Myostatin (MSTN) Gene Indel Variation and Its Associations with Body Traits in Shaanbei White Cashmere Goat
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Phenotypic Traits Recording, and Genomic DNA Isolation
2.2. Primer Design, PCR Amplification, and Genotyping
2.3. Statistical Analyses
3. Results
3.1. Identification and Genetic Parameter Analysis of Indel Variation
3.2. Associations between Indel Variations and Growth Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.J.; Ling, Y.H.; Wang, L.J.; Hang, Y.F.; Guo, X.F.; Zhang, Y.H.; Ding, J.P.; Zhang, X.R. Polymorphisms of the myostatin gene (MSTN) and its relationship with growth traits in goat breeds. Genet. Mol. Res. 2013, 12, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Dalvit, C.; De Marchi, M.; Cassandro, M. Genetic traceability of livestock products: A review. Meat Sci. 2007, 77, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.D.; Greenwood, P.L.; Pethick, D.W.; Ferguson, D.M. Genetic and environmental effects on meat quality. Meat Sci. 2010, 86, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.K.; Yang, B.G.; Duan, X.H.; Na, R.S.; Han, Y.G.; Zeng, Y. Association analysis of sixty-seven single nucleotide polymorphisms with litter size in Dazu black goats. Anim. Genet. 2019. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, B.; Bai, F.; Wu, F.; Zhou, Z.; Lai, Z.; Li, S.; Qu, K.; Jia, Y.; Lei, C.; et al. Two novel SNPs in RET gene are associated with cattle body measurement traits. Animals 2019, 9, 836. [Google Scholar] [CrossRef]
- Shi, B.; Jiang, Y.; Chen, Y.; Zhao, Z.; Zhou, H.; Luo, Y.; Hu, J.; Hickford, J.G.H. Variation in the Fatty Acid Synthase Gene (FASN) and Its Association with milk traits in gannan yaks. Aniamls 2019, 9, 613. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.M.; Garrick, D.J. Increasing the accuracy of genomic prediction in pure-bred Limousin beef cattle by including cross-bred Limousin data and accounting for an F94L variant in MSTN. Anim. Genet. 2019. [Google Scholar] [CrossRef]
- E, G.X.; Duan, X.H.; Zhang, J.H.; Huang, Y.F.; Zhao, Y.J.; Na, R.S.; Zhao, Z.Q.; Ma, Y.H.; Chu, M.X.; Basang, W.D.; et al. Genome-wide selection signatures analysis of litter size in Dazu black goats using single-nucleotide polymorphism. 3 Biotech. 2019. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, S.; He, L.; Zhu, H.; Wang, Z.; Yan, H.; Huang, Y.; Dang, R.; Lei, C.; Chen, H.; et al. A 14-bp functional deletion within the CMTM2 gene is significantly associated with litter size in goat. Theriogenology 2019, 139, 49–57. [Google Scholar] [CrossRef]
- La, Y.; Liu, Q.; Zhang, L.; Chu, M. Single nucleotide polymorphisms in SLC5A1, CCNA1, and ABCC1 and the association with litter size in small-tail Han sheep. Animals 2019, 9, 432. [Google Scholar] [CrossRef]
- Zhou, M.; Pan, Z.; Cao, X.; Guo, X.; He, X.; Sun, Q.; Di, R.; Hu, W.; Wang, X.; Zhang, X.; et al. Single nucleotide polymorphisms in the HIRA gene affect litter size in small tail Han sheep. Animals 2018, 8, 71. [Google Scholar] [CrossRef]
- Goshu, H.A.; Wu, X.Y.; Chu, M.; Bao, P.J.; Ding, X.Z.; Yan, P. Copy number variations of KLF6 modulate gene transcription and growth traits in Chinese Datong yak (Bos Grunniens). Animals 2018, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Liu, D.L.; Tang, S.Q.; Tan, X.X.; Han, R.L.; Tian, Y.D.; Li, H.; Li, G.X.; Li, W.T.; Liu, X.J.; et al. A multiallelic indel in the promoter region of the Cyclin-dependent kinase inhibitor 3 gene is significantly associated with body weight and carcass traits in chickens. Poult. Sci. 2019, 98, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Zhou, H.T.; Luo, Y.; Zhao, M.L.; Gong, H.; Hao, Z.Y.; Hu, J.; Hickford, J.G.H. Variation in the caprine KAP24-1 gene affects cashmere fibre diameter. Animals 2019, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.H.; Li, W.Y.; Liu, D.L.; Liang, K.; Wang, X.G.; Li, H.; Jiang, R.R.; Tian, Y.D.; Kang, X.T.; Li, Z.J. Two indels mutation in the promoter region of the QPCTL gene is significantly associated with body weight and carcass traits in chickens. Anim. Genet. 2019, 50, 279–282. [Google Scholar] [CrossRef]
- Ji, S.; Losinski, R.L.; Cornelius, S.G.; Frank, G.R.; Willis, G.M.; Gerrard, D.E.; Depreux, F.F.; Spurlock, M.E. Myostatin expression in porcine tissues: Tissue specificity and developmental and postnatal regulation. Am. Physiol. Soc. 1998, 275, 1265–1273. [Google Scholar] [CrossRef]
- Wolfman, N.M.; McPherron, A.C.; Pappano, W.N.; Davies, M.V.; Song, K.; Tomkinson, K.N.; Wright, J.F.; Zhao, L.; Sebaid, S.M.; Greenspan, D.S.; et al. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc. Natl. Acad. Sci. USA 2003, 100, 15842–15846. [Google Scholar] [CrossRef]
- Beyer, T.A.; Narimatsu, M.; Weiss, A.; David, L.; Wrana, J.L. The TGFβ superfamily in stem cell biology and early mammalian embryonic development. Biochim. Biophys. Acta 2013, 1830, 2268–2279. [Google Scholar] [CrossRef]
- Liu, H.H.; Mao, H.G.; Dong, X.Y.; Cao, H.Y.; Liu, K.; Yin, Z.Z. Expression of MSTN gene and its correlation with pectoralis muscle fiber traits in the domestic pigeons (Columba livia). Poult. Sci. 2019. [Google Scholar] [CrossRef]
- Thomas, M.; Langley, B.; Berry, C.; Sharma, M.; Kirk, S.; Bass, J.; Kambadur, R. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J. Biol. Chem. 2000, 275, 40235–40243. [Google Scholar] [CrossRef]
- Kambadur, R.; Sharma, M.; Smith, T.P.; Bass, J.J. Mutations in myostatin (GDF8) in Double-Muscled Belgian Blue and Piedmontese Cattle. Genome Res. 2015, 9, 910–916. [Google Scholar]
- Kambadur, R.; Sharma, M.; Smith, T.P.; Bass, J.J. Mutations in myostatin (GDF8) in double-musclód Belgian Blue and Piedmontese cattle. Genome Res. 1997, 7, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Grobet, L.; Martin, L.; Jcelet, D.; Pirottin, D.; Brouwers, B.; Riquet, J.; Schoeberlein, A.; Dunner, S.; Ménissier, F.; Massabanda, J.; et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat. Genet. 1997, 17, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Grobet, L.; Poncelet, D.; Royo, L.J.; Brouwers, B.; Pirottin, D.; Michaux, C.; Menissier, F.; Zanotti, M.; Dunner, S.; Georges, M. Molecular definition of an allelic series of mutations disrupting the myostatin function and causing double- muscling in cattle. Mamm. Genome 1998, 9, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Marchitelli, C.; Savarese, M.C.; Crisa, A.; Nardone, A.; Marsan, P.A.; Valentini, A. Double muscling in Marchigiana beef breed is caused by a stop codon in the third exon of myostatin gene. Mamm. Genome 2003, 14, 392–395. [Google Scholar] [CrossRef]
- Grobet, L.; Pirottin, D.; Farnir, F.; Poncelet, D.; Royo, L.J.; Brouwers, B.; Christians, E.; Desmecht, D.; Coignoul, F.; Kahn, R.; et al. Modulating skeletal muscle mass by postnatal, muscle- specific inactivation of the myostatin gene. Genesis 2003, 35, 227–238. [Google Scholar] [CrossRef]
- Tang, L.; Yan, Z.; Wan, Y.; Han, W.; Zhang, Y. Myostatin DNA vaccine increases skeletal muscle mass and endurance in mice. Muscle Nerv. 2007, 36, 342–348. [Google Scholar] [CrossRef]
- Amrutlal, K.; Patel, A.K.; Tripathi, U.A.; Shah, R.K.; Joshi, C.G. Myostatin knockdown and its effect on myogenic gene expression program in stably transfected goat myoblasts. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 587–596. [Google Scholar]
- Guo, X.; Wang, X.; Liang, B.; Di, R.; Liu, Q.; Hu, W.; He, X.; Zhang, J.; Zhang, X.; Chu, M. Differential expression of circular RNAs in polytocous and monotocous uters during the reproductive cycle of sheep. Animals 2018, 8, 160. [Google Scholar] [CrossRef]
- Hemlata, J.; Sanjeev, S.; Megha, K.; Sarkhel, B.C. Knockdown of the myostatin gene by RNA interference in caprine fibroblast cells. J. Biotechnol. 2010, 145, 99–102. [Google Scholar]
- Wang, X.L.; Yu, H.H.; Lei, A.M.; Zhou, J.K.; Zeng, W.X.; Zhu, H.J.; Dong, Z.M.; Niu, Y.Y.; Shi, B.B.; Cai, B.; et al. Generation of gene-modified goats targeting MSTN and FGF5 via zygote injection of CRISPR/Cas9 system. Sci. Rep. 2015, 5, 13878. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niu, Y.; Zhou, J.; Zhu, J.; Ma, B.; Yu, H.; Yan, H.; Hua, J.; Huang, X.; Qu, L.; et al. CRISPR/Cas9-mediated MSTN disruption and heritable mutagenesis in goats causes increased body mass. Anim. Genet. 2017, 49, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.L.; Lu, R.; Yuan, Y.G.; Zhang, T.; Song, S.Z.; Qi, Z.Q.; Shao, B.; Zhu, M.M.; Mi, F.; Cheng, Y. Efficient TALEN-mediated myostatin gene editing in goats. BMC Dev. Biol. 2016, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Yuan, L.; Deng, J.; Chen, M.; Wang, Y.; Zeng, L.Z.; Lai, L. Efficient generation of myostatin gene mutated rabbit by CRISPR/Cas9. Sci. Rep. 2016, 26, 25029. [Google Scholar] [CrossRef] [PubMed]
- Kobolák, J.; Gócza, E. The role of the myostatin protein in meat quality: A review. Arch. Anim. Breed. 2002, 45, 159–170. [Google Scholar] [CrossRef]
- Aiello, D.; Patel, K.; Lasagna, G. The myostatin gene: An overview of mechanisms of action and its relevance to livestock animals. Anim. Genet. 2018, 49, 505–519. [Google Scholar] [CrossRef]
- Khani, K.; Abdolmohammadi, A.; Foroutanifar, S.; Zebarjadi, A. Assessment of polymorphisms in mysostatin gene and their allele substitution effects showed weak association with growth traits in Iranian Markhoz goats. J. Agric. Sci. 2017, 155, 519–526. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumar, R.; Kumar, S.; Mitra, A. Characterization of 5’ upsteam region and investigation of TTTTA deletion in 5’UTR of myostatin (MSTN) gene in Indian goat breeds. Anim. Biotechnol. 2014, 25, 55–68. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Liu, Y.; Xu, D.Q.; Wen, Q.Y.; Li, X.; Zhang, W.M.; Yang, L.G. Polymorphisms of myostatin gene (MSTN) in four goat breeds and their effects on Boer goat growth performance. Mol. Biol. Rep. 2012, 39, 3081–3087. [Google Scholar] [CrossRef]
- Li, X.L.; Liu, Z.Z.; Zhou, R.Y.; Zheng, G.R.; Gong, Y.F.; Li, L.H. Deletion of TTTTA in 5’UTR of goat MSTN gene and its distribution in different population groups and genetic effect on body weight at different ages. Front. Agric. China 2008, 2, 103–109. [Google Scholar] [CrossRef]
- Liu, A.Y. Progress in the production performance of Shaanbei white Cashmere goat. China Herbiv. Sci. 2018, 27, 449–455. [Google Scholar]
- Wu, X.; Zhou, X.; Ding, X.; Chu, M.; Liang, C.; Pei, J.; Xiong, L.; Bao, P.; Guo, X.; Yan, P. The selection of reference genes for quantitative real-time PCR in the ashidan yak mammary gland during lactation and dry period. Animals 2019, 9, 943. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Du, X.; Li, Q.; Pan, Z.; Wu, W.; Liu, H.; Li, Q. Variants in BMP7 and BMP15 3’-UTRs associated with reproductive traits in a large white pig population. Animals 2019, 9, 905. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhang, J.; Li, W.; Wang, W.; Li, F.; Yue, X. Association of Polymorphisms in candidate genes with the litter size in two sheep breeds. Animals. 2019, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Aljanabi, S.M.; Martinez, I. Universal and rapid salt-extraction of high-quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, Y.; Bai, Y.; Yang, H.; Yan, H.; Liu, J.; Shi, L.; Song, X.; Li, L.; Dong, S.; et al. Relationship between SNPs of POU1F1 gene and litter size and growth traits in Shaanbei white cashmere goats. Animals 2019, 9, 114. [Google Scholar] [CrossRef]
- Chen, M.; Yan, H.; Wang, K.; Cui, Y.; Chen, R.; Liu, W.; Zhu, H.; Qu, L.; Pan, C. Goat SPEF2: Expression profile, indel variants identification and association analysis with litter size. Theriogenology 2019, 139, 147–155. [Google Scholar] [CrossRef]
- Nei, M.; Roychoudhury, A.K. Sampling variances of heterozygosity and genetic distance. Genetics 1974, 76, 379–390. [Google Scholar]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Wang, Z.; Zhang, X.L.; Jiang, E.H.; Yan, H.L.; Zhu, H.J.; Chen, H.; Liu, J.W.; Qu, L.; Pan, C.Y.; Lan, X.Y. InDels within caprine IGF2BP1 intron 2 and the 3’-untranslated regions are associated with goat growth traits. Anim. Genet. 2019. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lee, S.J. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA 1997, 94, 12457–12461. [Google Scholar] [CrossRef]
- Miranda, M.E.; Aigues, M.Y.; Boscher, M.Y.; Menissier, F.; Cortes, O.; Nunner, S. Simultaneous genotyping to detect myostatin gene polymorphism in beef cattle breeds. J. Anim. Breed. Genet. 2002, 119, 361–366. [Google Scholar] [CrossRef]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeya, A.S.; Glass, D.J. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 2009, 296, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Hifzan, R.M.; Idris, I.; Yaakub, H. Growth pattern for body weight, height at withers and body length of kalahari red goats. Pak. J. Pharm. 2015, 18, 200–203. [Google Scholar] [CrossRef] [PubMed]

| Sample Sizes | Frequencies | Homo | Hetero | Ne | PIC | HWE p-Value | |
|---|---|---|---|---|---|---|---|
| Genotypes | Alleles | ||||||
| N = 1074 | DD 0.890 | D 0.943 | 0.896 | 0.104 | 1.116 | 0.098 | 0.055 |
| ID 0.110 | I 0.057 | ||||||
| II 0.000 | |||||||
| Growth Traits | Observed Genotypes (LSM a ± SE) | p-Values | |
|---|---|---|---|
| DD | ID | ||
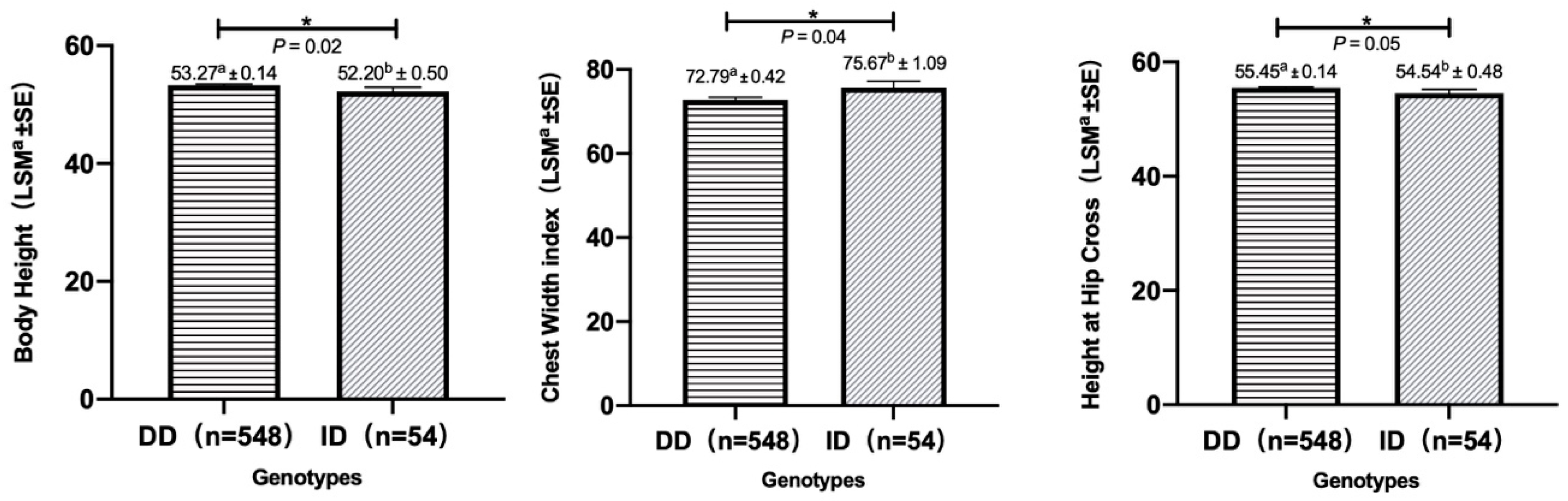
| Body Height (cm) | 53.27 a ± 0.14 (n = 548) | 52.20 b ± 0.50 (n = 54) | 0.02 |
| Height at Hip Cross (cm) | 55.45 a ± 0.14 (n = 548) | 54.54 b ± 0.48 (n = 54) | 0.05 |
| Body Length (cm) | 61.70 ± 0.20 (n = 548) | 60.73 ± 0.48 (n = 54) | 0.13 |
| Hip Width (cm) | 12.85 ± 0.07 (n = 548) | 12.96 ± 0.30 (n = 54) | 0.72 |
| Chest Width (cm) | 18.48 ± 0.11 (n = 548) | 19.10 ± 0.36 (n = 54) | 0.09 |
| Cannon bone Circumference (cm) | 7.38 ± 0.03 (n = 548) | 7.32 ± 0.10 (n = 54) | 0.47 |
| Chest Depth (cm) | 25.44 ± 0.11 (n = 548) | 25.25 ± 0.31 (n = 54) | 0.54 |
| Body Weight (kg) | 36.22 ± 0.93 (n = 73) | 35.70 ± 2.36 (n = 11) | 0.84 |
| Heart Girth (cm) | 72.11 ± 0.31 (n = 548) | 72.30 ± 0.94 (n = 54) | 0.92 |
| Body Trunk index | 118.36 ± 1.77 (n = 548) | 119.07 ± 1.29 (n = 54) | 0.90 |
| Body Length index | 115.98 ± 0.47 (n = 548) | 116.89 ± 1.42 (n = 54) | 0.57 |
| Heart Girth index | 135.67 ± 0.69 (n = 548) | 139.27 ± 2.41 (n = 54) | 0.12 |
| Cannon bone Circumference index | 13.89 ± 0.07 (n = 548) | 14.08 ± 0.22 (n = 54) | 0.43 |
| Chest Width index | 72.79 a ± 0.42 (n = 548) | 75.67 b ± 1.09 (n = 54) | 0.04 |
| Hip Width index | 147.57 ± 2.97 (n = 548) | 149.50 ± 2.89 (n = 54) | 0.84 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, Y.; Feng, B.; Wang, Z.; Zhu, H.; Qu, L.; Lan, X.; Pan, C.; Song, X. Myostatin (MSTN) Gene Indel Variation and Its Associations with Body Traits in Shaanbei White Cashmere Goat. Animals 2020, 10, 168. https://doi.org/10.3390/ani10010168
Bi Y, Feng B, Wang Z, Zhu H, Qu L, Lan X, Pan C, Song X. Myostatin (MSTN) Gene Indel Variation and Its Associations with Body Traits in Shaanbei White Cashmere Goat. Animals. 2020; 10(1):168. https://doi.org/10.3390/ani10010168
Chicago/Turabian StyleBi, Yi, Bo Feng, Zhen Wang, Haijing Zhu, Lei Qu, Xianyong Lan, Chuanying Pan, and Xiaoyue Song. 2020. "Myostatin (MSTN) Gene Indel Variation and Its Associations with Body Traits in Shaanbei White Cashmere Goat" Animals 10, no. 1: 168. https://doi.org/10.3390/ani10010168
APA StyleBi, Y., Feng, B., Wang, Z., Zhu, H., Qu, L., Lan, X., Pan, C., & Song, X. (2020). Myostatin (MSTN) Gene Indel Variation and Its Associations with Body Traits in Shaanbei White Cashmere Goat. Animals, 10(1), 168. https://doi.org/10.3390/ani10010168




