Composition and Function of Chicken Gut Microbiota
Simple Summary
Abstract
1. Introduction
2. Composition of Gut Microbiota in Adult Chickens
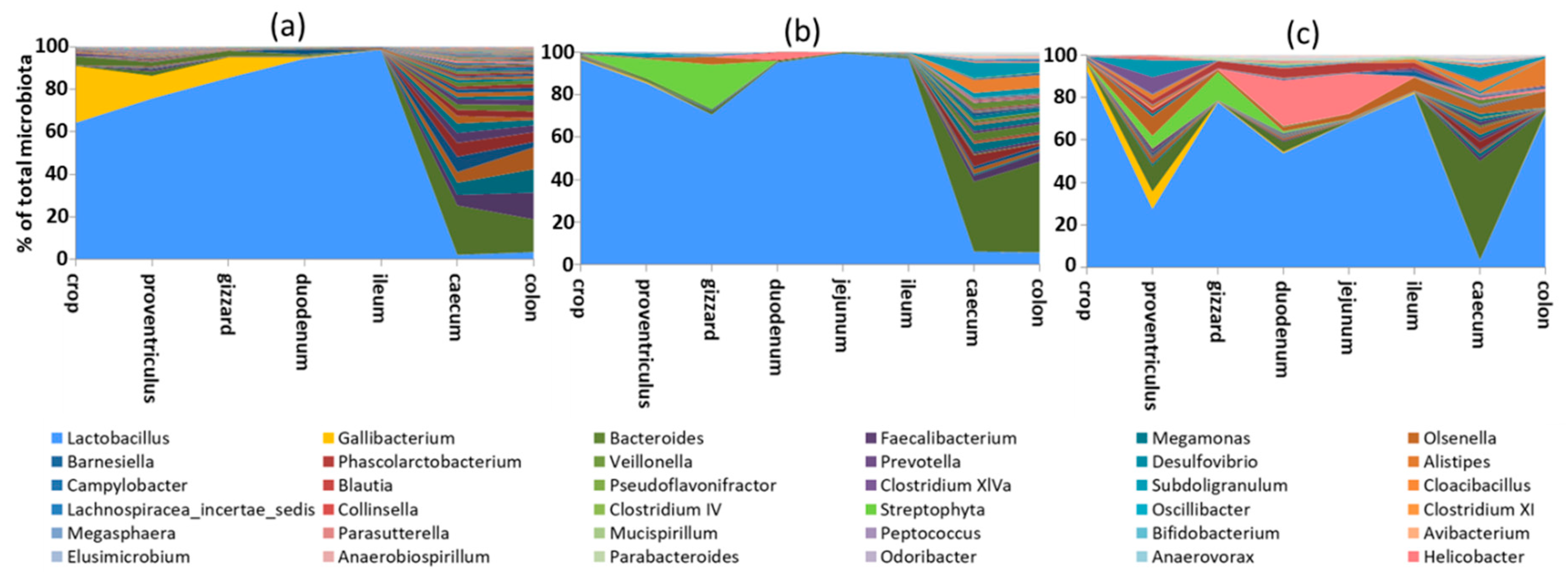
2.1. Crop Microbiota
2.2. Stomach Microbiota
2.3. Small Intestine Microbiota
2.4. Microbiota in the Caecum
2.5. Colonic and Faecal Microbiota
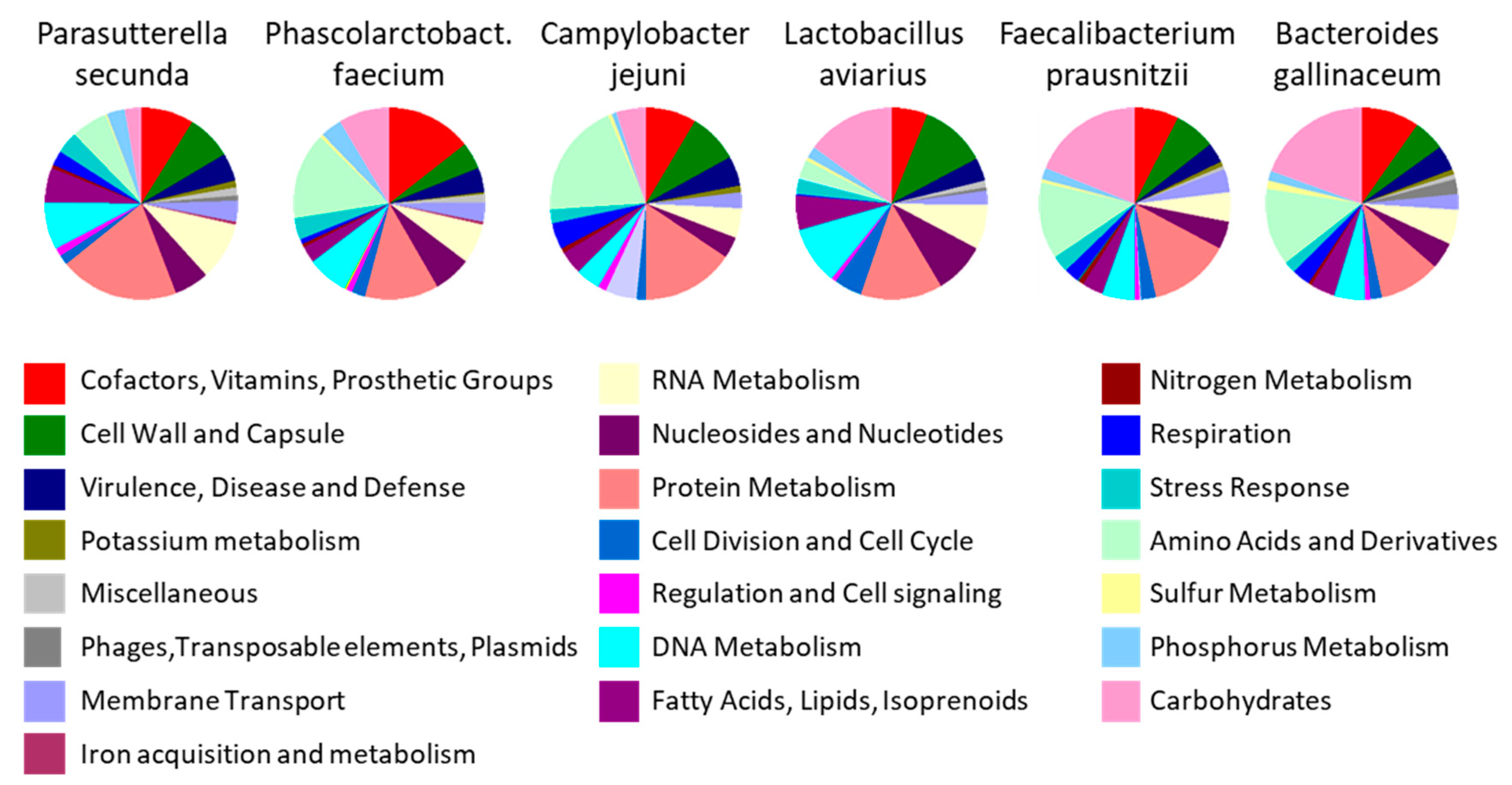
2.6. Major Bacterial Taxa Colonising Chicken Intestinal Tract
2.7. Other Bacteria Colonising the Chicken Intestinal Tract
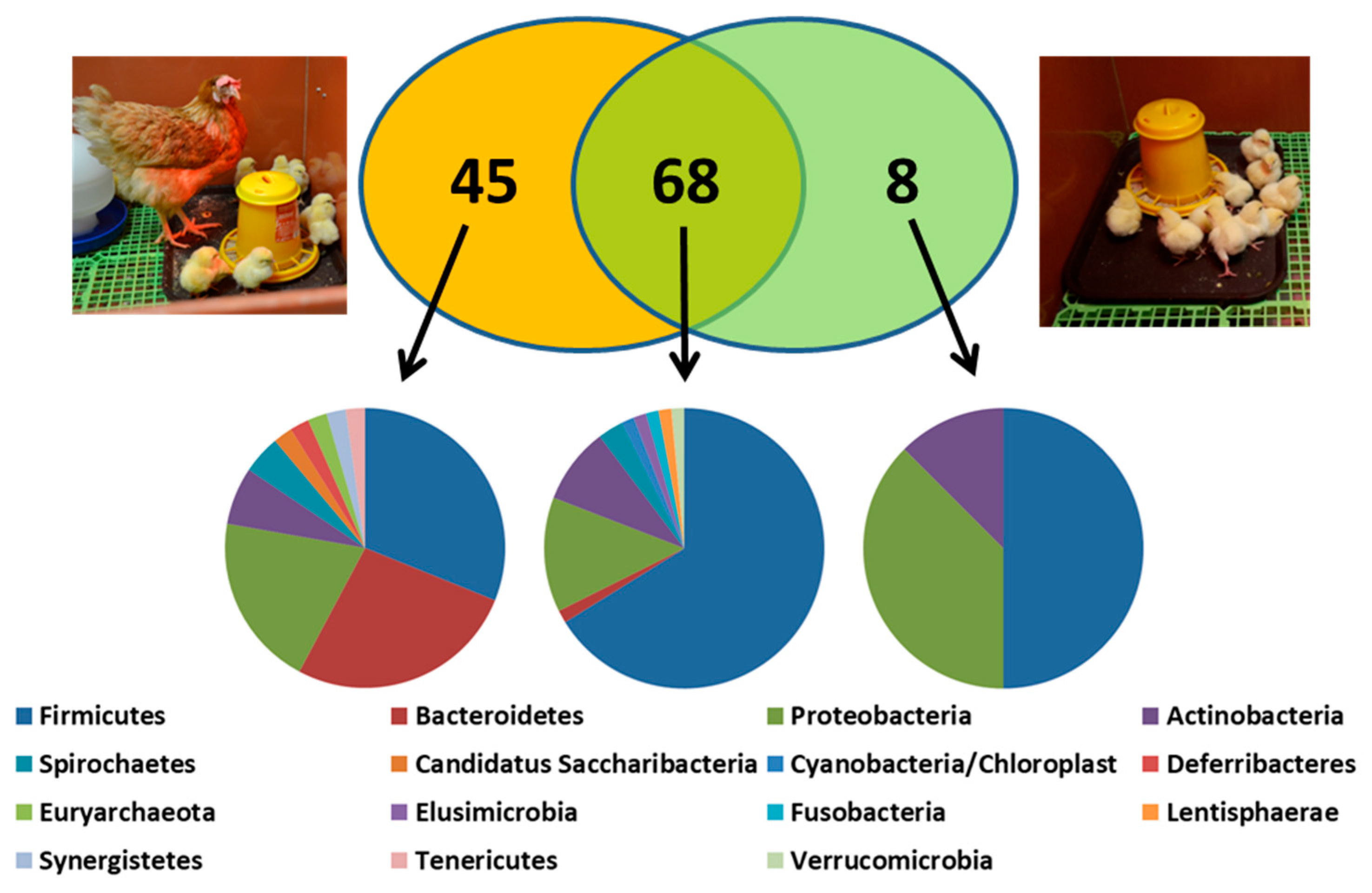
3. How Different Is Chicken Gut Microbiota to Gut Microbiota of Other Warm-Blooded Animals?
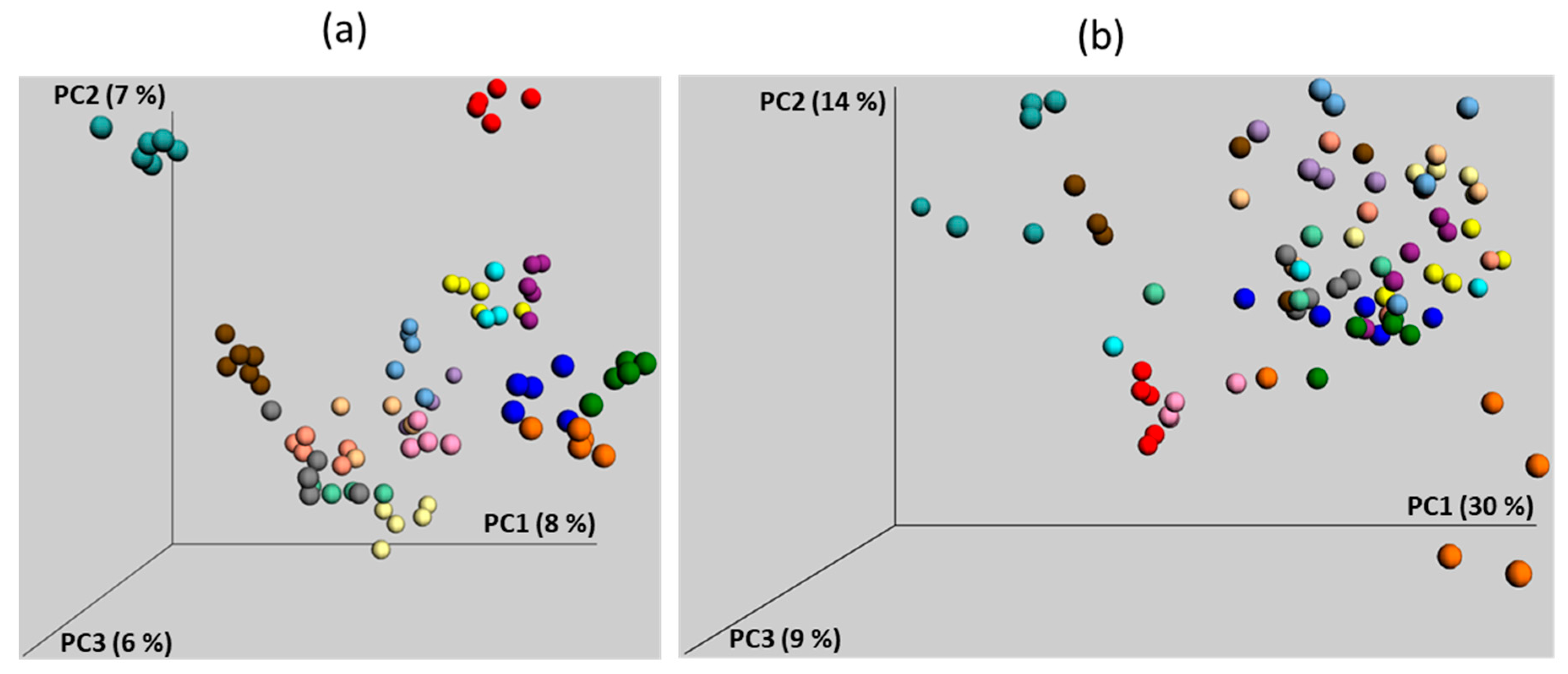
4. Development of Chicken Gut Microbiota
4.1. Development of Gut Microbiota in Commercially Hatched Chickens
4.2. Gut Microbiota Development in Chicks in Contact with Adult Hens
5. Probiotics and Competitive Exclusion
6. Common Issues in Experimental Design of Studies on Chicken Gut Microbiota
7. Future Challenges
8. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Stanley, D.; Geier, M.S.; Chen, H.; Hughes, R.J.; Moore, R.J. Comparison of fecal and cecal microbiotas reveals qualitative similarities but quantitative differences. BMC Microbiol. 2015, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Hughes, R.J.; Geier, M.S.; Moore, R.J. Bacteria within the Gastrointestinal Tract Microbiota Correlated with Improved Growth and Feed Conversion: Challenges Presented for the Identification of Performance Enhancing Probiotic Bacteria. Front. Microbiol. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Willson, N.L.; Nattrass, G.S.; Hughes, R.J.; Moore, R.J.; Stanley, D.; Hynd, P.I.; Forder, R.E.A. Correlations between intestinal innate immune genes and cecal microbiota highlight potential for probiotic development for immune modulation in poultry. Appl. Microbiol. Biotechnol. 2018, 102, 9317–9329. [Google Scholar] [CrossRef] [PubMed]
- Videnska, P.; Sedlar, K.; Lukac, M.; Faldynova, M.; Gerzova, L.; Cejkova, D.; Sisak, F.; Rychlik, I. Succession and replacement of bacterial populations in the caecum of egg laying hens over their whole life. PLoS ONE 2014, 9, e115142. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kang, S.K.; Heo, Y.J.; Shin, D.W.; Park, T.E.; Han, G.G.; Jin, G.D.; Lee, H.B.; Jung, E.; Kim, H.S.; et al. Influence of Flaxseed Oil on Fecal Microbiota, Egg Quality and Fatty Acid Composition of Egg Yolks in Laying Hens. Curr. Microbiol. 2016, 72, 259–266. [Google Scholar] [CrossRef]
- Qi, Z.; Shi, S.; Tu, J.; Li, S. Comparative metagenomic sequencing analysis of cecum microbiotal diversity and function in broilers and layers. 3 Biotech 2019, 9, 316. [Google Scholar] [CrossRef]
- Long, C.; Wang, J.; Zhang, H.J.; Wu, S.G.; Qi, G.H. Effects of dietary rapeseed meal supplementation on cecal microbiota in laying hens with different flavin-containing monooxygenase 3 genotypes. Poult. Sci. 2017, 96, 1748–1758. [Google Scholar] [CrossRef]
- Videnska, P.; Faldynova, M.; Juricova, H.; Babak, V.; Sisak, F.; Havlickova, H.; Rychlik, I. Chicken faecal microbiota and disturbances induced by single or repeated therapy with tetracycline and streptomycin. BMC Vet. Res. 2013, 9, 30. [Google Scholar] [CrossRef]
- Wang, J.; Fan, H.; Han, Y.; Wei, J.P.; Zhao, J.Z.; Zhou, Z.J. Pyrosequencing of the broiler chicken gastrointestinal tract reveals the regional similarity and dissimilarity of microbial community. Can. J. Anim. Sci. 2017, 97, 302–313. [Google Scholar] [CrossRef]
- Kollarcikova, M.; Kubasova, T.; Karasova, D.; Crhanova, M.; Cejkova, D.; Sisak, F.; Rychlik, I. Use of 16S rRNA gene sequencing for prediction of new opportunistic pathogens in chicken ileal and cecal microbiota. Poult. Sci. 2019, 98, 2347–2353. [Google Scholar] [CrossRef]
- Yan, W.; Sun, C.; Zheng, J.; Wen, C.; Ji, C.; Zhang, D.; Chen, Y.; Hou, Z.; Yang, N. Efficacy of Fecal Sampling as a Gut Proxy in the Study of Chicken Gut Microbiota. Front. Microbiol. 2019, 10, 2126. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Nordentoft, S.; Molbak, L.; Bjerrum, L.; De Vylder, J.; Van Immerseel, F.; Pedersen, K. The influence of the cage system and colonisation of Salmonella Enteritidis on the microbial gut flora of laying hens studied by T-RFLP and 454 pyrosequencing. BMC Microbiol. 2011, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Candela, M.; Vitali, B.; Matteuzzi, D.; Brigidi, P. Evaluation of the rrn operon copy number in Bifidobacterium using real-time PCR. Lett. Appl. Microbiol. 2004, 38, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Rothrock, M.J., Jr.; Locatelli, A.; Feye, K.M.; Caudill, A.J.; Guard, J.; Hiett, K.; Ricke, S.C. A Microbiomic Analysis of a Pasture-Raised Broiler Flock Elucidates Foodborne Pathogen Ecology Along the Farm-To-Fork Continuum. Front. Vet. Sci. 2019, 6, 260. [Google Scholar] [CrossRef]
- Gao, P.; Ma, C.; Sun, Z.; Wang, L.; Huang, S.; Su, X.; Xu, J.; Zhang, H. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome 2017, 5, 91. [Google Scholar] [CrossRef]
- Svihus, B.; Hetland, H.; Choct, M.; Sundby, F. Passage rate through the anterior digestive tract of broiler chickens fed on diets with ground and whole wheat. Br. Poult. Sci. 2002, 43, 662–668. [Google Scholar] [CrossRef]
- Hughes, R.J. Relationship between digesta transit time and apparent metabolisable energy value of wheat in chickens. Br. Poult. Sci. 2008, 49, 716–720. [Google Scholar] [CrossRef]
- Warriss, P.D.; Wilkins, L.J.; Brown, S.N.; Phillips, A.J.; Allen, V. Defaecation and weight of the gastrointestinal tract contents after feed and water withdrawal in broilers. Br. Poult. Sci. 2004, 45, 61–66. [Google Scholar] [CrossRef]
- Svihus, B.; Choct, M.; Classen, H.L. Function and nutritional roles of the avian caeca: A review. World Poult. Sci. J. 2013, 69, 249–263. [Google Scholar] [CrossRef]
- Hinton, A., Jr.; Buhr, R.J.; Ingram, K.D. Physical, chemical, and microbiological changes in the ceca of broiler chickens subjected to incremental feed withdrawal. Poult. Sci. 2000, 79, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Duke, G.E. Relationship of cecal and colonic motility to diet, habitat, and cecal anatomy in several avian species. J. Exp. Zool. Suppl. 1989, 3, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Duke, G.E.; Eccleston, E.; Kirkwood, S.; Louis, C.F.; Bedbury, H.P. Cellulose digestion by domestic turkeys fed low or high fiber diets. J. Nutr. 1984, 114, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Varmuzova, K.; Kubasova, T.; Davidova-Gerzova, L.; Sisak, F.; Havlickova, H.; Sebkova, A.; Faldynova, M.; Rychlik, I. Composition of Gut Microbiota Influences Resistance of Newly Hatched Chickens to Salmonella Enteritidis Infection. Front. Microbiol. 2016, 7, 957. [Google Scholar] [CrossRef]
- Han, Z.; Willer, T.; Pielsticker, C.; Gerzova, L.; Rychlik, I.; Rautenschlein, S. Differences in host breed and diet influence colonization by Campylobacter jejuni and induction of local immune responses in chicken. Gut Pathog. 2016, 8, 56. [Google Scholar] [CrossRef]
- Polansky, O.; Sekelova, Z.; Faldynova, M.; Sebkova, A.; Sisak, F.; Rychlik, I. Important Metabolic Pathways and Biological Processes Expressed by Chicken Cecal Microbiota. Appl. Environ. Microbiol. 2016, 82, 1569–1576. [Google Scholar] [CrossRef]
- Line, J.E.; Hiett, K.L.; Guard-Bouldin, J.; Seal, B.S. Differential carbon source utilization by Campylobacter jejuni 11168 in response to growth temperature variation. J. Microbiol. Methods 2010, 80, 198–202. [Google Scholar] [CrossRef]
- Mohammed, K.A.; Miles, R.J.; Halablab, M.A. The pattern and kinetics of substrate metabolism of Campylobacter jejuni and Campylobacter coli. Lett. Appl. Microbiol. 2004, 39, 261–266. [Google Scholar] [CrossRef]
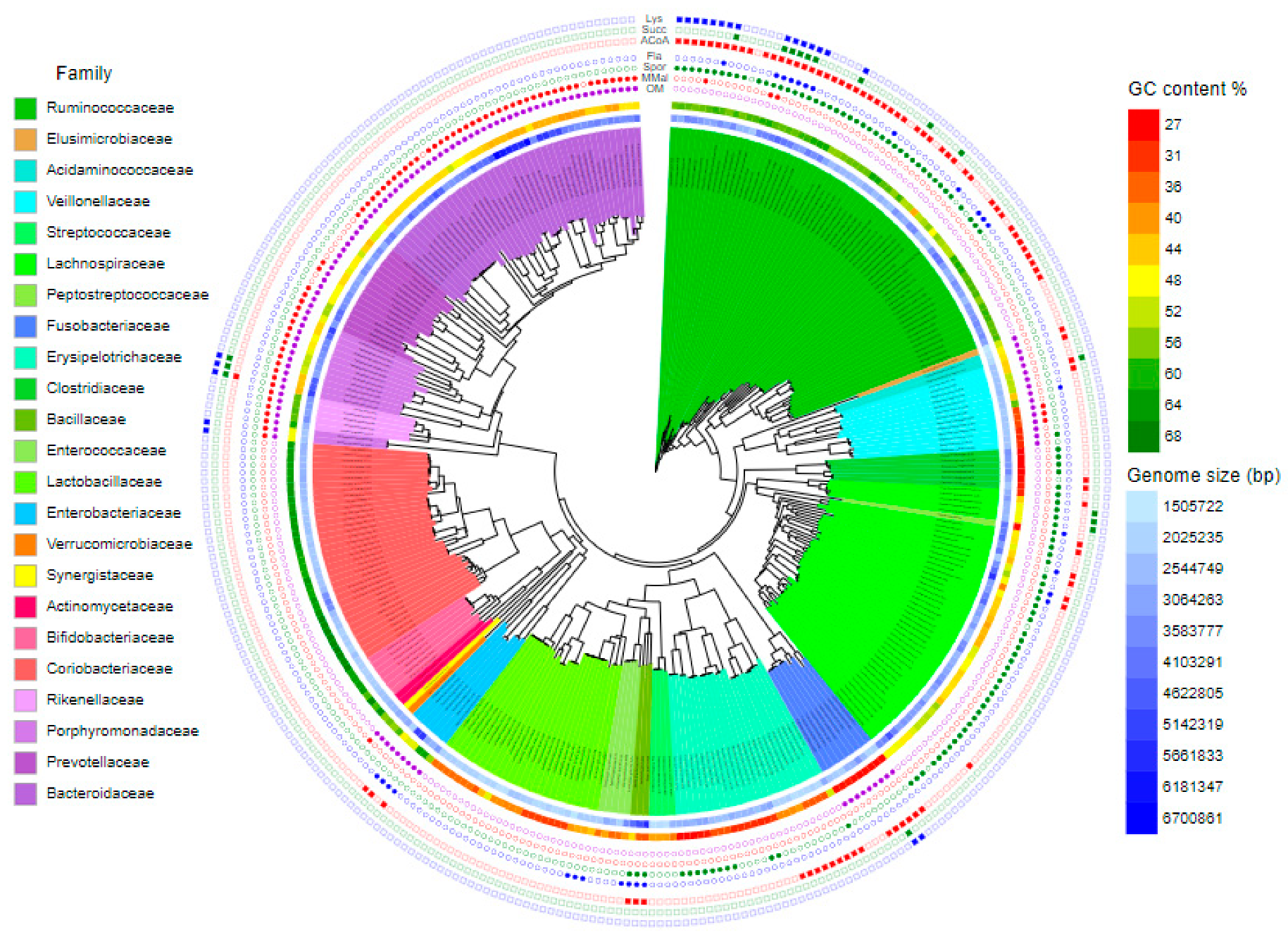
- Crhanova, M.; Karasova, D.; Juricova, H.; Matiasovicova, J.; Jahodarova, E.; Kubasova, T.; Seidlerova, Z.; Cizek, A.; Rychlik, I. Systematic Culturomics Shows that Half of Chicken Caecal Microbiota Members can be Grown in Vitro Except for Two Lineages of Clostridiales and a Single Lineage of Bacteroidetes. Microorganisms 2019, 7, 496. [Google Scholar] [CrossRef]
- Medvecky, M.; Cejkova, D.; Polansky, O.; Karasova, D.; Kubasova, T.; Cizek, A.; Rychlik, I. Whole genome sequencing and function prediction of 133 gut anaerobes isolated from chicken caecum in pure cultures. BMC Genom. 2018, 19, 561. [Google Scholar] [CrossRef]
- Sergeant, M.J.; Constantinidou, C.; Cogan, T.A.; Bedford, M.R.; Penn, C.W.; Pallen, M.J. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS ONE 2014, 9, e91941. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Duncan, S.H.; Stams, A.J.; van Dijl, J.M.; Flint, H.J.; Harmsen, H.J. The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic-anoxic interphases. ISME J. 2012, 6, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, V.; Wang, J.; Van Parys, A.; Haesebrouck, F.; Joossens, M.; Falony, G.; Raes, J.; Ducatelle, R.; Van Immerseel, F. The Probiotic Butyricicoccus pullicaecorum Reduces Feed Conversion and Protects from Potentially Harmful Intestinal Microorganisms and Necrotic Enteritis in Broilers. Front. Microbiol. 2016, 7, 1416. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Elizondo, S.; Ilhan, Z.E.; Garcia-Pena, E.I.; Krajmalnik-Brown, R. Insights into Butyrate Production in a Controlled Fermentation System via Gene Predictions. mSystems 2017, 2, e00051-17. [Google Scholar] [CrossRef]
- Vital, M.; Karch, A.; Pieper, D.H. Colonic Butyrate-Producing Communities in Humans: An Overview Using Omics Data. mSystems 2017, 2, e00130-17. [Google Scholar] [CrossRef]
- Thiennimitr, P.; Winter, S.E.; Winter, M.G.; Xavier, M.N.; Tolstikov, V.; Huseby, D.L.; Sterzenbach, T.; Tsolis, R.M.; Roth, J.R.; Baumler, A.J. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc. Natl. Acad. Sci. USA 2011, 108, 17480–17485. [Google Scholar] [CrossRef]
- Winter, S.E.; Thiennimitr, P.; Winter, M.G.; Butler, B.P.; Huseby, D.L.; Crawford, R.W.; Russell, J.M.; Bevins, C.L.; Adams, L.G.; Tsolis, R.M.; et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 2010, 467, 426–429. [Google Scholar] [CrossRef]
- Adamberg, S.; Tomson, K.; Vija, H.; Puurand, M.; Kabanova, N.; Visnapuu, T.; Jogi, E.; Alamae, T.; Adamberg, K. Degradation of Fructans and Production of Propionic Acid by Bacteroides thetaiotaomicron are Enhanced by the Shortage of Amino Acids. Front. Nutr. 2014, 1, 21. [Google Scholar] [CrossRef]
- Isar, J.; Agarwal, L.; Saran, S.; Saxena, R.K. Succinic acid production from Bacteroides fragilis: Process optimization and scale up in a bioreactor. Anaerobe 2006, 12, 231–237. [Google Scholar] [CrossRef]
- Strobel, H.J. Vitamin B12-dependent propionate production by the ruminal bacterium Prevotella ruminicola 23. Appl. Environ. Microbiol. 1992, 58, 2331–2333. [Google Scholar] [CrossRef]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef] [PubMed]
- Gorvitovskaia, A.; Holmes, S.P.; Huse, S.M. Interpreting Prevotella and Bacteroides as biomarkers of diet and lifestyle. Microbiome 2016, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Gerzova, L.; Babak, V.; Sedlar, K.; Faldynova, M.; Videnska, P.; Cejkova, D.; Jensen, A.N.; Denis, M.; Kerouanton, A.; Ricci, A.; et al. Characterization of Antibiotic Resistance Gene Abundance and Microbiota Composition in Feces of Organic and Conventional Pigs from Four EU Countries. PLoS ONE 2015, 10, e0132892. [Google Scholar] [CrossRef] [PubMed]
- Mulder, I.E.; Schmidt, B.; Stokes, C.R.; Lewis, M.; Bailey, M.; Aminov, R.I.; Prosser, J.I.; Gill, B.P.; Pluske, J.R.; Mayer, C.D.; et al. Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces. BMC Biol. 2009, 7, 79. [Google Scholar] [CrossRef]
- Kubasova, T.; Davidova-Gerzova, L.; Merlot, E.; Medvecky, M.; Polansky, O.; Gardan-Salmon, D.; Quesnel, H.; Rychlik, I. Housing Systems Influence Gut Microbiota Composition of Sows but Not of Their Piglets. PLoS ONE 2017, 12, e0170051. [Google Scholar] [CrossRef]
- Kubasova, T.; Kollarcikova, M.; Crhanova, M.; Karasova, D.; Cejkova, D.; Sebkova, A.; Matiasovicova, J.; Faldynova, M.; Sisak, F.; Babak, V.; et al. Gut anaerobes capable of chicken caecum colonisation. Microorganisms 2019, 7, 597. [Google Scholar] [CrossRef]
- Noda, S.; Shimizu, D.; Yuki, M.; Kitade, O.; Ohkuma, M. Host-Symbiont Cospeciation of Termite-Gut Cellulolytic Protists of the Genera Teranympha and Eucomonympha and their Treponema Endosymbionts. Microbes Environ. 2018, 33, 26–33. [Google Scholar] [CrossRef]
- Angelakis, E.; Bachar, D.; Yasir, M.; Musso, D.; Djossou, F.; Gaborit, B.; Brah, S.; Diallo, A.; Ndombe, G.M.; Mediannikov, O.; et al. Treponema species enrich the gut microbiota of traditional rural populations but are absent from urban individuals. New Microbes New Infect. 2019, 27, 14–21. [Google Scholar] [CrossRef]
- Obregon-Tito, A.J.; Tito, R.Y.; Metcalf, J.; Sankaranarayanan, K.; Clemente, J.C.; Ursell, L.K.; Zech Xu, Z.; Van Treuren, W.; Knight, R.; Gaffney, P.M.; et al. Subsistence strategies in traditional societies distinguish gut microbiomes. Nat. Commun. 2015, 6, 6505. [Google Scholar] [CrossRef]
- Niu, Q.; Li, P.; Hao, S.; Kim, S.W.; Du, T.; Hua, J.; Huang, R. Characteristics of Gut Microbiota in Sows and Their Relationship with Apparent Nutrient Digestibility. Int. J. Mol. Sci. 2019, 20, 870. [Google Scholar] [CrossRef]
- Kubasova, T.; Davidova-Gerzova, L.; Babak, V.; Cejkova, D.; Montagne, L.; Le-Floc’h, N.; Rychlik, I. Effects of host genetics and environmental conditions on fecal microbiota composition of pigs. PLoS ONE 2018, 13, e0201901. [Google Scholar] [CrossRef] [PubMed]
- He, X.; McLean, J.S.; Edlund, A.; Yooseph, S.; Hall, A.P.; Liu, S.Y.; Dorrestein, P.C.; Esquenazi, E.; Hunter, R.C.; Cheng, G.; et al. Cultivation of a human-associated TM7 phylotype reveals a reduced genome and epibiotic parasitic lifestyle. Proc. Natl. Acad. Sci. USA 2015, 112, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Kubasova, T.; Kollarcikova, M.; Crhanova, M.; Karasova, D.; Cejkova, D.; Sebkova, A.; Matiasovicova, J.; Faldynova, M.; Pokorna, A.; Cizek, A.; et al. Contact with adult hen affects development of caecal microbiota in newly hatched chicks. PLoS ONE 2019, 14, e0212446. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Swanson, K.S. Gut microbiota of humans, dogs and cats: Current knowledge and future opportunities and challenges. Br. J. Nutr. 2015, 113, S6–S17. [Google Scholar] [CrossRef]
- Mathur, R.; Barlow, G.M. Obesity and the microbiome. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1087–1099. [Google Scholar] [CrossRef]
- Xi, Y.; Shuling, N.; Kunyuan, T.; Qiuyang, Z.; Hewen, D.; ChenCheng, G.; Tianhe, Y.; Liancheng, L.; Xin, F. Characteristics of the intestinal flora of specific pathogen free chickens with age. Microb. Pathog. 2019, 132, 325–334. [Google Scholar] [CrossRef]
- Siegerstetter, S.C.; Petri, R.M.; Magowan, E.; Lawlor, P.G.; Zebeli, Q.; O’Connell, N.E.; Metzler-Zebeli, B.U. Fecal Microbiota Transplant from Highly Feed-Efficient Donors Shows Little Effect on Age-Related Changes in Feed-Efficiency-Associated Fecal Microbiota from Chickens. Appl. Environ. Microbiol. 2018, 84, e02330-17. [Google Scholar] [CrossRef]
- Zou, A.; Sharif, S.; Parkinson, J. Lactobacillus elicits a ‘Marmite effect’ on the chicken cecal microbiome. NPJ Biofilms Microbiomes 2018, 4, 27. [Google Scholar] [CrossRef]
- Stanley, D.; Geier, M.S.; Hughes, R.J.; Denman, S.E.; Moore, R.J. Highly variable microbiota development in the chicken gastrointestinal tract. PLoS ONE 2013, 8, e84290. [Google Scholar] [CrossRef]
- Volf, J.; Polansky, O.; Varmuzova, K.; Gerzova, L.; Sekelova, Z.; Faldynova, M.; Babak, V.; Medvecky, M.; Smith, A.L.; Kaspers, B.; et al. Transient and Prolonged Response of Chicken Cecum Mucosa to Colonization with Different Gut Microbiota. PLoS ONE 2016, 11, e0163932. [Google Scholar] [CrossRef]
- Thomas, M.; Wongkuna, S.; Ghimire, S.; Kumar, R.; Antony, L.; Doerner, K.C.; Singery, A.; Nelson, E.; Woyengo, T.; Chankhamhaengdecha, S.; et al. Gut Microbial Dynamics during Conventionalization of Germfree Chicken. mSphere 2019, 4, e00035-19. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Mann, E.; Dzieciol, M.; Hess, C.; Schmitz-Esser, S.; Wagner, M.; Hess, M. Age-Related Differences in the Luminal and Mucosa-Associated Gut Microbiome of Broiler Chickens and Shifts Associated with Campylobacter jejuni Infection. Front. Cell. Infect. Microbiol. 2016, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Ranjitkar, S.; Lawley, B.; Tannock, G.; Engberg, R.M. Bacterial Succession in the Broiler Gastrointestinal Tract. Appl. Environ. Microbiol. 2016, 82, 2399–2410. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.; Nurmi, E. Prevention of the growth of Salmonella infantis in chicks by the flora of the alimentary tract of chickens. Br. Poult. Sci. 1973, 14, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Cressman, M.D.; Yu, Z.; Nelson, M.C.; Moeller, S.J.; Lilburn, M.S.; Zerby, H.N. Interrelations between the microbiotas in the litter and in the intestines of commercial broiler chickens. Appl. Environ. Microbiol. 2010, 76, 6572–6582. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lilburn, M.; Yu, Z. Intestinal Microbiota of Broiler Chickens as Affected by Litter Management Regimens. Front. Microbiol. 2016, 7, 593. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Chimenti, M.S.; Farnell, M.; Tabler, T.; Bair, T.; Bray, J.L.; Nonnenmann, M.W. High throughput genomic sequencing of bioaerosols in broiler chicken production facilities. Microb. Biotechnol. 2016, 9, 782–791. [Google Scholar] [CrossRef]
- Baldwin, S.; Hughes, R.J.; Hao Van, T.T.; Moore, R.J.; Stanley, D. At-hatch administration of probiotic to chickens can introduce beneficial changes in gut microbiota. PLoS ONE 2018, 13, e0194825. [Google Scholar] [CrossRef]
- Beirao, B.C.B.; Ingberman, M.; Favaro, C., Jr.; Mesa, D.; Bittencourt, L.C.; Fascina, V.B.; Caron, L.F. Effect of an Enterococcus faecium probiotic on specific IgA following live Salmonella Enteritidis vaccination of layer chickens. Avian Pathol. 2018, 47, 325–333. [Google Scholar] [CrossRef]
- Mazanko, M.S.; Gorlov, I.F.; Prazdnova, E.V.; Makarenko, M.S.; Usatov, A.V.; Bren, A.B.; Chistyakov, V.A.; Tutelyan, A.V.; Komarova, Z.B.; Mosolova, N.I.; et al. Bacillus Probiotic Supplementations Improve Laying Performance, Egg Quality, Hatching of Laying Hens, and Sperm Quality of Roosters. Probiotics Antimicrob. Proteins 2018, 10, 367–373. [Google Scholar] [CrossRef]
- Weinack, O.M.; Snoeyenbos, G.H.; Soerjadi-Liem, A.S. Further studies on competitive exclusion of Salmonella typhimurium by lactobacilli in chickens. Avian Dis. 1985, 29, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Adler, H.E.; DaMassa, A.J. Effect of ingested Lactobacilli on Salmonella infantis and Escherichia coli and on intestinal flora, pasted vents, and chick growth. Avian Dis. 1980, 24, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Milbradt, E.L.; Zamae, J.R.; Araujo Junior, J.P.; Mazza, P.; Padovani, C.R.; Carvalho, V.R.; Sanfelice, C.; Rodrigues, D.M.; Okamoto, A.S.; Andreatti Filho, R.L. Control of Salmonella Enteritidis in turkeys using organic acids and competitive exclusion product. J. Appl. Microbiol. 2014, 117, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.J.; Ferreira, C.S.; Knobl, T.; Moreno, A.M.; Bacarro, M.R.; Chen, M.; Robach, M.; Mead, G.C. Comparison of three commercial competitive-exclusion products for controlling Salmonella colonization of broilers in Brazil. J. Food Prot. 2003, 66, 490–492. [Google Scholar] [CrossRef]
- Nakamura, A.; Ota, Y.; Mizukami, A.; Ito, T.; Ngwai, Y.B.; Adachi, Y. Evaluation of aviguard, a commercial competitive exclusion product for efficacy and after-effect on the antibody response of chicks to Salmonella. Poult. Sci. 2002, 81, 1653–1660. [Google Scholar] [CrossRef]
- Palmu, L.; Camelin, I. The use of competitive exclusion in broilers to reduce the level of Salmonella contamination on the farm and at the processing plant. Poult. Sci. 1997, 76, 1501–1505. [Google Scholar] [CrossRef]
- Methner, U.; Barrow, P.A.; Martin, G.; Meyer, H. Comparative study of the protective effect against Salmonella colonisation in newly hatched SPF chickens using live, attenuated Salmonella vaccine strains, wild-type Salmonella strains or a competitive exclusion product. Int. J. Food Microbiol. 1997, 35, 223–230. [Google Scholar] [CrossRef]
- Van Immerseel, F.; De Buck, J.; Pasmans, F.; Velge, P.; Bottreau, E.; Fievez, V.; Haesebrouck, F.; Ducatelle, R. Invasion of Salmonella enteritidis in avian intestinal epithelial cells in vitro is influenced by short-chain fatty acids. Int. J. Food Microbiol. 2003, 85, 237–248. [Google Scholar] [CrossRef]
- Winter, S.E.; Baumler, A.J. A breathtaking feat: To compete with the gut microbiota, Salmonella drives its host to provide a respiratory electron acceptor. Gut Microbes 2011, 2, 58–60. [Google Scholar] [CrossRef]
- Ludvigsen, J.; Svihus, B.; Rudi, K. Rearing Room Affects the Non-dominant Chicken Cecum Microbiota, While Diet Affects the Dominant Microbiota. Front. Vet. Sci. 2016, 3, 16. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, Y.; Guo, S.; Tan, J. Effects of Clostridium butyricum and Enterococcus faecium on growth performance, lipid metabolism, and cecal microbiota of broiler chickens. Appl. Microbiol. Biotechnol. 2013, 97, 6477–6488. [Google Scholar] [CrossRef] [PubMed]
- Tayeri, V.; Seidavi, A.; Asadpour, L.; Phillips, C.J.C. A comparison of the effects of antibiotics, probiotics, synbiotics and prebiotics on the performance and carcass characteristics of broilers. Vet. Res. Commun. 2018, 42, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhen, W.; Geng, Y.; Wang, Z.; Guo, Y. Pretreatment with probiotic Enterococcus faecium NCIMB 11181 ameliorates necrotic enteritis-induced intestinal barrier injury in broiler chickens. Sci. Rep. 2019, 9, 10256. [Google Scholar] [CrossRef] [PubMed]
- Latorre, J.D.; Hernandez-Velasco, X.; Vicente, J.L.; Wolfenden, R.; Hargis, B.M.; Tellez, G. Effects of the inclusion of a Bacillus direct-fed microbial on performance parameters, bone quality, recovered gut microflora, and intestinal morphology in broilers consuming a grower diet containing corn distillers dried grains with solubles. Poult. Sci. 2017, 96, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Brisbin, J.T.; Gong, J.; Orouji, S.; Esufali, J.; Mallick, A.I.; Parvizi, P.; Shewen, P.E.; Sharif, S. Oral treatment of chickens with lactobacilli influences elicitation of immune responses. Clin. Vaccine Immunol. 2011, 18, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, H.R.; Gong, J.; Gyles, C.L.; Hayes, M.A.; Zhou, H.; Sanei, B.; Chambers, J.R.; Sharif, S. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. 2006, 13, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, J.; Velkers, F.C.; Bouwstra, R.J.; Beerens, N.; Stegeman, J.A.; de Boer, W.F.; Elbers, A.R.W.; van Hooft, P.; Feberwee, A.; Bossers, A.; et al. Limited changes in the fecal microbiome composition of laying hens after oral inoculation with wild duck feces. Poult. Sci. 2019, 98, 6542–6551. [Google Scholar] [CrossRef]
- Papanicolas, L.E.; Choo, J.M.; Wang, Y.; Leong, L.E.X.; Costello, S.P.; Gordon, D.L.; Wesselingh, S.L.; Rogers, G.B. Bacterial viability in faecal transplants: Which bacteria survive? EBioMedicine 2019, 41, 509–516. [Google Scholar] [CrossRef]
- Donaldson, E.E.; Stanley, D.; Hughes, R.J.; Moore, R.J. The time-course of broiler intestinal microbiota development after administration of cecal contents to incubating eggs. PeerJ 2017, 5, e3587. [Google Scholar] [CrossRef]
- Ferrario, C.; Alessandri, G.; Mancabelli, L.; Gering, E.; Mangifesta, M.; Milani, C.; Lugli, G.A.; Viappiani, A.; Duranti, S.; Turroni, F.; et al. Untangling the cecal microbiota of feral chickens by culturomic and metagenomic analyses. Environ. Microbiol. 2017, 19, 4771–4783. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, H.; Zhang, L.; Su, Y.; Shi, D.; Xiao, H.; Tian, Y. High-throughput sequencing technology to reveal the composition and function of cecal microbiota in Dagu chicken. BMC Microbiol. 2016, 16, 259. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.F.; Laursen, R.P.; Larnkjaer, A.; Molgaard, C.; Michaelsen, K.F.; Frokiaer, H.; Bahl, M.I.; Licht, T.R. Faecalibacterium Gut Colonization Is Accelerated by Presence of Older Siblings. mSphere 2017, 2, e00448-17. [Google Scholar] [CrossRef] [PubMed]
- Lagier, J.C.; Khelaifia, S.; Alou, M.T.; Ndongo, S.; Dione, N.; Hugon, P.; Caputo, A.; Cadoret, F.; Traore, S.I.; Seck, E.H.; et al. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat. Microbiol. 2016, 1, 16203. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.T.; Whelan, F.J.; Herath, I.; Lee, C.H.; Collins, S.M.; Bercik, P.; Surette, M.G. Capturing the diversity of the human gut microbiota through culture-enriched molecular profiling. Genome Med. 2016, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Browne, H.P.; Forster, S.C.; Anonye, B.O.; Kumar, N.; Neville, B.A.; Stares, M.D.; Goulding, D.; Lawley, T.D. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature 2016, 533, 543–546. [Google Scholar] [CrossRef]
| Phylum | Order | Family | Transferred Genera | Non-Transferred Genera |
|---|---|---|---|---|
| Actinobacteria | Bifidobacteriales | Bifidobacteriaceae | Bifidobacterium | - |
| Coriobacteriales | Coriobacteriaceae | Olsenella, Collinsella | Eggerthella | |
| Bacteroidetes | Bacteroidales | Bacteroidaceae | Bacteroides | - |
| Bacteroidales incertae sedis | Phocaeicola | - | ||
| Porphyromonadaceae | Parabacteroides, Barnesiella, Odoribacter, Butyricimonas, Tannerella | |||
| Prevotellaceae | Prevotella, Paraprevotella, Hallella | - | ||
| Rikenellaceae | Alistipes, Rikenella | - | ||
| Firmicutes | Clostridiales | Ruminococcaceae | Faecalibacterium, Subdoligranulum, Gemmiger | - |
| Lachnospiraceae | Dorea, Acetitomaculum | Clostridium XlVa, Ruminococcus2, Anaerostipes, Blautia | ||
| Peptococcaceae 1 | Peptococcus | - | ||
| Eubacteriaceae | Eubacterium | - | ||
| Defluviitaleaceae | Defluviitalea | - | ||
| Clostridiales Incertae Sedis XII | Guggenheimella | - | ||
| Erysipelotrichales | Erysipelotrichaceae | Erysipelotrichaceae incertae sedis | - | |
| Selenomonadales | Acidaminococcaceae | Phascolarctobacterium | - | |
| Veillonellaceae | Megamonas, Megasphaera, Dialister | - | ||
| Proteobacteria | Burkholderiales | Sutterellaceae | Sutterella, Parasutterella | - |
| Campylobacterales | Helicobacteraceae | Helicobacter | - | |
| Campylobacteraceae | Campylobacter | - | ||
| Desulfovibrionales | Desulfovibrionaceae | Desulfovibrio, Bilophila | - | |
| Aeromonadales | Succinivibrionaceae | Anaerobiospirillum, Succinatimonas, Succinivibrio | - | |
| Enterobacteriales | Enterobacteriaceae | - | Escherichia, Proteus, Salmonella | |
| Deferribacteres | Deferribacterales | Deferribacteraceae | Mucispirillum | - |
| Spirochaetes | Spirochaetales | Spirochaetaceae | Treponema, Spirochaeta | - |
| Synergistetes | Synergistales | Synergistaceae | Cloacibacillus | - |
| Tenericutes | Anaeroplasmatales | Anaeroplasmataceae | Asteroleplasma | - |
| Candidatus Saccharibacteria | - | - | Saccharibacteria genera incertae sedis | - |
| Euryarchaeota | Methanobacteriales | Methanobacteriaceae | Methanobrevibacter | - |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rychlik, I. Composition and Function of Chicken Gut Microbiota. Animals 2020, 10, 103. https://doi.org/10.3390/ani10010103
Rychlik I. Composition and Function of Chicken Gut Microbiota. Animals. 2020; 10(1):103. https://doi.org/10.3390/ani10010103
Chicago/Turabian StyleRychlik, Ivan. 2020. "Composition and Function of Chicken Gut Microbiota" Animals 10, no. 1: 103. https://doi.org/10.3390/ani10010103
APA StyleRychlik, I. (2020). Composition and Function of Chicken Gut Microbiota. Animals, 10(1), 103. https://doi.org/10.3390/ani10010103




