Development and Potential Application of Ras Domain Containing Protein from Haemonchus contortus for Diagnosis of Goat Infection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Expression of Recombinant Proteins
2.2. Animals and Parasites
2.3. Serum Sample
2.4. Immunoblot Analysis of Recombinant Antigens
2.5. Indirect ELISA Procedure
2.6. Estimation of Cut-off Value, Sensitivity, and Specificity
2.7. Measurement of Repeatability
2.8. Field Evaluation of the Indirect ELISA
3. Results
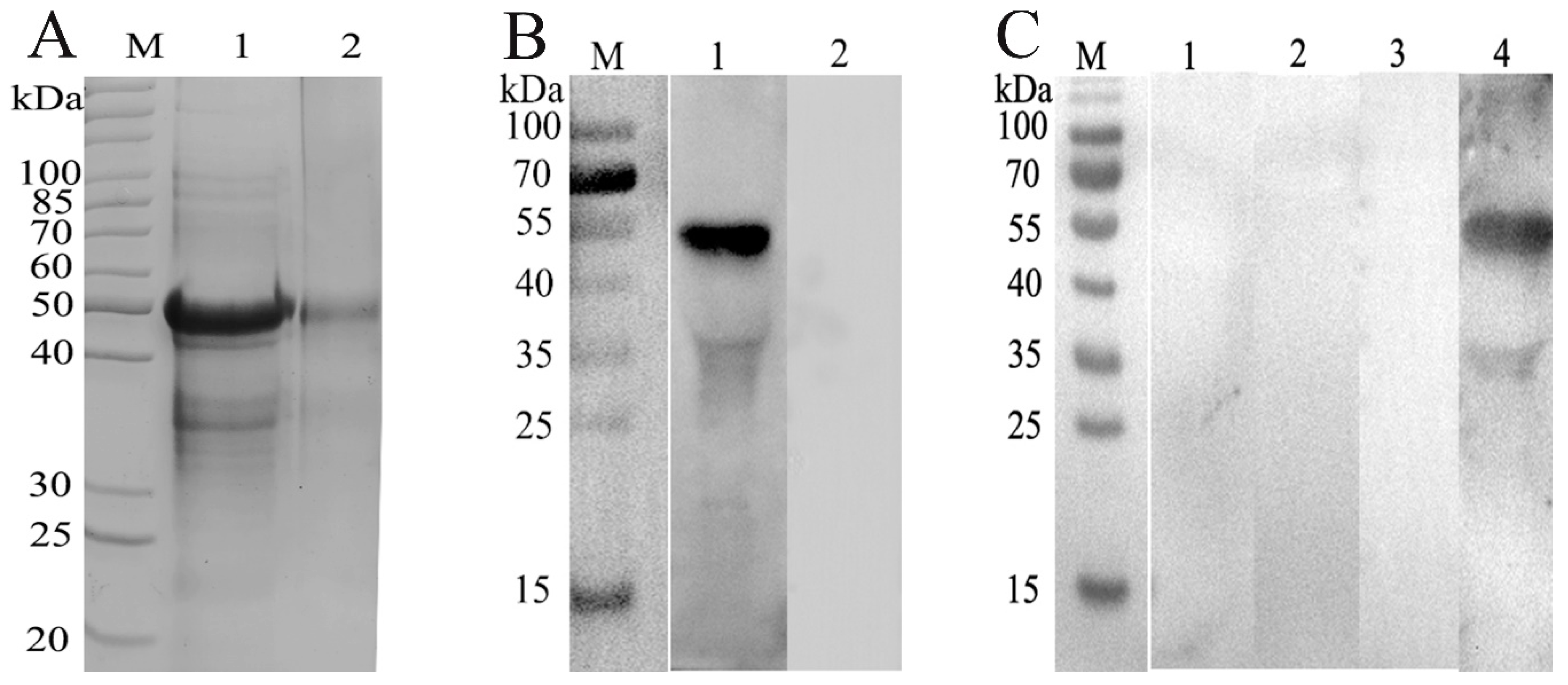
3.1. Expression, Purification, and Western Blot Analysis of rHcRas
3.2. Serodiagnostic Potential of rHcRas
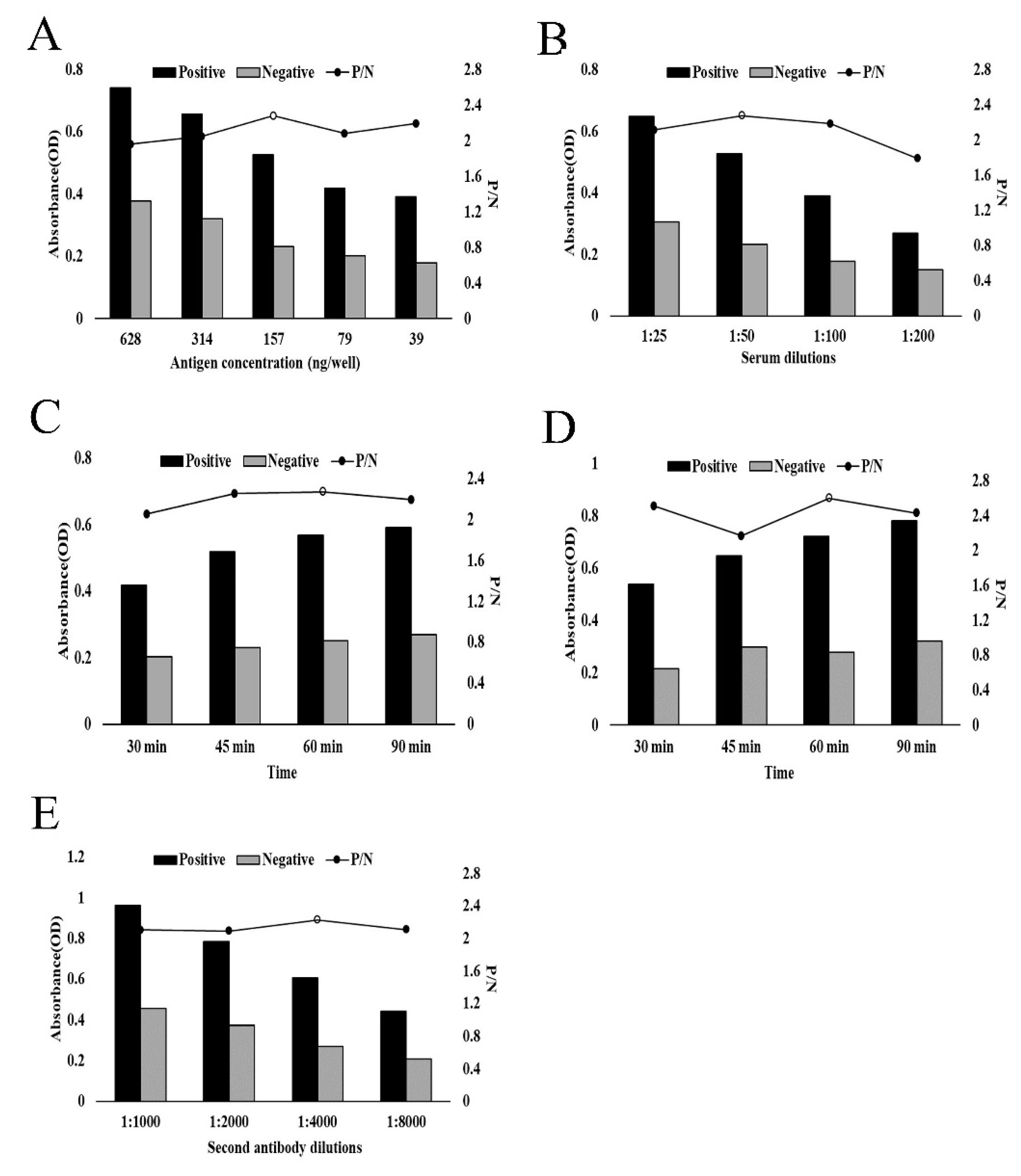
3.3. Development of Indirect ELISA
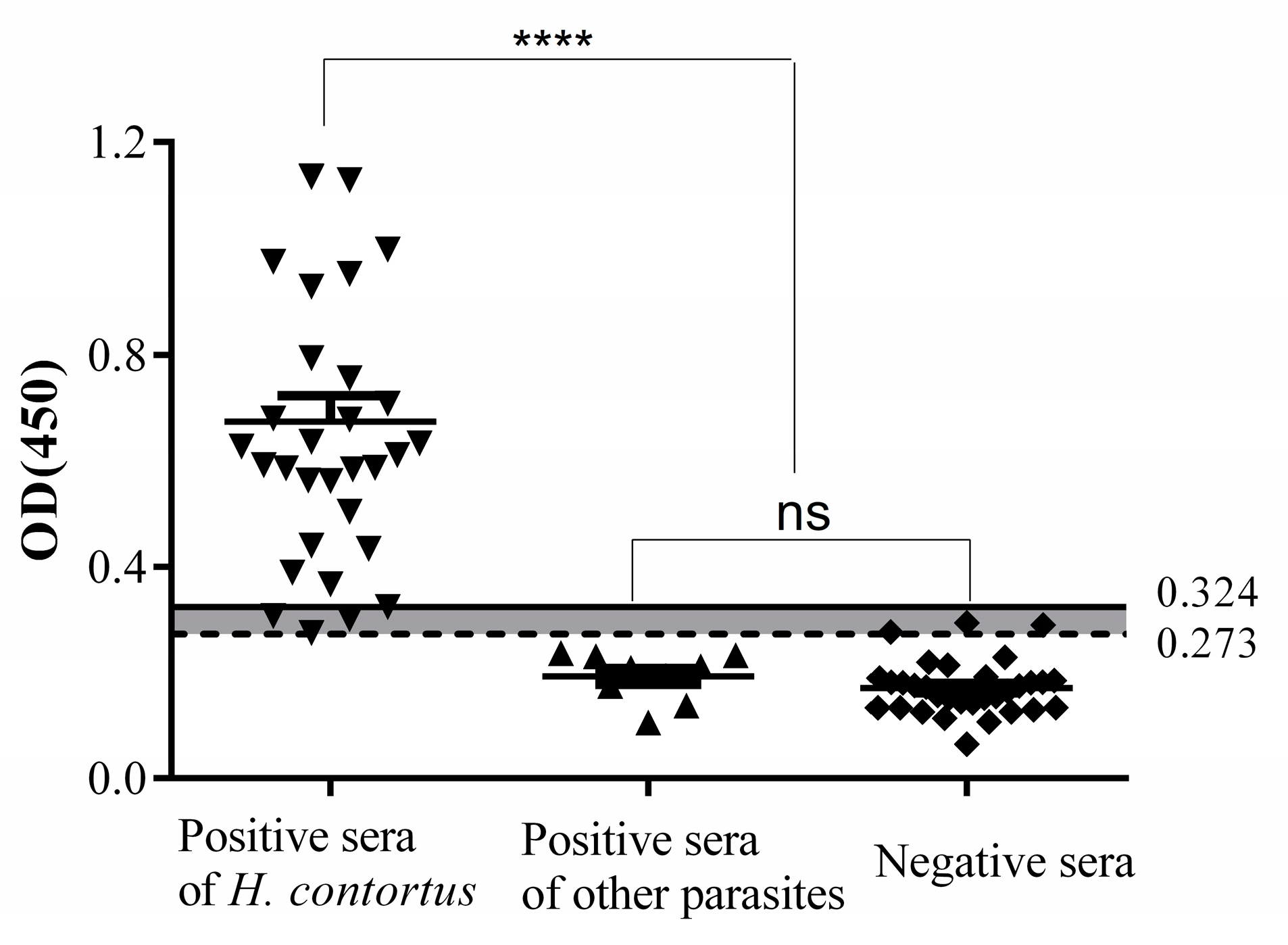
3.4. Cut-Off Value of Indirect ELISA
3.5. Determination of Repeatability
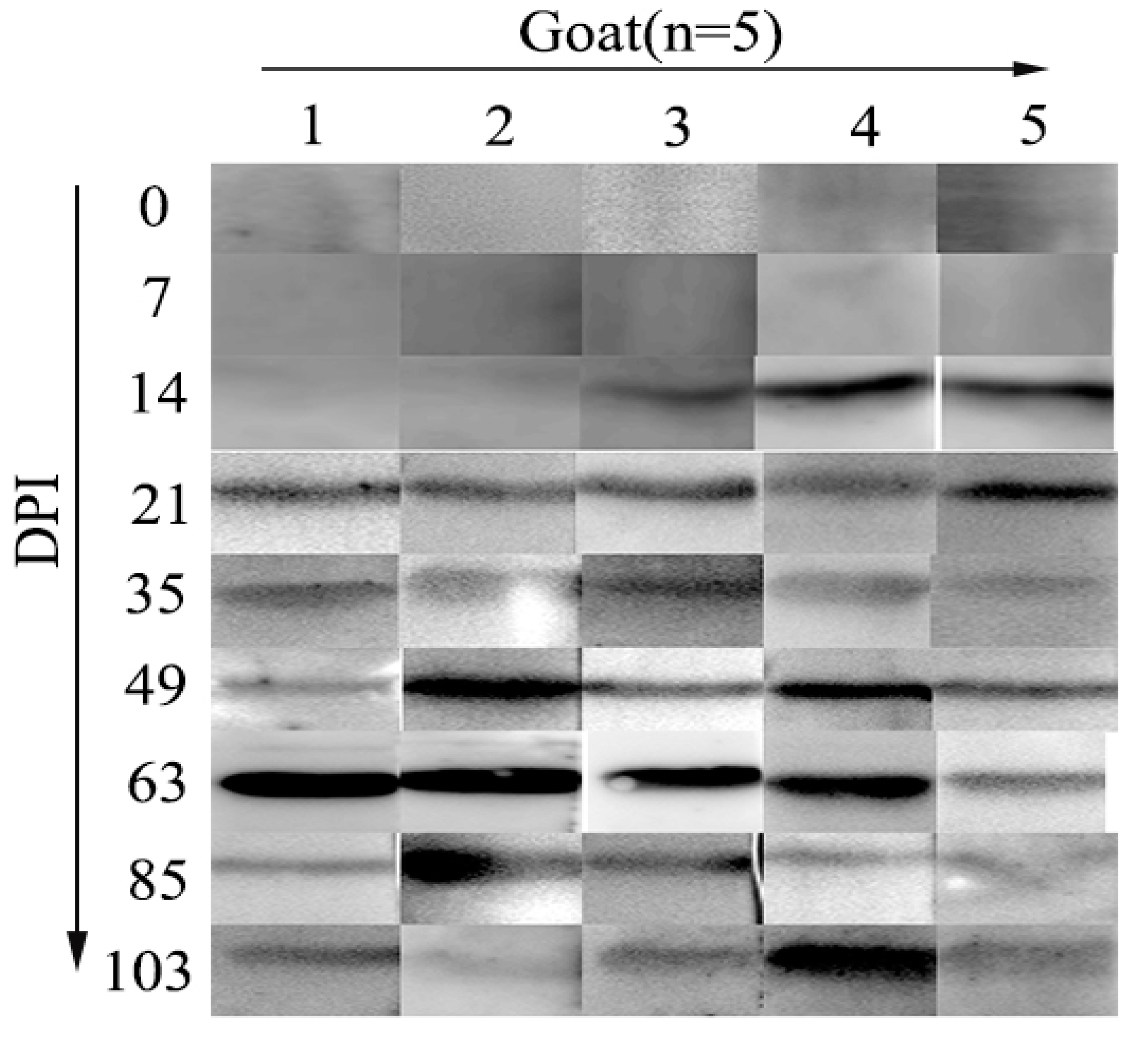
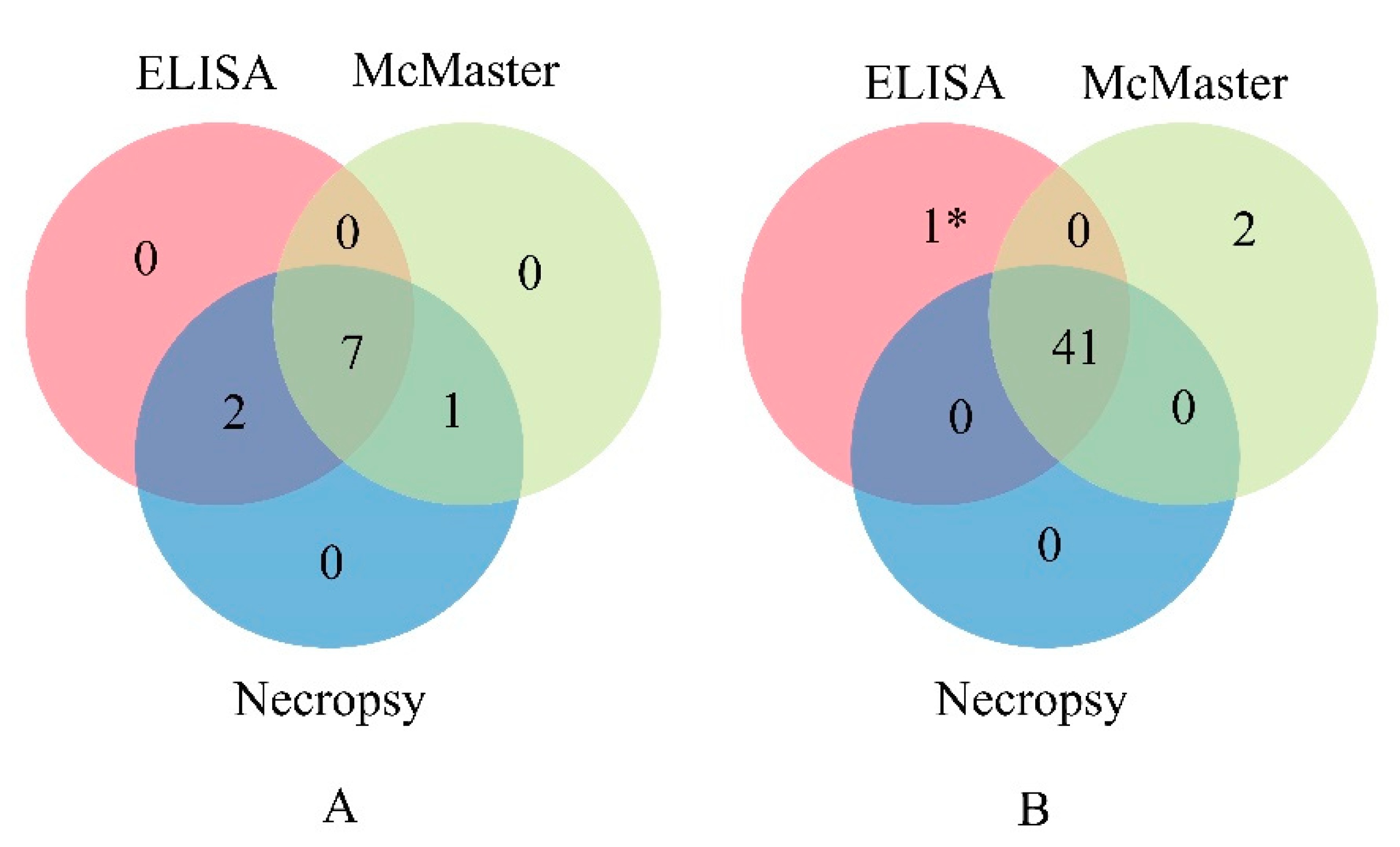
3.6. Diagnosis of H. Contortus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Besier, R.B.; Kahn, L.P.; Sargison, N.D.; Van Wyk, J.A. Diagnosis, Treatment and Management of Haemonchus Contortus in Small Ruminants. Adv. Parasitol. 2016, 93, 181–238. [Google Scholar] [PubMed]
- Cabardo, D.E., Jr.; Portugaliza, H.P. Anthelmintic Activity of Moringa Oleifera Seed Aqueous and Ethanolic Extracts against Haemonchus Contortus Eggs and Third Stage Larvae. Int. J. Vet. Sci Med. 2017, 5, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Kandil, O.M.; Hendawy, S.H.M.; Namaky, A.H.; Gabrashanska, M.P.; Nanev, V.N. Evaluation of Different Haemonchus Contortus Antigens for Diagnosis of Sheep Haemonchosis by Elisa and Their Cross Reactivity with Other Helminthes. J. Parasit. Dis. 2017, 41, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Ljungstrom, S.; Melville, L.; Skuce, P.J.; Hoglund, J. Comparison of Four Diagnostic Methods for Detection and Relative Quantification of Haemonchus Contortus Eggs in Feces Samples. Front. Vet. Sci. 2018, 4, 239. [Google Scholar] [CrossRef]
- Yang, X.; Qi, M.W.; Zhang, Z.Z.; Gao, C.; Wang, C.Q.; Lei, W.Q.; Tan, L.; Zhao, J.L.; Fang, R.; Hu, M. Development and Evaluation of a Loop-Mediated Isothermal Amplification (Lamp) Assay for the Detection of Haemonchus Contortus in Goat Fecal Samples. J. Parasitol. 2017, 103, 161–167. [Google Scholar] [CrossRef]
- Naqvi, M.A.; Naqvi, S.Z.; Memon, M.A.; Aimulajiang, K.; Haseeb, M.; Xu, L.; Song, X.; Li, X.; Yan, R. Combined Use of Indirect Elisa and Western Blotting with Recombinant Hepatocellular Carcinoma-Associated Antigen 59 Is a Potential Immunodiagnostic Tool for the Detection of Prepatent Haemonchus Contortus Infection in Goat. Animals 2019, 13, E548. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Giri, B.R.; Li, X.; He, X.; Jing, Z.; Cheng, G. Preliminary Evaluation of the Diagnostic Potential of Schistosoma Japonicum Extracellular Vesicle Proteins for Schistosomiasis Japonica. Acta Trop. 2019, 201, 105184. [Google Scholar] [CrossRef]
- Naqvi, M.A.; Jamil, T.; Naqvi, S.Z.; Memon, M.A.; Aimulajiang, K.; Aleem, M.T.; Ehsan, M.; Xu, L.; Song, X.; Li, X.; et al. Immunodiagnostic Potential of Recombinant Tropomyosin During Prepatent Haemonchus Contortus Infection in Goat. Res. Vet. Sci. 2019, 128, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Aimulajiang, K.; Naqvi, M.A.-U.-H.; Chu, W.; Lu, M.; Tian, X.; Bu, Y.; Memon, M.A.; Li, X.; Xu, L.; Song, X.; et al. Adhesion-Regulating Molecule from Haemonchus Contortus: Potential Antigen for Diagnosis of Early Infection in Goats. Pathogens 2019, 9, 34. [Google Scholar] [CrossRef]
- Gadahi, J.A.; Wang, S.; Bo, G.; Ehsan, M.; Yan, R.; Song, X.; Xu, L.; Li, X. Proteomic Analysis of the Excretory and Secretory Proteins of Haemonchus Contortus (HcESP) Binding to Goat Pbmcs in Vivo Revealed Stage-Specific Binding Profiles. PLoS ONE 2016, 7, e0159796. [Google Scholar] [CrossRef]
- Bae, J.W.; Kim, S.H.; Kim, D.H.; Ha, J.J.; Yi, J.K.; Hwang, S.; Ryu, B.Y.; Pang, M.G.; Kwon, W.S. Ras-Related Proteins (Rab) Are Key Proteins Related to Male Fertility Following a Unique Activation Mechanism. Reprod. Biol. 2019, 4, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Akao, T.; Kakehi, Y.; Wu, X.X.; Kinoshita, H.; Takahashi, T.; Ogawa, O.; Kato, T.; Yoshida, O. Semi-Quantitative Analysis of Telomerase Activity of Exfoliated Cells in Urine of Patients with Urothelial Cancers: Causative Factors Affecting Sensitivity and Specificity. Urol. Oncol. 1997, 3, 118–124. [Google Scholar] [CrossRef]
- Debajyoti, G.; Bernstein, J. Development of a Progesterone-Specific Ige Assay for Diagnosing Patients with Suspected Progestogen Hypersensitivity. Ann. Allergy Asthma Immunol. 2019, 6, 616–622. [Google Scholar]
- Deo, V.K.; Inagaki, Y.; Murhandarwati, E.H.; Asmara, W.; Miyazaki, T.; Kato, T.; Park, E.Y. Sero-Diagnostic Potential of Plasmodium Falciparum Recombinant Merozoite Surface Protein (Msp)-3 Expressed in Silkworm. Parasitol. Int. 2019, 72, 1–6. [Google Scholar] [CrossRef]
- Jansen, F.; Dorny, P.; Berkvens, D.; Van Hul, A.; Van den Broeck, N.; Makay, C.; Praet, N.; Gabriel, S. Assessment of the Repeatability and Border-Plate Effects of the B158/B60 Enzyme-Linked-Immunosorbent Assay for the Detection of Circulating Antigens (Ag-Elisa) of Taenia Saginata. Vet. Parasitol. 2016, 227, 69–72. [Google Scholar] [CrossRef]
- Lopes, L.G.; Silva, M.H.; Figueiredo, A.; Canuto, K.M.; Brito, E.S.; Ribeiro, P.R.V.; Souza, A.S.Q.; Barioni-Junior, W.; Esteves, S.N.; Chagas, A.C.S. The Intake of Dry Cashew Apple Fiber Reduced Fecal Egg Counts in Haemonchus Contortus-Infected Sheep. Exp. Parasitol. 2018, 195, 38–43. [Google Scholar] [CrossRef]
- Paswan, J.K.; Kumar, K.; Kumar, A.; Kumar, S.; Chandramoni. Effect of Feeding Acacia Nilotica Pod Meal on Hematobiochemical Profile and Fecal Egg Count in Goats. Vet. World 2016, 9, 1400–1406. [Google Scholar] [CrossRef][Green Version]
- Khan, S.; Zhao, X.; Hou, Y.; Yuan, C.; Li, Y.; Luo, X.; Liu, J.; Feng, X. Analysis of Genome-Wide Snps Based on 2b-Rad Sequencing of Pooled Samples Reveals Signature of Selection in Different Populations of Haemonchus Contortus. J. Biosci. 2019, 44, 97. [Google Scholar] [CrossRef]
- Han, K.; Xu, L.; Yan, R.; Song, X.; Li, X. Molecular Cloning, Expression and Characterization of Enolase from Adult Haemonchus Contortus. Res. Vet. Sci. 2012, 92, 259–265. [Google Scholar] [CrossRef]
- Kumar, N.; Das, B.; Jadav, M.M.; Solanki, B.J. Immunodiagnostic Potency of Homologous Antigens for Natural Haemonchus Contortus Infection in Small Ruminants in Plate and Paper Enzyme Linked Immunosorbent Assay. Indian, J. Anim. Res. 2016, 52, 1–9. [Google Scholar]
- Gowda, A.K. Sero-Prevalence of Haemonchus Contortus Infection in Sheep by Indirect-Elisa Using Somatic Antigen. J. Parasit. Dis. 2016, 40, 464–468. [Google Scholar] [PubMed]
- Bai, X.C.; Jiang, Q.F.; Xu, M.; Liang, X. Rassf1 Promotes Cardiomyocyte Apoptosis after Acute Myocardial Infarction and Is Regulated by Mir-125b. J. Cell. Biochem. 2019, 8, 489–496. [Google Scholar]
- Munoz-Maldonado, C.; Zimmer, Y.; Medova, M. A Comparative Analysis of Individual Ras Mutations in Cancer Biology. Front. Oncol. 2019, 9, 1088. [Google Scholar] [CrossRef] [PubMed]
- Schallig, H.D.; van Leeuwen, M.A.; Hendrikx, W.M. Immune Responses of Texel Sheep to Excretory/Secretory Products of Adult Haemonchus Contortus. Parasitology 1994, 108, 351–357. [Google Scholar]
- Xu, J.; Peeling, R.W.; Chen, J.X.; Wu, X.H.; Wu, Z.D.; Wang, S.P.; Feng, T.; Chen, S.H.; Li, H.; Guo, J.G.; et al. Evaluation of Immunoassays for the Diagnosis of Schistosoma Japonicum Infection Using Archived Sera. PLoS Negl. Trop. Dis. 2011, 5, e949. [Google Scholar] [CrossRef]
- Lv, C.; Hong, Y.; Fu, Z.; Lu, K.; Cao, X.; Wang, T.; Zhu, C.; Li, H.; Xu, R.; Jia, B.; et al. Evaluation of Recombinant Multi-Epitope Proteins for Diagnosis of Goat Schistosomiasis by Enzyme-Linked Immunosorbent Assay. Parasit. Vectors 2016, 9, 135. [Google Scholar]
- Schallig, H.D.; Hornok, S.; Cornelissen, J.B. Comparison of Two Enzyme Immunoassays for the Detection of Haemonchus Contortus Infections in Sheep. Vet. Parasitol. 1995, 57, 329–338. [Google Scholar] [CrossRef]
- Lin, A.V. Indirect Elisa. Methods Mol. Biol. 2015, 1318, 51–59. [Google Scholar]
- Muller, N.; Frei, E.; Nuñez, S.; Gottstein, B. Improved Serodiagnosis of Alveolar Echinococcosis of Humans Using an in Vitro-Produced Echinococcus Multilocularis Antigen. J. Parasitol. 2007, 134, 879–888. [Google Scholar] [CrossRef]
- Wattanaphansak, S.; Asawakarn, T.; Gebhart, C.J.; Deen, J. Development and Validation of an Enzyme-Linked Immunosorbent Assay for the Diagnosis of Porcine Proliferative Enteropathy. J. Vet. Diagn. Invest. 2008, 20, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ahirwar, R.; Rehman, I.; Nahar, P. Moderate Reagent Mixing on an Orbital Shaker Reduces the Incubation Time of Enzyme-Linked Immunosorbent Assay. Anal. Biochem. 2017, 528, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Azri, F.A.; Sukor, R.; Selamat, J.; Abu Bakar, F.; Yusof, N.A.; Hajian, R. Electrochemical Immunosensor for Detection of Aflatoxin B₁ Based on Indirect Competitive Elisa. Toxins 2018, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Kandil, O.M.; Gamil, I.S.; Hendawy, S.H.M.; Medhat, F.; El-Habit, O.H. Efficacy of Glutathione-S-Transferase Purified Antigen of the Gastro-Intestinal Nematode Haemonchus Contortus in Diagnosis of Sheep Haemonchosis. J. Parasit. Dis. 2017, 41, 968–975. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aimulajiang, K.; Cao, M.; Liao, S.; Naqvi, M.A.-u.-H.; Tian, X.; Li, Z.; Lu, M.; Lakho, S.A.; Li, X.; Xu, L.; et al. Development and Potential Application of Ras Domain Containing Protein from Haemonchus contortus for Diagnosis of Goat Infection. Animals 2020, 10, 138. https://doi.org/10.3390/ani10010138
Aimulajiang K, Cao M, Liao S, Naqvi MA-u-H, Tian X, Li Z, Lu M, Lakho SA, Li X, Xu L, et al. Development and Potential Application of Ras Domain Containing Protein from Haemonchus contortus for Diagnosis of Goat Infection. Animals. 2020; 10(1):138. https://doi.org/10.3390/ani10010138
Chicago/Turabian StyleAimulajiang, Kalibixiati, Man Cao, Shuyi Liao, Muhammad Ali-ul-Husnain Naqvi, Xiaowei Tian, Zehua Li, Mingmin Lu, Shakeel Ahmed Lakho, Xiangrui Li, Lixin Xu, and et al. 2020. "Development and Potential Application of Ras Domain Containing Protein from Haemonchus contortus for Diagnosis of Goat Infection" Animals 10, no. 1: 138. https://doi.org/10.3390/ani10010138
APA StyleAimulajiang, K., Cao, M., Liao, S., Naqvi, M. A.-u.-H., Tian, X., Li, Z., Lu, M., Lakho, S. A., Li, X., Xu, L., Song, X., & Yan, R. (2020). Development and Potential Application of Ras Domain Containing Protein from Haemonchus contortus for Diagnosis of Goat Infection. Animals, 10(1), 138. https://doi.org/10.3390/ani10010138







