place using heat shrink plastic. The loggers were inserted into the rectum and held in place using veterinary tape (Tensoplast® Vet, BSN Medical Inc., Hamburg, Germany) to attach the exposed end of the logger to the underside of the tail. After intra-rectal loggers were in place, all cattle (n = 60) were returned to grazing pasture adjacent to the handling facility. Data loggers were programed to record TREC at 20 s intervals from 08:00 h on day 2, 1 h prior to the handling procedure until 2 h post handling.
2.3. Handling Procedure
At 08:00 h, on day 2, cattle were relocated from the adjoining paddock and marshalled into holding yards within the cattle handling facility, used on day 1. Cattle were given 30 min to settle down and return to basal activity prior to marshalling into a single-file race. Cattle were then exposed to a standardized handling procedure, typical of routine husbandry, consisting of three components: (1) cattle being confined in a single animal-weighing box (Ramage Engineering, Guyra, Australia, fitted with TRUTEST weighing cells and the XR3000 indicator panel) with solid sides, for 30 s (stage 1); then (2) being confined within a crush (MK5, Ramage Engineering, Guyra, Australia) for 30 s (stage 2); and then (3) being restrained in a head bail for 60 s (stage 3). The duration of the handling procedure was 2.3 min for each animal. A similar procedure utilized by Lee et al. [35], involving restraint in a head bail, was shown to elicit an acute stress response as quantified by increased cortisol and beta-endorphin concentrations. All cattle were subjected to the handling procedure, which occurred over approximately 3 h on day 2.
2.4. Temperament Assessments
During the handling procedure on day 2, cattle temperaments were evaluated by utilizing three methodologies: (1) agitometer score (AG), (2) crush score (CS), and (3) flight speed (FS). Agitometer scores were determined via a purposely built agitometer attached to the weigh box via a metal bracket, modified from Blache and Ferguson [36]. The agitometer was designed and developed by Blache and Ferguson [36] and is used to measure the vibrations over a 30 s period, providing a numerical value of cattle movement within the weigh box, during stage 1 of the standardized handling procedure, as described above in Section 2.3. The agitometer was calibrated with a specifically engineered unit that mimicked the action of animals through four ‘feet’ via spring-loaded solenoids, as described by Blache and Ferguson [36].
Crush scores were evaluated by a single trained observer over the entire 30 s period, whilst the cattle remained unrestrained within the crush, during stage 2 of the standardized handling procedure, as described above, in Section 2.3. Crush scores were evaluated by using a 5-point subjective scale scoring system, as described in Table 1.

Table 1.
Cattle crush scoring descriptions 1.
Flight speeds were determined for all cattle on exit from the crush. Flight times were recorded for the time taken for cattle to traverse through two pairs of infrared sensors 1.8 m apart (FarmTek Electronic Timers, Wylie, TX, USA). These data were used to calculate FS (m/s) as described by Burdick et al. [39] (FS = m/time, s), and flight speed data were then used for statistical analysis.
2.5. Statistical Analysis
Seven data loggers failed to download data, and six data loggers provided data that were deemed erroneous, i.e., TREC <20 °C. Additionally, two animals physically displaced the infrared sensors that were recording flight time; thus, data from a total of 15 animals were excluded from analysis. Data analyzed incorporated information from 22 heifers and 23 steers. All data exploration and statistical analyses were conducted in R [40].
Rectal temperatures were aligned for each animal, so that time zero (T0) occurred at entry into the weighing box. Individual TREC data sets were generated to encompass a 2.5 h period consisting of data from 30 min prior to entry into the weigh box to 2 h post-entry into the weigh box. Baseline TREC was considered as time −30 min, i.e., the first measurement of TREC 30 min prior to entry into the weigh box. Change in TREC was considered the difference in TREC for each 20 s time point for comparison to baseline TREC (T-30).
Agitometer scores were used to categorize animals into three temperament categories (AGCAT): 1, calm, ≤33 (n = 16; ♀ = 6, ♂ = 10); 2, intermediate, 45 ≤ 82 (n = 15; ♀ = 10, ♂ = 5); and 3, temperamental, ≥86 (n = 14; ♀ = 6, ♂ = 8). Similarly, FS were then classified into three temperament categories (FSCAT): 1, calm, ≤1.6 m/s (n = 13; ♀ = 2, ♂ = 11); 2, intermediate, 1.7 m/s ≤2.5 m/s (n = 23; ♀ = 14, ♂ = 9); and 3, temperamental, ≥2.6 m/s (n = 9; ♀ = 6, ♂ = 3). Agitometer and flight speed categories were developed by applying a percentile ranking technique, based on the data generated within this study [12,39,41]. Crush scores of 5 were not observed within the current study; thus, CS were grouped into 4 categories, as previously described: (1) calm (n = 14, ♀ = 4, ♂ = 10); (2) slightly restless (n = 16, ♀ = 7, ♂ = 9); (3) restless (n = 11; ♀ = 9, ♂ = 2); and (4) nervous (n = 4; ♀ = 3, ♂ = 1).
Initially, Pearson’s correlation coefficients were used to evaluate the relationship between temperament measures. Then, the influence of sex on temperament traits were evaluated by Welch’s two-sample t-test. Pearson’s correlation coefficients were then used to evaluate the relationship between temperament traits (AG, CS, and FS) and TREC at T-30 and at one-minute intervals between T0 to T10.
Due to the proximate relationship and highly correlated nature of TREC measures, the relationship between TREC, sex, and temperament traits (CS, AGCAT, and FSCAT) were modeled by using a first-order autoregressive repeated measures model, within the “nlme” package [42]. The influence of temperament traits on TREC were investigated individually, as previous studies have established correlations between temperament traits. Each model incorporated time (each 20 s interval); a temperament trait (CS, AGCAT, or FSCAT); sex; time × sex; time × temperament trait (CS, AGCAT, or FSCAT); and time × temperament trait (CS, AGCAT, or FSCAT) × sex as fixed effects. Animal identification was incorporated as a random effect in all models. Initially live weight was evaluated as a covariate within all models, but did not contribute significance (p ≥ 0.05); thus, live weight was excluded from the final models.
Statistical significance was set at p < 0.05, and tendencies were determined if p ≥ 0.05 and ≤ 0.10. The strength of the Pearson’s correlation coefficients was identified as weak (r ≤ 0.30), moderate (r = 0.30 ≤ 0.50), and strong (r ≥ 0.50), as described by Cohen [43].
3. Results
Rectal temperature, irrespective of sex and temperament traits, was influenced by time (p < 0.0001). Pooled TREC data indicated that maximum TREC (39.3 ± 0.04 °C) occurred 4 min after entry into the weighing box (T0; p = 0.008) and remained elevated until 5.7 min. There were no differences in the TREC between heifers (39.4 ± 0.07 °C) and steers (39.2 ± 0.05 °C; Figure 1), nor were there differences in the change in TREC between heifers and steers from baseline TREC (p = 0.596; Figure 2). Change in TREC peaked between 3.3 and 5.0 min and at 5.7 min for steers (0.24 °C) and heifers (0.28 °C), respectively, although the change in TREC of heifers was 0.27 °C between 4.3 and 5.3 min (Figure 2). Post-handling, TREC showed a steady rate of decline (−0.01 °C) between 6.0 and 90.4 min, stabilizing at 38.9 ± 0.03 °C (Figure 1 and Figure 2). After 90.4 min, TREC remained within a temperature range between 38.88 ± 0.03 °C and 38.92 ± 0.04 °C, until data loggers were removed, 120 min post-handling (Figure 1 and Figure 2).
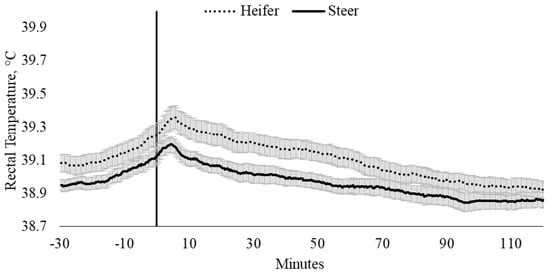
Figure 1.
Twenty-seconds mean (±SEM) rectal temperature (TREC, °C) of heifers (dotted line) and steers (solid line), 30 min prior to 2 h post-handling, also showing the time from weighing box (solid black vertical line).
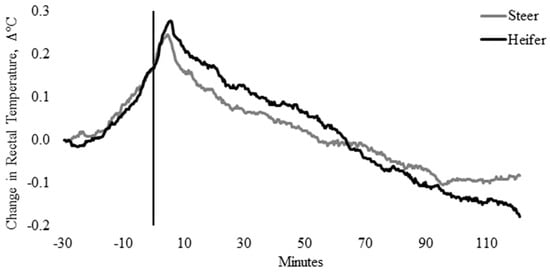
Figure 2.
Change in twenty-seconds rectal temperature (±SEM) from baseline rectal temperature (TREC, °C) for heifers (solid black line) and steers (solid gray line), 30 min prior to 2 h post-handling, also showing the time from weighing box (solid black vertical line).
There were no relationships identified between FS and CS (r = 0.09; p = 0.28) or FS and AG (r = 0.05; p = 0.36). Heifers had greater CS (p = 0.017) and FS (p = 0.008) when compared to steers, however, there were no sex differences in AG (p = 0.75; Table 2).

Table 2.
Mean (±SEM) agitometer score (AG), crush score (CS) s, and flight speed (FS, m/s) for heifers, steers, and pooled data.
There were strong to moderate associations between T-30 TREC and FS (r = 0.51; p = 0.0002). There was a weak relationship between CS and T-30 TREC (r = 0.16; p = 0.16). However, there were no relationships with T-30 TREC and AG (r = −0.15; p = 0.84).
There were strong to moderate associations between FS (r ≥ 0.50; p ≤ 0.0001) and TREC between T0 and T10 (Table 3). Similarly, there were moderate associations amongst TREC between T0 and T10 and CS (r ≥ 0.31; p ≤ 0.01; Table 3). There were no relationships between TREC and AG (r ≤ 0.03; p ≥ 0.42) at any time point between T0 and T10, highlighting that the correlations are not changing over this period (Table 3).

Table 3.
Pearson’s correlation coefficients between rectal temperature and agitometer score (AG 1), flight speed (FS 1, m/s), and crush score (CS) between the time of weighing (T0) and 10 min post-weighing (T10).
Crush score had a strong relationship with TREC (CS, p = 0.007). There were limited differences between the TREC of CS scores 1 (calm) and 2 (slightly restless), where the minimum and maximum temperature differences were −0.10 and 0.11 °C, respectively (Figure 3); however, as the CS category increased, there was an increase in the difference in TREC (min, 0.01 °C; max, 0.52 °C; Figure 3). The influence of CS on TREC were particularly evident between cattle scored as category 1 (calm; n = 14) when compared with cattle scored as category 4 (nervous, n = 4), where cattle that were scored in category 1 exhibited a 0.18 °C increase in TREC, whilst cattle scored within category 4 had a TREC increase of 0.44 °C (Figure 3).
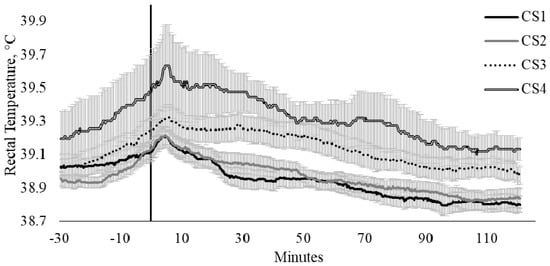
Figure 3.
Twenty-seconds mean (±SEM) rectal temperature (TREC, °C) of cattle crush scores (CS) of 1 (CS1; n = 14; ♀ = 4, ♂ = 10); 2 (CS2; n = 16; ♀ = 7, ♂ = 9); 3 (CS3; n = 11; ♀ = 9, ♂ = 2); and 4 (CS4; n = 4; ♀ = 3, ♂ = 1).
Agitometer category had a tendency (p = 0.087) to influence TREC (Figure 4). Interestingly, cattle classified as intermediate temperaments within the AGCAT (AG scores, 45 ≤ 82) were inclined to have higher TREC than cattle with lower (≤ 33) and higher (≥ 86) AG scores (Figure 4).
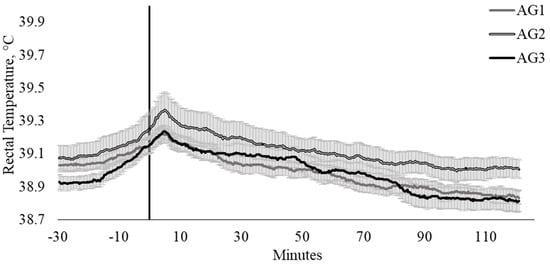
Figure 4.
Twenty-seconds mean (±SEM) rectal temperature (TREC, °C) of cattle with agitometer scores ≤ 33 (AG1; n = 16; ♀ = 6, ♂ = 10); 45 ≤ 82 (AG2; n = 15; ♀ = 10, ♂ = 5); and ≥ 86 (AG3; n = 14; ♀ = 6, ♂ = 8).
Similarly, FSCAT had a tendency to influence TREC (p = 0.080) in these cattle (Figure 5). Cattle within FSCAT 3 (FS ≥ 2.6 m/s) generally had higher TREC when compared with FSCAT 1 (FS ≤ 1.6 m/s) and 2 (FS 1.7 ≥ 2.5 m/s). The influence of FS on TREC appears to exist between FSCAT 1 and 2 in comparison to FSCAT 3, where cattle that were within FSCAT 1 and 2 exhibited a 0.22 and 0.24 °C increase in TREC, whilst cattle within category 3 had a TREC increase of 0.36 °C.
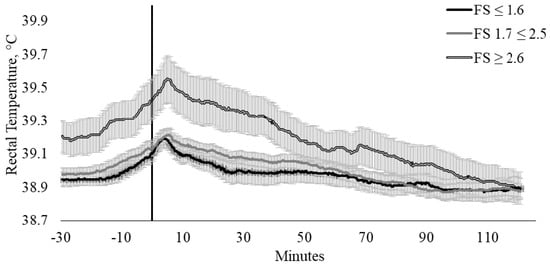
Figure 5.
Twenty-seconds mean (±SEM) rectal temperature (TREC, °C) of cattle with flight speeds of ≤ 1.6 m/s (FS ≤ 1.6; n = 13; ♀ = 2, ♂ = 11); 1.7 ≤ 2.5 m/s (FS 1.7 ≤ 2.5; n = 23; ♀ = 14, ♂ = 9); and ≥ 2.6 m/s (FS ≥ 2.6 m/s; n = 9; ♀ = 6, ♂ = 3).
Sex did not influence TREC when modeled with AGCAT (p = 0.178), CS (p = 0.306), or FSCAT (p = 0.126). There were no interactions between time × temperament trait, time × sex, or temperament trait × sex within the AGCAT, FSCAT, or CS models (Table 3). However, there were significant time × temperament trait × sex interactions within the CS (p = 0.0003; Figure 6) and FSCAT (p = 0.043; Figure 7) models (Table 4).
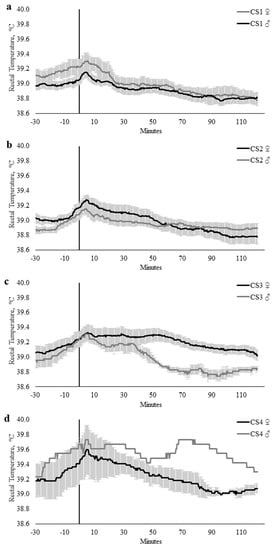
Figure 6.
Twenty-seconds mean (±SEM) rectal temperature (TREC, °C) of heifers (gray line) and steers (black line) with crush scores of (a) 1 (CS1; n = 14; ♀ = 4, ♂ = 10); (b) 2 (CS2; n = 16; ♀ = 7, ♂ = 9); (c) 3 (CS3; n = 11; ♀ = 9, ♂ = 2); and (d) 4 (CS4; n = 4; ♀ = 3, ♂ = 1).
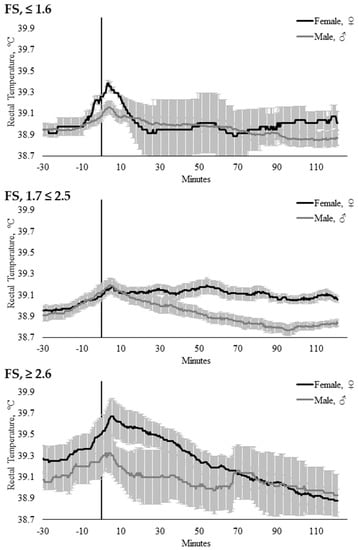
Figure 7.
Twenty-seconds mean (±SEM) rectal temperature (TREC, °C) of heifers (gray line) and steers (black line) with flight speeds of FS ≤ 1.6 (n = 13; ♀ = 2, ♂ = 11); FS 1.7 ≤ 2.5 (n = 23; ♀ = 14, ♂ = 9); and FS ≥ 2.6 (n = 9; ♀ = 6, ♂ = 3).

Table 4.
Interactions (p-values) for agitometer score category (AGCAT), crush score (CS), and flight speed category (FSCAT) models.
4. Discussion
Handling cattle can be considered a stressor eliciting a series of responses to stressful stimuli, including SIH [28,29,30,32,35]. Within the current study, maximum TREC (39.3 ± 0.04 °C) occurred between 4 and 5.7 min after entry into the weighing box (T0), and then exhibited a steady rate of decline until 90.4 min, stabilizing at 38.9 ± 0.03 °C. The handling procedure had a total duration of 2.3 min; thus, TREC peaked 2 to 3.7 min after the conclusion of the handling procedure. These results indicate that the change in TREC from the onset of handling was characterizing SIH in these cattle via the short-term increase in TREC that occurred within minutes of the handling procedure commencing [26,44,45]. This is supported by the findings of Sanger et al. [28], concluding that shearing sheep resulted in SIH between 4 and 14 min post-shearing. Therefore, the results presented here provide further evidence that handling is perceived as a stressful event by cattle, which is associated with the induction of a series of responses, including SIH [28,29,30,32,35].
There have been numerous studies investigating the impact of various stressors on body-temperature responses in cattle [6,7,22,28,30,46,47]; however, studies investigating the influence of temperament on body temperature are limited. In the current study, AG and FS were used to classify cattle into temperament categories within this study, in line with previous studies [12,41]. The AG device registers vibrations from movement and sound, thus differing from the mechanical movement device which has been previously used in cattle [48,49]. Although the use of this evaluation method was novel for cattle, the AG has been successfully used in sheep studies [36,50,51]. Within the current study, there was no relationship between FS and AG. Similarly, Blache and Ferguson [36] reported a limited relationship (r < 0.01) between flight time and AG in sheep. Within the current study, there were weak to moderate relationships between AG and CS. However, it is not clear what aspects of temperament AG may be evaluating, indicating that FS and AG describe different components of temperament [36]. Further studies investigating the use of AG in cattle, and sheep, and its relationship with other measures of temperament, are required, in order to validate its use in cattle.
Within the current study, CS appeared to have the greatest influence on TREC. When evaluating the impact of CS on TREC, there was a clear divergence in TREC responses of cattle across the CS categories. This is particularly evident when comparing the TREC of cattle that were scored within category 1 (calm; n = 14) and category 4 (nervous, n = 4). Similarly, Burdick et al. [7], established that, 30 min following the commencement of transport, temperamental bulls had a greater maximal TREC. However, the magnitude of TREC increase was comparable between temperament classes [7], which were also observed within the current study. Whilst somewhat preliminary in nature, the results from the current study suggest that peak body temperature following handling is higher in cattle that are identified to have more excitable temperaments, even though the magnitude of temperature increase is comparable across temperament classes. Overall, the findings of the current study and those of Burdick et al. [7] suggest that temperament classifications may allow for the early identification of cattle with increases in stress responsiveness, manifesting as elevated SIH. However, further studies with greater cattle numbers across temperament scores are required to definitively establish the relationship that exists between temperament traits and SIH.
Recently, there has been some conjecture regarding what aspects of temperament FS and CS are evaluating [12,30,52,53,54]. MacKay et al. [54] suggested that CS evaluate cattle’s response to handling, whereas FS may provide a measure of fearfulness. Moreover, King et al. [12] suggested that FS and CS were related to the stress responsiveness of cattle. However, a recent study by Lee et al. [30] concluded that pharmacologically induced anxiety did not influence FS or CS in cattle, suggesting that these temperament traits do not measure aspects related to anxiety. As such, FS may be a better methodology to evaluate cattle temperament, as its evaluation is objective, whereas subjective evaluations such as CS may allow for human error or bias to influence temperament evaluations [41]. Moreover, FS can be evaluated without restricting animal movement or inducing fear responses from being confined within a crush [37,39] or from social isolation [55].
Within the current study, heifers had greater CS and FS when compared with the steers, which is consistent with previous studies [16,38,56], although some studies have suggested that FS does not differ between bulls and heifers [39,57]. Regardless, sex did not influence TREC in these cattle, regardless of temperament traits, nor were there time × sex or temperament trait × sex influences (Table 4). Furthermore, within this study, a relationship between FS and CS was not observed, whilst previous studies had identified weak relationships (r ≤ 0.3) between FS and CS [12,18,41,58,59,60]. Therefore, the results presented here provide further evidence that FS and CS are potentially evaluating different aspects of cattle behavior that contribute to temperament [2,18].
In this study, there were strong to moderate associations of TREC between T0 and T10 with FS. Burdick et al. [7] also established a strong relationship between FS and maximum TREC, and FS tended to be correlated with minimum TREC. The current study also established moderate relationships between CS and TREC between T0 and T10. Furthermore, there was a strong to moderate association with T-30 TREC and FS identified within this study. Burdick et al. [7] established that temperamental bulls had higher TREC when compared with bulls that were classified as calm and intermediate temperaments (p < 0.05) prior to transportation. Similarly, Burdick et al. [6] concluded that temperament influenced TREC in Brahman bulls, where bulls classified as temperamental had higher TREC (p < 0.001) prior to a lipopolysaccharide challenge. The relationship between T-30 TREC and FS and CS within this study suggests that these temperament traits may have systemic influence on body temperature regulation, i.e., that cattle with faster FS and higher CS have higher body temperature when not being handled or exposed to stressful stimuli. This may provide a biological rationalization for the elevated TREC in bulls classified as temperamental prior to transport and a lipopolysaccharide challenge as described by Burdick et al. [7] and Burdick et al. [6]. Burrow [13] reported a moderate negative phenotypic correlation (r = −0.24) between flight time and TREC, suggesting that cattle with lower TREC have higher flight time. However, the correlations described by Burrow [13] were collected in accordance with the Iberia heat tolerance test, where TREC is collected after cattle have been exposed to ambient temperatures ≥ 30 °C, without access to shade, for a minimum of 3 h [61]; thus, TREC may have been confounded by heat load [25,61,62]. These findings suggest that further investigations are required to determine the relationship between temperament traits and the regulation of body temperature under conditions classified as non-stressful. This is especially pertinent given that heritability estimates for FS and flight time have been described as moderate to high (≥0.3) [13,16,17,18]. Investigating the influence of temperament traits on body temperature may be of importance in the changing global environment [63], as the results from this study suggest that cattle with more excitable temperaments may have higher basal TREC, which may indicate that these animals may have an increased susceptibility to heat load. However, further studies are required to quantify the relationship between temperament and body temperature, and the influence that this may have on an animals’ susceptibility to heat load.
Interestingly, within the current study, TREC 120 min post-handling was 0.13 °C lower than 30 min prior to the handling procedure. Intra-rectal loggers were inserted the day prior to handling, to negate handling and data logger insertion influences on TREC. However, these cattle were kept in grazing pastures adjacent to the handling facility and required mustering from the paddock prior to the commencement of the handling procedure. Beatty et al. [47] highlighted that transport, shearing and sorting elevated the core body temperature of sheep. Similarly, Mader et al. [22] reported that moving feedlot cattle from home pens to handling facilities (between 150 and 900 m) increased tympanic temperature between 0.3 and 0.8 °C, within 30 min of cattle movement. Therefore, it is likely that basal TREC described within this study does not provide a true estimate of basal TREC, as the TREC presented here may be confounded by relocation and handling events prior to the commencement of the handling procedure. Thus, it is anticipated that this accounts for the elevated TREC prior to the commencement of the handling procedure. Additionally, it is important to consider that body temperature exhibits a circadian rhythm [64,65,66], which may influence the magnitude of the increase in body temperature within SIH studies. Future studies investigating SIH should consider evaluating the underlying circadian rhythm of body temperature prior to exposure to stressful stimuli. Furthermore, given that body temperature is highly regulated [21], understanding the circadian rhythm would allow for SIH to be evaluated by expressing the change in body temperature from a quantified underlying rhythmic temperature. This may provide a more concise estimate of the impact of stressors on body temperature and the time required for body temperature to return to a baseline temperature after exposure to stressors. Quantifying the influence of stressors may provide a more consistent estimate of the impact of stressors on body temperature, thus providing a more reliable physiological evaluation of the impact of stressors on cattle. Furthermore, evaluating temperament is an important consideration for all facets of cattle production, as cattle with excitable/poor/reactive temperaments have been associated with decreased average daily gain [1,2], greater hypothalamic–pituitary–adrenal axis response to handling [9], and reduced carcass quality [2]. Thus, cattle that are considered to have temperamental or excitable temperaments may have reduced welfare regardless of the management procedure being conducted.
5. Conclusions
The variations in TREC observed within this study suggest that the standardized handling procedure elicited a temperature response that was characteristic of SIH associated with psychological stress. Basal TREC differed between temperament classes within FS and CS traits, suggesting that there is an underlying influence of temperament traits on body temperature. Cattle with excitable temperaments, as evaluated by FS and CS, had higher TREC following handling; however, the magnitude of the temperature increase was comparable across temperament classes. Further studies are required to definitively quantify the relationship between temperament traits, body temperature, and SIH. Additionally, these results suggest that the impact of relocation, handling and temperament need to be considered when evaluating body temperature in cattle. Overall, these results further highlight the importance of selecting animals for calm temperaments, as cattle with excitable temperaments may exhibit a greater stress response during routine handling procedures.
Author Contributions
C.L., I.G.C., H.E.S., and A.M.L. contributed to the conceptualization and experimental design of the study; A.M.L. and H.E.S. conducted the study; A.M.L. analyzed the data, with support from I.G.C.; A.M.L. wrote the manuscript; I.G.C., H.E.S., and C.L. contributed to manuscript revisions prior to submission. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by funding from Meat & Livestock Australia Ltd. (North Sydney, NSW, Australia) and the Commonwealth Scientific and Industrial Research Organisation (CSIRO, Canberra, ACT, Australia), through the Animal Welfare Strategic Partnership Program.
Acknowledgments
The authors would like to acknowledge Jim Lea, Dom Niemeyer, and Troy Kalinowski for their assistance with cattle handling and care throughout the duration of this study. The authors extend their gratitude to Laurie Piper and Allan Lisle for their advice and guidance regarding statistical analysis. The authors also wish to acknowledge Sonja Dominic and Sabine Schmoelzl for their comments and suggestions on improving this manuscript prior to submission.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Vetters, M.D.D.; Engle, T.E.; Ahola, J.K.; Grandin, T. Comparison of flight speed and exit score as measurements of temperament in beef cattle. J. Anim. Sci. 2013, 91, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Cafe, L.M.; Robinson, D.L.; Ferguson, D.M.; McIntyre, B.L.; Geesink, G.H.; Greenwood, P.L. Cattle temperament: Persistence of assessments and associations with productivity, efficiency, carcass and meat quality traits. J. Anim. Sci. 2011, 89, 1452–1465. [Google Scholar] [CrossRef]
- Voisinet, B.D.; Grandin, T.; O’Connor, S.F.; Tatum, J.D.; Deesing, M.J. Bos indicus-cross feedlot cattle with excitable temperaments have tougher meat and a higher incidence of borderline dark cutters. Meat Sci. 1997, 46, 367–377. [Google Scholar] [CrossRef]
- Fell, L.R.; Colditz, I.G.; Walker, K.H.; Watson, D.L. Associations between temperament, performance and immune function in cattle entering a commercial feedlot. Aust. J. Exp. Agric. 1999, 39, 795–802. [Google Scholar] [CrossRef]
- Hine, B.C.; Ingham, A.B.; Dominik, S.; Colditz, I.G. Ian Colditz—Mentor for Postdoctoral Fellow. MLA Final Report B. STU. 0244; Meat and Livestock Australia: Sydney, Australia, 2016. [Google Scholar]
- Burdick, N.C.; Carroll, J.A.; Hulbert, L.E.; Dailey, J.W.; Ballou, M.A.; Randel, R.D.; Willard, S.T.; Vann, R.C.; Welsh, T.H. Temperament influences endotoxin-induced changes in rectal temperature, sickness behavior, and plasma epinephrine concentrations in bulls. Innate Immun. 2011, 17, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Burdick, N.C.; Carroll, J.A.; Hulbert, L.E.; Dailey, J.W.; Willard, S.T.; Vann, R.C.; Welsh, T.H.; Randel, R.D. Relationships between temperament and transportation with rectal temperature and serum concentrations of cortisol and epinephrine in bulls. Livest. Sci. 2010, 129, 166–172. [Google Scholar] [CrossRef]
- Curley, K.O.; Neuendorff, D.A.; Lewis, A.W.; Cleere, J.J.; Welsh, T.H.; Randel, R.D. Functional characteristics of the bovine hypothalamic–pituitary–adrenal axis vary with temperament. Horm. Behav. 2008, 53, 20–27. [Google Scholar] [CrossRef]
- Cafe, L.M.; Robinson, D.L.; Ferguson, D.M.; Geesink, G.H.; Greenwood, P.L. Temperament and hypothalamic-pituitary-adrenal axis function are related and combine to affect growth, efficiency, carcass, and meat quality traits in Brahman steers. Domest. Anim. Endocrinol. 2011, 40, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.F.; Bill, E. Kunkle Interdisciplinary Beef Symposium: Temperament and acclimation to human handling influence growth, health, and reproductive responses in Bos taurus and Bos indicus cattle. J. Anim. Sci. 2014, 92, 5325–5333. [Google Scholar] [CrossRef]
- Collier, R.J.; Renquist, B.J.; Xiao, Y. A 100-Year Review: Stress physiology including heat stress. J. Dairy Sci. 2017, 100, 10367–10380. [Google Scholar] [CrossRef] [PubMed]
- King, D.A.; Schuehle Pfeiffer, C.E.; Randel, R.D.; Welsh, T.H.; Oliphint, R.A.; Baird, B.E.; Curley, K.O.; Vann, R.C.; Hale, D.S.; Savell, J.W. Influence of animal temperament and stress responsiveness on the carcass quality and beef tenderness of feedlot cattle. Meat Sci. 2006, 74, 546–556. [Google Scholar] [CrossRef]
- Burrow, H.M. Variances and covariances between productive and adaptive traits and temperament in a composite breed of tropical beef cattle. Livest. Prod. Sci. 2001, 70, 213–233. [Google Scholar] [CrossRef]
- Porto-Neto, L.R.; Reverter, A.; Prayaga, K.C.; Chan, E.K.F.; Johnston, D.J.; Hawken, R.J.; Fordyce, G.; Garcia, J.F.; Sonstegard, T.S.; Bolormaa, S.; et al. The Genetic Architecture of Climatic Adaptation of Tropical Cattle. PLoS ONE 2014, 9, e113284. [Google Scholar] [CrossRef] [PubMed]
- Haskell, M.J.; Simm, G.; Turner, S.P. Genetic selection for temperament traits in dairy and beef cattle. Front. Genet. 2014, 5, 368. [Google Scholar] [CrossRef] [PubMed]
- Prayaga, K.C.; Henshall, J.M. Adaptability in tropical beef cattle: Genetic parameters of growth, adaptive and temperament traits in a crossbred population. Aust. J. Exp. Agric. 2005, 45, 971–983. [Google Scholar] [CrossRef]
- Kadel, M.J.; Johnston, D.J.; Burrow, H.M.; Graser, H.-U.; Ferguson, D.M. Genetics of flight time and other measures of temperament and their value as selection criteria for improving meat quality traits in tropically adapted breeds of beef cattle. Aust. J. Agric. Res. 2006, 57, 1029–1035. [Google Scholar] [CrossRef]
- Burrow, H.M.; Corbet, N.J. Genetic and environmental factors affecting temperament of zebu and zebu-derived beef cattle grazed at pasture in the tropics. Aust. J. Agric. Res. 2000, 51, 155–162. [Google Scholar] [CrossRef]
- Dikmen, S.; Cole, J.B.; Null, D.J.; Hansen, P.J. Heritability of rectal temperature and genetic correlations with production and reproduction traits in dairy cattle. J. Dairy Sci. 2012, 95, 3401–3405. [Google Scholar] [CrossRef]
- Turner, H.G. Variation in rectal temperature of cattle in a tropical environment and its relation to growth rate. Anim. Sci. 1984, 38, 417–427. [Google Scholar] [CrossRef]
- Robertshaw, D. Heat Loss of Cattle. In Stress Physiology in Livestock; Yousef, M.K., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1985; Volume I, pp. 55–66. [Google Scholar]
- Mader, T.L.; Davis, M.S.; Kreikemeier, W.M. Case Study: Tympanic Temperature and Behavior Associated with Moving Feedlot Cattle. Prof. Anim. Sci. 2005, 21, 339–344. [Google Scholar] [CrossRef]
- Czerkawski, J.W. A novel estimate of the magnitude of heat produced in the rumen. Br. J. Nutr. 1980, 42, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Rose-Dye, T.K.; Burciaga-Robles, L.O.; Krehbiel, C.R.; Step, D.L.; Fulton, R.W.; Confer, A.W.; Richards, C.J. Rumen temperature change monitored with remote rumen temperature boluses after challenges with bovine viral diarrhea virus and Mannheimia haemolytica. J. Anim. Sci. 2011, 89, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Mader, T.L.; Gaughan, J.B.; Johnson, L.J.; Hahn, G.L. Tympanic temperature in confined beef cattle exposed to excessive heat load. Int. J. Biometeorol. 2010, 54, 629–635. [Google Scholar] [CrossRef]
- Bouwknecht, J.A.; Olivier, B.; Paylor, R.E. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: A review of pharmacological and genetic studies in the mouse. Neurosci. Biobehav. Rev. 2007, 31, 41–59. [Google Scholar] [CrossRef]
- Oka, T.; Oka, K. Mechanisms of psychogenic fever. Adv. Neuroimmune Biol. 2012, 3, 3–17. [Google Scholar] [CrossRef]
- Sanger, M.E.; Doyle, R.E.; Hinch, G.N.; Lee, C. Sheep exhibit a positive judgement bias and stress-induced hyperthermia following shearing. Appl. Anim. Behav. Sci. 2011, 131, 94–103. [Google Scholar] [CrossRef]
- Pedernera-Romano, C.; De La Torre, J.R.; Badiella, L.; Manteca, X. Associations between open-field behaviour and stress-induced hyperthermia in two breeds of sheep. Anim. Welf. 2011, 20, 339–346. [Google Scholar]
- Lee, C.; Cafe, L.M.; Robinson, S.L.; Doyle, R.E.; Lea, J.M.; Small, A.H.; Colditz, I.G. Anxiety influences attention bias but not flight speed and crush score in beef cattle. Appl. Anim. Behav. Sci. 2018, 205, 210–215. [Google Scholar] [CrossRef]
- Boivin, X.; Lensink, J.; Tallet, C.; Veissier, I. Stockmanship and farm animal welfare. Anim. Welf. 2003, 12, 479–492. [Google Scholar]
- Grandin, T. Assessment of stress during handling and transport. J. Anim. Sci. 1997, 75, 249–257. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Australian Code for the Care and Use of Animals for Scientific Purposes, 8th ed.; National Health and Medical Research Council: Canberra, ACT, Australia, 2013.
- Lea, J.M.; Niemeyer, D.D.O.; Reed, M.T.; Fisher, A.D.; Ferguson, D.M. Development and validation of a simple technique for logging body temperature in free-ranging cattle. Aust. J. Exp. Agric. 2008, 48, 741–745. [Google Scholar] [CrossRef]
- Lee, C.; Fisher, A.D.; Reed, M.T.; Henshall, J.M. The effect of low energy electric shock on cortisol, β-endorphin, heart rate and behaviour of cattle. Appl. Anim. Behav. Sci. 2008, 113, 32–42. [Google Scholar] [CrossRef]
- Blache, D.; Ferguson, D. Increasing Sheep Meat Production Efficiency and Animal Welfare by Selection for Temperament; MLA Final Report SHGEN.025; Meat and Livestock Australia Sydney: Sydney, Australia, 2005. [Google Scholar]
- Grandin, T. Behavioral agitation during handling of cattle is persistent over time. Appl. Anim. Behav. Sci. 1993, 36, 1–9. [Google Scholar] [CrossRef]
- Voisinet, B.D.; Grandin, T.; Tatum, J.D.; O’Connor, S.F.; Struthers, J.J. Feedlot cattle with calm temperaments have higher average daily gains than cattle with excitable temperaments. J. Anim. Sci. 1997, 75, 892–896. [Google Scholar] [CrossRef]
- Burdick, N.C.; Agado, B.; White, J.C.; Matheney, K.J.; Neuendorff, D.A.; Riley, D.G.; Vann, R.C.; Welsh, J.T.H.; Randel, R.D. Technical note: Evolution of exit velocity in suckling Brahman calves. J. Anim. Sci. 2011, 89, 233–236. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 18 January 2020).
- Curley, K.O.; Paschal, J.C.; Welsh, T.H.; Randel, R.D. Technical note: Exit velocity as a measure of cattle temperament is repeatable and associated with serum concentration of cortisol in Brahman bulls. J. Anim. Sci. 2006, 84, 3100–3103. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. nlme: Linear and Non-Linear Mixed-Effects Models. 2019. Available online: https://CRAN.R-project.org/package=nlme (accessed on 18 January 2020).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: London, UK, 2013. [Google Scholar]
- Spooren, W.P.J.M.; Schoeffter, P.; Gasparini, F.; Kuhn, R.; Gentsch, C. Pharmacological and endocrinological characterisation of stress-induced hyperthermia in singly housed mice using classical and candidate anxiolytics (LY314582, MPEP and NKP608). Eur. J. Pharmacol. 2002, 435, 161–170. [Google Scholar] [CrossRef]
- Vinkers, C.H.; Groenink, L.; van Bogaert, M.J.V.; Westphal, K.G.C.; Kalkman, C.J.; van Oorschot, R.; Oosting, R.S.; Olivier, B.; Korte, S.M. Stress-induced hyperthermia and infection-induced fever: Two of a kind? Physiol. Behav. 2009, 98, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Burdick, N.C.; Randel, R.D.; Carroll, J.A.; Welsh, T.H. Interactions between Temperament, Stress, and Immune Function in Cattle. Int. J. Zool. 2011, 2011, 373197. [Google Scholar] [CrossRef]
- Beatty, D.T.; Barnes, A.; Fleming, P.A.; Taylor, E.; Maloney, S.K. The effect of fleece on core and rumen temperature in sheep. J. Therm. Biol. 2008, 33, 437–443. [Google Scholar] [CrossRef]
- Sebastian, T.; Watts, J.; Stookey, J.; Buchanan, F.; Waldner, C. Temperament in beef cattle: Methods of measurement and their relationship to production. Can. J. Anim. Sci. 2011, 91, 557–565. [Google Scholar] [CrossRef]
- Stookey, J.; Nickel, T.; Hanson, J.; Vandenbosch, S. A movement-measuring-device for objectively measuring temperament in beef cattle and for use in determining factors that influence handling. J. Anim. Sci. 1994, 72, 207. [Google Scholar]
- Plush, K.J.; Hebart, M.L.; Brien, F.D.; Hynd, P.I. The genetics of temperament in Merino sheep and relationships with lamb survival. Appl. Anim. Behav. Sci. 2011, 134, 130–135. [Google Scholar] [CrossRef]
- Doughty, A.K.; Horton, B.J.; Huyen, N.T.D.; Ballagh, C.R.; Corkrey, R.; Hinch, G.N. The influence of lameness and individuality on movement patterns in sheep. Behav. Process. 2018, 151, 34–38. [Google Scholar] [CrossRef]
- Parham, J.T.; Tanner, A.E.; Barkley, K.; Pullen, L.; Wahlberg, M.L.; Swecker, W.S.; Lewis, R.M. Temperamental cattle acclimate more substantially to repeated handling. Appl. Anim. Behav. Sci. 2019, 212, 36–43. [Google Scholar] [CrossRef]
- Bruno, K.A.; Vanzant, E.S.; Vanzant, K.A.; McLeod, K.R. Relationships of a novel objective chute score and exit velocity with growth performance of receiving cattle. J. Anim. Sci. 2016, 94, 4819–4831. [Google Scholar] [CrossRef]
- MacKay, J.R.D.; Turner, S.P.; Hyslop, J.; Deag, J.M.; Haskell, M.J. Short-term temperament tests in beef cattle relate to long-term measures of behavior recorded in the home pen. J. Anim. Sci. 2013, 91, 4917–4924. [Google Scholar] [CrossRef]
- Kilgour, R.J.; Melville, G.J.; Greenwood, P.L. Individual differences in the reaction of beef cattle to situations involving social isolation, close proximity of humans, restraint and novelty. Appl. Anim. Behav. Sci. 2006, 99, 21–40. [Google Scholar] [CrossRef]
- Burrow, H. Measurements of temperament and their relationships with performance traits. Anim. Breed. Abstr. 1997, 65, 477–495. [Google Scholar]
- Burdick, N.C.; Banta, J.P.; Neuendorff, D.A.; White, J.C.; Vann, R.C.; Laurenz, J.C.; Welsh, J.T.H.; Randel, R.D. Interrelationships among growth, endocrine, immune, and temperament variables in neonatal Brahman calves. J. Anim. Sci. 2009, 87, 3202–3210. [Google Scholar] [CrossRef]
- Gibbons, J.M.; Lawrence, A.B.; Haskell, M.J. Consistency of flight speed and response to restraint in a crush in dairy cattle. Appl. Anim. Behav. Sci. 2011, 131, 15–20. [Google Scholar] [CrossRef]
- Stockman, C.A.; McGilchrist, P.; Collins, T.; Barnes, A.L.; Miller, D.; Wickham, S.L.; Greenwood, P.L.; Cafe, L.M.; Blache, D.; Wemelsfelder, F.; et al. Qualitative Behavioural Assessment of Angus steers during pre-slaughter handling and relationship with temperament and physiological responses. Appl. Anim. Behav. Sci. 2012, 142, 125–133. [Google Scholar] [CrossRef]
- Turner, S.P.; Navajas, E.A.; Hyslop, J.J.; Ross, D.W.; Richardson, R.I.; Prieto, N.; Bell, M.; Jack, M.C.; Roehe, R. Associations between response to handling and growth and meat quality in frequently handled Bos taurus beef cattle. J. Anim. Sci. 2011, 89, 4239–4248. [Google Scholar] [CrossRef]
- Dowling, D. An experimental study of heat tolerance of cattle. Aust. J. Agric. Res. 1956, 7, 469–481. [Google Scholar] [CrossRef]
- Brown-Brandl, T.M.; Eigenberg, R.A.; Nienaber, J.A.; Hahn, G.L. Dynamic Response Indicators of Heat Stress in Shaded and Non-shaded Feedlot Cattle, Part 1: Analyses of Indicators. Biosyst. Eng. 2005, 90, 451–462. [Google Scholar] [CrossRef]
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.S.; Bernabucci, U. Effects of climate changes on animal production and sustainability of livestock systems. Livest. Sci. 2010, 130, 57–69. [Google Scholar] [CrossRef]
- Bitman, J.; Lefcourt, A.; Wood, D.L.; Stroud, B. Circadian and Ultradian Temperature Rhythms of Lactating Dairy Cows. J. Dairy Sci. 1984, 67, 1014–1023. [Google Scholar] [CrossRef]
- Piccione, G.; Caola, G.; Refinetti, R. Daily and estrous rhythmicity of body temperature in domestic cattle. BMC Physiol. 2003, 3, 7. [Google Scholar] [CrossRef]
- Lefcourt, A.M.; Huntington, J.B.; Akers, R.M.; Wood, D.L.; Bitman, J. Circadian and ultradian rhythms of body temperature and peripheral concentrations of insulin and nitrogen in lactating dairy cows. Domest. Anim. Endocrinol. 1999, 16, 41–55. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).