Novel Approach to Dental Biofilm Management through Guided Biofilm Therapy (GBT): A Review
Abstract
:1. Introduction
2. Dental Biofilm and Its Relation to Periodontal and Peri-Implant Diseases
3. Rationale and Approaches for Non-Surgical Management of Dental Biofilm
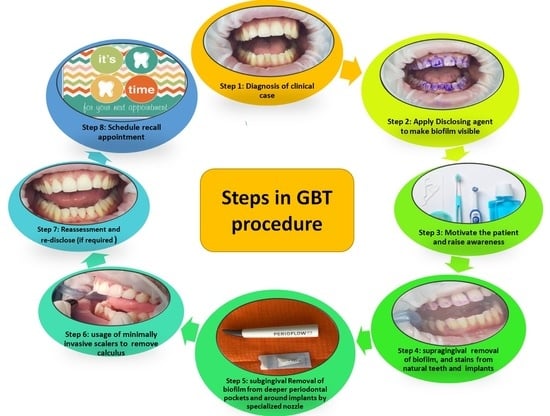
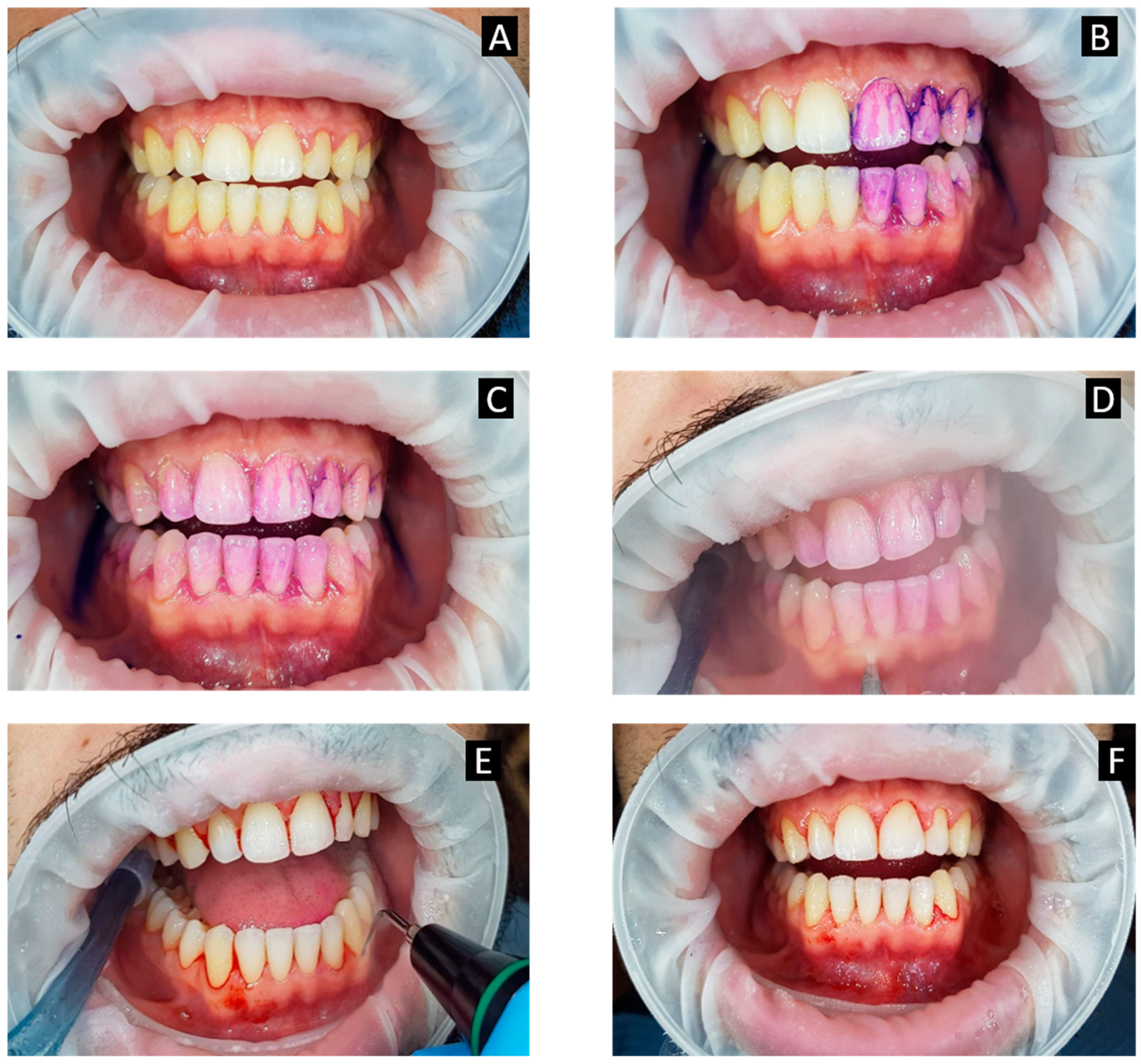
4. Guided Biofilm Therapy
5. Role of Disclosing Agent
- (a)
- It should be used with caution on the restorative material as it can cause staining;
- (b)
- It should not be applied before the application of a sealant;
- (c)
- Solutions containing alcohol should not be stored for more than 2 to 3 months as the alcohol will evaporate, making the solution too concentrated;
- (d)
- Clinical assessments of soft tissue color, such as gingival status and gingival bleeding index, should be performed prior to the use of the plaque detector as dyeing the solution may mask the clinical status of the tissues;
- (e)
- Always assess for any kind of allergies of patients before using the detectors in any form [44].
6. Air Polishing Devices
7. Air Abrasive Powders
7.1. Sodium Bicarbonate (NaHCO₃)
7.2. Glycine Powder
7.3. Erythitol Powder
8. Guided Biofilm Therapy in Periodontal Disease and Peri-Implant Disease
- The use of a plaque disclosing agent allows the operator to determine the patient compliance in executing proper oral hygiene practices. It also allows the patient to visualize areas that were neglected;
- The use of an air-polishing device can remove the disclosed plaque effectively and safely without causing soft tissue damage compared to conventional rubber cups, especially during subgingival plaque removal;
- The removal of plaque using air polishing prior to ultrasonic scaling provides better visible access to calculus deposits. Instead of the indiscriminate use of ultrasonic scalers for the entire dentition, the operator can now target the use of ultrasonic scalers on sites with mineralized deposits. This minimizes soft tissue damage and CAL caused by ultrasonic scaling at sites with shallow pocket depths. From the patient’s perspective, this translates to lesser discomfort and sensitivity experienced during ultrasonic scaling. Overall, treatment time is also reduced;
- A second plaque disclosure provides quality control and assurance to the patient as well as the operator.
9. Limitations and Future Recommendation
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and perio-dontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and peri-odontal disease. J. Clin. Periodontol. 2017, 44, S5–S11. [Google Scholar] [CrossRef]
- Meto, A.; Colombari, B.; Castagnoli, A.; Sarti, M.; Denti, L.; Blasi, E. Efficacy of a Copper–Calcium–Hydroxide Solution in Reducing Microbial Plaque on Orthodontic Clear Aligners: A Case Report. Eur. J. Dent. 2019, 13, 478–484. [Google Scholar] [CrossRef] [Green Version]
- Lasserre, J.F.; Brecx, M.C.; Toma, S. Oral Microbes, Biofilms and Their Role in Periodontal and Peri-Implant Diseases. Materials 2018, 11, 1802. [Google Scholar] [CrossRef] [Green Version]
- Lindhe, J.; Westfelt, E.; Nyman, S.; Socransky, S.S.; Haffajee, A.D. Long-term effect of surgical/non-surgical treatment of periodontal disease. J. Clin. Periodontol. 1984, 11, 448–458. [Google Scholar] [CrossRef]
- Sultan, D.A.; Hill, R.G.; Gillam, D.G. Air-polishing in subgingival root debridement: A critical literature review. J. Dent. Oral Biol. 2017, 2, 1065. Available online: https://www.gavinpublishers.com/article/view/air-polishing-in-subgingival-root-debridement-during-supportive-periodontal-care-a-review (accessed on 15 August 2021).
- Rabbani, G.M.; Ash, M.M., Jr.; Caffesse, R.G. The effectiveness of subgingival scaling and root planing in calculus removal. J. Periodontol. 1981, 52, 119–123. [Google Scholar] [CrossRef]
- Eaton, K.A.; Kieser, J.B.; Davies, R.M. The removal of root surface deposits. J. Clin. Periodontol. 1985, 12, 141–152. [Google Scholar] [CrossRef]
- Mensi, M.; Scotti, E.; Sordillo, A.; Agosti, R.; Calza, S. Plaque disclosing agent as a guide for professional biofilm removal: A randomized controlled clinical trial. Int. J. Dent. Hyg. 2020, 18, 285–294. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Zhang, C.F.; Samaranayake, L.P. Dental plaque biofilm in oral health and disease. Chin. J. Dent. Res. 2011, 14, 87–94. Available online: https://europepmc.org/article/MED/22319749 (accessed on 15 August 2021).
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44 (Suppl. 18), S12–S22. [Google Scholar] [CrossRef]
- Nimbulkar, G.; Garacha, V.; Shetty, V.; Bhor, K.; Srivastava, K.C.; Shrivastava, D.; Sghaireen, M.G. Microbiological and Clinical evaluation of Neem gel and Chlorhexidine gel on Dental Plaque and Gingivitis in 20–30 Years Old Adults: A Randomized Parallel-Armed, Double-Blinded Controlled Trial. J. Pharm. Bioallied. Sci. 2020, 12 (Suppl. 1), S345–S351. [Google Scholar] [CrossRef]
- Larsen, T.; Fiehn, N.E. Dental biofilm infections-an update. APMIS 2017, 125, 376–384. [Google Scholar] [CrossRef]
- Rode Sde, M.; Gimenez, X.; Montoya, V.C.; Gómez, M.; Blanc, S.L.; Medina, M.; Salinas, E.; Pedroza, J.; Zaldivar-Chiapa, R.M.; Pannuti, C.M.; et al. Daily biofilm control and oral health: Consensus on the epidemiological challenge-Latin American Advisory Panel. Braz. Oral Res. 2012, 26 (Suppl. 1), 133–143. [Google Scholar] [CrossRef]
- Berger, D.; Rakhamimova, A.; Pollack, A.; Loewy, Z. Oral Biofilms: Development, Control, and Analysis. High Throughput 2018, 7, 24. [Google Scholar] [CrossRef]
- Shrivastava, D.; Srivastava, K.C.; Ganji, K.K.; Alam, M.K.; Al Zoubi, I.; Sghaireen, M.G. Quantitative Assessment of Gingival Inflammation in Patients Undergoing Nonsurgical Periodontal Therapy Using Photometric CIELab Analysis. BioMed Res. Int. 2021, 30, 2021. [Google Scholar] [CrossRef]
- Shrivastava, D.; Srivastava, K.C.; Dayakara, J.K.; Sghaireen, M.G.; Gudipaneni, R.K.; Al-Johani, K.; Baig, M.N.; Khurshid, Z. BactericidalActivity of Crevicular Polymorphonuclear Neutrophils in Chronic Periodontitis Patients and Healthy Subjects under the Influence of Areca Nut Extract: An In Vitro Study. Appl. Sci. 2020, 10, 5008. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; Garcia-Sesnich, J.; Vernal, R.; Hernandez, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355. [Google Scholar] [CrossRef] [Green Version]
- Amano, A. Host-parasite interactions in periodontitis: Microbial pathogenicity and innate immunity. Periodontology 2000 2010, 54, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, M.; Kurihara, H. Molecular Mechanisms of Periodontal Disease. Int. J. Mol. Sci. 2021, 22, 930. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Dhir, S. Biofilm and dental implant: The microbial link. J. Indian Soc. Periodontol. 2013, 17, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Lang, N.P. Microbial aspects of implant dentistry. Periodontology 2000 1994, 4, 74–80. [Google Scholar] [CrossRef]
- Sahni, K.; Khashai, F.; Forghany, A.; Krasieva, T.; Wilder-Smith, P. Exploring Mechanisms of Biofilm Removal. Dentistry (Sunnyvale) 2016, 6, 371. [Google Scholar] [CrossRef]
- Fatima, T.; Khurshid, Z.; Rehman, A.; Imran, E.; Srivastava, K.C.; Shrivastava, D. Gingival Crevicular Fluid (GCF): A Diagnostic Tool for the Detection of Periodontal Health and Diseases. Molecules 2021, 26, 1208. [Google Scholar] [CrossRef]
- Ng, E.; Byun, R.; Spahr, A.; Divnic-Resnik, T. The efficacy of air polishing devices in supportive periodontal therapy: A systematic review and meta-analysis. Quintessence Int. 2018, 49, 453–467. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R. Supportive periodontal therapy. Periodontology 2000 2004, 36, 179–195. [Google Scholar] [CrossRef]
- Cortés-Acha, B.; Figueiredo, R.; Seminago, R.; Roig, F.J.; Llorens, C.; Valmaseda-Castellón, E. Microbiota Analysis of Biofilms on Experimental Abutments Mimicking Dental Implants: An In Vivo Model. J. Periodontol. 2017, 88, 1090–1104. [Google Scholar] [CrossRef] [PubMed]
- Schultz-Haudt, S.; Bruce, M.A.; Bibby, B.G. Bacterial factors in nonspecific gingivitis. J. Dent. Res. 1954, 33, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J. Clinical and microbiological aspects of chemotherapeutic agents used according to the specific plaque hypothesis. J. Dent. Res. 1979, 58, 2404–2412. [Google Scholar] [CrossRef] [Green Version]
- Meto, A.; Colombari, B.; Odorici, A.; Giva, L.B.; Pericolini, E.; Regina, A.L.; Blasi, E. Antibacterial Effects of MicroRepair® BIOMA-Based Toothpaste and Chewing Gum on Orthodontic Elastics Contaminated In Vitro with Saliva from Healthy Donors: A Pilot Study. Appl. Sci. 2020, 10, 6721. [Google Scholar] [CrossRef]
- Park, E.J.; Kwon, E.Y.; Kim, H.J.; Lee, J.Y.; Choi, J.; Joo, J.Y. Clinical and microbiological effects of the supplementary use of an erythritol powder air-polishing device in non-surgical periodontal therapy: A randomized clinical trial. J. Periodontal. Implant Sci. 2018, 48, 295–304. [Google Scholar] [CrossRef]
- Fleischer, H.C.; Mellonig, J.T.; Brayer, W.K.; Gray, J.L.; Barnett, J.D. Scaling and root planing efficacy in multirooted teeth. J. Periodontol. 1989, 60, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Wennberg, A.; Fischer, R.G.; Attström, R. Clinical evaluation of pulp and dentine sensitivity after supragingival and subgingival scaling. Endod. Dent. Traumatol. 1991, 7, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, G. Periodontal response to mechanical non-surgical therapy: A review. J. Periodontol. 1992, 63, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Boyd, L.D.; Mallonee, L.F.; Wyche, C.J.; Halaris, J.F. Wilkins’ Clinical Practice of the Dental Hygienist, 13th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2016. [Google Scholar]
- Montevecchi, M.; Checchi, V.; Gatto, M.R.; Klein, S.; Checchi, L. The use of a disclosing agent during resective periodontal surgery for improved removal of biofilm. Open Dent. J. 2012, 6, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Nepale, M.B.; Varma, S.; Suragimath, G.; Abbayya, K.; Zope, S.; Kale, V. A prospective case-control study to assess and compare the role of disclosing agent in improving the patient compliance in plaque control. J. Oral Res. Rev. 2014, 6, 45. [Google Scholar]
- Barrows, J.N.; Lipman, A.L.; Bailey, C.J. Color additives: FDA’s regulatory process and historical perspectives. Food Saf. Mag. 2003, 1. Available online: https://www.food-safety.com/articles/4207-color-additives-fdas-regulatory-process-and-historical-perspectives (accessed on 15 August 2021).
- Allam, K.V.; Kumar, G.P. Colorants-the cosmetics for the pharmaceutical dosage forms. Int. J. Pharm. Pharm. Sci. 2011, 3 (Suppl. 3), 9. [Google Scholar]
- Datta, D.; Kumar, S.R.; Narayanan, A.; Selvamary, A.L.; Sujatha, A. Disclosing solutions used in dentistry. World J. Pharm. Res. 2017, 6, 1648–1656. [Google Scholar] [CrossRef] [Green Version]
- Block, P.L.; Lobene, R.R.; Derdivanis, J.P. A two-tone dye test for dental plaque. J. Periodontol. 1972, 43, 423–426. [Google Scholar] [CrossRef]
- Checchi, L.; Forteleoni, G.; Pelliccioni, G.A.; Loriga, G. Plaque removal with variable instrumentation. J.Clin. Periodontol. 1997, 24, 715–717. [Google Scholar] [CrossRef]
- Tan, A.E.; Wade, A.B. The role of visual feedback by a disclosing agent in plaque control. J. Clin. Periodontol. 1980, 7, 140–148. [Google Scholar] [CrossRef]
- Petersilka, G.J. Subgingival air-polishing in the treatment of periodontal biofilm infections. Periodontology 2000 2011, 55, 124–142. [Google Scholar] [CrossRef] [PubMed]
- Petersilka, G.J.; Schenck, U.; Flemmig, T.F. Powder emission rates of four air polishing devices. J. Clin. Periodontol. 2002, 29, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Donnet, M.; Fournier, M.; Schmidlin, P.R.; Lussi, A. A Novel Method to Measure the Powder Consumption of Dental Air-Polishing Devices. Appl. Sci. 2021, 11, 1101. [Google Scholar] [CrossRef]
- Prof, Û.; Nardi, G.M. Système d’aéro-POLISSAGE COMBI Touch. Available online: www.mectron.fr ou mectronfrance@mectron.fr (accessed on 15 August 2021).
- Momber, A.; Kovacevic, R. Principles of Abrasive Water Jet Machining; 9.6; Springer: New York, NY, USA, 1998. [Google Scholar]
- Barnes, C.M. The management of aerosols with airpolishing delivery systems. J. Dent. Hyg. 1991, 65, 280–282. [Google Scholar] [PubMed]
- Barnes, C.M. An In-Depth Look at Air Polishing; University of Nebraska Medical Center: Omaha, NE, USA, 2010. [Google Scholar]
- Petersilka, G.J.; Bell, M.; Mehl, A.; Hickel, R.; Flemmig, T.F. Root defects following air polishing. J. Clin. Periodontol. 2003, 30, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Conserva, E.; Pisciotta, A.; Bertoni, L.; Bertani, G.; Meto, A.; Colombari, B.; Blasi, E.; Bellini, P.; de Pol, A.; Consolo, U.; et al. Evaluation of biological response of STRO-1/c-Kit enriched human dental pulp stem cells to titanium surfaces treated with two different cleaning systems. Int. J. Mol. Sci. 2019, 20, 1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meto, A.; Conserva, E.; Liccardi, F.; Colombari, B.; Consolo, U.; Blasi, E. Differential efficacy of two dental implant decontamination techniques in reducing microbial biofilm and re-growth onto titanium disks in vitro. Appl. Sci. 2019, 9, 3191. [Google Scholar] [CrossRef] [Green Version]
- Munro, I.C.; Berndt, W.O.; Borzelleca, J.F.; Flamm, G.; Lynch, B.S.; Kennepohl, E.; Bär, E.A.; Modderman, J. Erythritol: An interpretive summary of biochemical, metabolic, toxicological and clinical data. Food Chem. Toxicol. 1998, 36, 1139–1174. [Google Scholar] [CrossRef]
- Hashino, E.; Kuboniwa, M.; Alghamdi, S.A.; Yamaguchi, M.; Yamamoto, R.; Cho, H.; Amano, A. Erythritol alters microstructure and metabolomic profiles of biofilm composed of Streptococcus gordonii and Porphyromonasgingivalis. Mol. Oral Microbiol. 2013, 28, 435–451. [Google Scholar] [CrossRef]
- Caygur, A.; Albaba, M.R.; Berberoglu, A.; Yilmaz, H.G. Efficacy of glycine powder air-polishing combined with scaling and root planing in the treatment of periodontitis and halitosis: A randomized clinical study. J. Int. Med. Res. 2017, 45, 1168–1174. [Google Scholar] [CrossRef]
- Hägi, T.T.; Hofmänner, P.; Salvi, G.E.; Ramseier, C.A.; Sculean, A. Clinical outcomes following subgingival application of a novel erythritol powder by means of air polishing in supportive periodontal therapy: A randomized, controlled clinical study. Quintessence Int. 2013, 44, 753–761. [Google Scholar] [CrossRef]
- Müller, N.; Moëne, R.; Cancela, J.A.; Mombelli, A. Subgingival air-polishing with erythritol during periodontal maintenance: Randomized clinical trial of twelve months. J. Clin. Periodontol. 2014, 41, 883–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhardt, B.; Klocke, A.; Neering, S.H.; Selbach, S.; Peters, U.; Flemmig, T.F.; Beikler, T. Microbiological dynamics of red complex bacteria following full-mouth air polishing in periodontally healthy subjects-a randomized clinical pilot study. Clin. Oral Investig. 2019, 23, 3905–3914. [Google Scholar] [CrossRef]
- Jentsch, H.F.R.; Flechsig, C.; Kette, B.; Eick, S. Adjunctive air-polishing with erythritol in nonsurgical periodontal therapy: A randomized clinical trial. BMC Oral Health 2020, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Hägi, T.T.; Hofmänner, P.; Eick, S.; Donnet, M.; Salvi, G.E.; Sculean, A.; Ramseier, C.A. The effects of erythritol air-polishing powder on microbiologic and clinical outcomes during supportive periodontal therapy: Six-month results of a randomized controlled clinical trial. Quintessence Int. 2015, 46, 31–41. [Google Scholar] [CrossRef]
- Tsang, Y.C.; Corbet, E.F.; Jin, L.J. Subgingival glycine powder air-polishing as an additional approach to nonsurgical periodontal therapy in subjects with untreated chronic periodontitis. J. Periodontal. Res. 2018, 53, 440–445. [Google Scholar] [CrossRef]
- Kargas, K.; Tsalikis, L.; Sakellari, D.; Menexes, G.; Konstantinidis, A. Pilot study on the clinical and microbiological effect of subgingival glycine powder air polishing using a cannula-like jet. Int. J. Dent. Hyg. 2015, 13, 161–169. [Google Scholar] [CrossRef]
- Flemmig, T.F.; Arushanov, D.; Daubert, D.; Rothen, M.; Mueller, G.; Leroux, B.G. Randomized controlled trial assessing efficacy and safety of glycine powder air polishing in moderate-to-deep periodontal pockets. J. Periodontol. 2012, 83, 444–452. [Google Scholar] [CrossRef]
- Wennström, J.L.; Dahlén, G.; Ramberg, P. Subgingival debridement of periodontal pockets by air polishing in comparison with ultrasonic instrumentation during maintenance therapy. J. Clin. Periodontol. 2011, 38, 820–827. [Google Scholar] [CrossRef]
- Petersilka, G.; Koch, R.; Vomhof, A.; Joda, T.; Harks, I.; Arweiler, N.; Ehmke, B. Retrospective analysis of the long-term effect of subgingival air polishing in supportive periodontal therapy. J. Clin. Periodontol. 2021, 48, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Janner, S.F.; Wittneben, J.G.; Brägger, U.; Ramseier, C.A.; Salvi, G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant. Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef]
- Lee, C.T.; Huang, Y.W.; Zhu, L.; Weltman, R. Prevalences of peri-implantitis and peri-implant mucositis: Systematic review and meta-analysis. J. Dent. 2017, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Zorzano, L.A.; Estefanía-Fresco, R.; Telletxea, O.; Bravo, M. Prevalence of peri-implant inflammatory disease in patients with a history of periodontal disease who receive supportive periodontal therapy. Clin. Oral. Implants Res. 2015, 26, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Hirooka, H.; Polyzois, I.; Kelekis-Cholakis, A.; Wang, H.L. Working Group 3. Diagnosis and non-surgical treatment of peri-implant diseases and maintenance care of patients with dental implants–Consensus report of working group 3. Int. Dent. J. 2019, 69, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Ramanauskaite, A.; Tervonen, T. The Efficacy of Supportive Peri-Implant Therapies in Preventing Peri-Implantitis and Implant Loss: A Systematic Review of the Literature. J. Oral Maxillofac. Res. 2016, 7, e12. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, F.; Becker, K.; Bastendorf, K.D.; Cardaropoli, D.; Chatfield, C.; Dunn, I.; Fletcher, P.; Einwag, J.; Louropoulou, A.; Mombelli, A.; et al. Recommendations on the clinical application of air polishing for the management of peri-implant mucositis and peri-implantitis. Quintessence Int. 2016, 47, 293–296. [Google Scholar] [CrossRef]
- Solderer, A.; Pippenger, B.E.; Donnet, M.; Wiedemeier, D.; Ramenzoni, L.L.; Schmidlin, P.R. Evaluation of air polishing with a sterile powder and mechanical debridement during regenerative surgical periimplantitis treatment: A study in dogs. Clin. Oral Investig. 2021, 25, 2609–2618. [Google Scholar] [CrossRef]
- Menini, M.; Setti, P.; Dellepiane, E.; Zunino, P.; Pera, P.; Pesce, P. Comparison of biofilm removal using glycine air polishing versus sodium bicarbonate air polishing or hand instrumentation on full-arch fixed implant rehabilitations: A split-mouth study. Quintessence Int. 2019, 50, 722–730. [Google Scholar] [CrossRef]
- De Siena, F.; Corbella, S.; Taschieri, S.; Del Fabbro, M.; Francetti, L. Adjunctive glycine powder air-polishing for the treatment of peri-implant mucositis: An observational clinical trial. Int. J. Dent. Hyg. 2015, 13, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Lupi, S.M.; Granati, M.; Butera, A.; Collesano, V.; Rodriguez, Y.; Baena, R. Air-abrasive debridement with glycine powder versus manual debridement and chlorhexidine administration for the maintenance of peri-implant health status: A six-month randomized clinical trial. Int. J. Dent. Hyg. 2017, 15, 287–294. [Google Scholar] [CrossRef]
- John, G.; Sahm, N.; Becker, J.; Schwarz, F. Nonsurgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine. Twelve-month follow-up of a prospective, randomized, controlled clinical study. Clin. Oral Investig. 2015, 19, 1807–1814. [Google Scholar] [CrossRef]
- Ji, Y.J.; Tang, Z.H.; Wang, R.; Cao, J.; Cao, C.F.; Jin, L.J. Effect of glycine powder air-polishing as an adjunct in the treatment of peri-implant mucositis: A pilot clinical trial. Clin. Oral Implants Res. 2014, 25, 683–689. [Google Scholar] [CrossRef]
- Al Ghazal, L.; O’Sullivan, J.; Claffey, N.; Polyzois, I. Comparison of two different techniques used for the maintenance of peri-implant soft tissue health: A pilot randomized clinical trial. Acta Odontol. Scand. 2017, 75, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Sahm, N.; Becker, J.; Santel, T.; Schwarz, F. Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: A prospective, randomized, controlled clinical study. J. Clin. Periodontol. 2011, 38, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Persson, G.R.; Roos-Jansåker, A.M.; Lindahl, C.; Renvert, S. Microbiologic results after non-surgical erbium-doped:yttrium, aluminum, and garnet laser or air-abrasive treatment of peri-implantitis: A randomized clinical trial. J. Periodontol. 2011, 82, 1267–1278. [Google Scholar] [CrossRef]
- Hentenaar, D.F.M.; De Waal, Y.C.M.; Stewart, R.E.; Van Winkelhoff, A.J.; Meijer, H.J.A.; Raghoebar, G.M. Erythritol airpolishing in the non-surgical treatment of peri-implantitis: A randomized controlled trial. Clin Oral Implants Res. 2021, 32, 840–852. [Google Scholar] [CrossRef]

| FEATURES | STANDARD NOZZLE | SUBGINGIVAL NOZZLE |
|---|---|---|
| USE | Supragingival and shallow subgingival (≥4 mm) | The United States Food and Drug Administration has approved these devices for subgingival use in periodontal pockets up to 5 mm in the U.S., and Health Canada has approved them for up to 10 mm in Canada. |
| TECHNIQUE | Position 3 mm away from the tooth and angled between 30–60° to labial surface of the anterior teeth. For the posterior teeth, it should be kept 80° for buccal surface and 90° for the occlusal surface. | Insert nozzle tip to the bottom of the pocket and pull the nozzle back 1 mm and later activate the spray. |
| MOTION | Move in a continuous half-circle “smiley face” motion progressing of the tooth (3–5 s) or up and down vertical stroke. | Move nozzle continuously in a vertical-incisal motion to cover the entire length until removed from pocket for about 5 s. |
| S. No | Author, Year | Objective | Subjects | Sample Size | Parameters | Outcome |
|---|---|---|---|---|---|---|
| 1 | Park, E.J. et al., 2018 [34] | Comparison of erythritol powder air-polishing device (EPAP) as a supplement to SRP therapy. | Human | Split mouth study design with twenty-one patients of moderate chronic periodontitis. | All patients received SRP (control) SRP+EPAP (test) on either jaw. Clinical and microbiological parameters were examined before treatment, 1 and 3 months post treatment. | Clinical parameters showed no significant difference between groups. However, counts of P. gingivalis were significantly lower in the test group at 1 month follow up period. Both parameters deteriorated at 3 months. |
| 2 | Caygur, A. et al., 2017 [59] | Comparison of glycine powder air-polishing (GPAP)combined with SRP in the treatment of periodontitis and halitosis. | Human | Randomized clinical trial with sixty chronic periodontitis patients. | Patients were randomly allocated into control (SRP) and test group (SRP + GPAP). Clinical parameters were recorded at baseline and 1 month post treatment; also, the volatile sulphur compounds at baseline, immediately after treatment, and at 7, 14, and 30 days. | Clinical parameters were significantly reduced in both groups. The volatile sulphur compounds (VSCs) were significantly different at 1 month compared with baseline in both groups. GPAP has no additional benefit and is shown equally effective. |
| 3 | Hägi, T.T. et al., 2013 [60] | Comparison of erythritol powder by means of an air-polishing (EPAP) device and of (SRP) during SPT up to 3 months. | Human | Randomized clinical trial with forty patients on SPT, after completion of active treatment of moderate or severe periodontitis. | Patients were randomly assigned to control and test group. Clinical parameters such as plaque indices, BOP, PPD, and CAL were recorded at baseline and at 3 months. Patient’s comfort using a visual analog scale was also recorded. | All clinical parameters showed non-significant improvement. However, patients in test group showed significantly lower visual analogue scale (VAS) scores. |
| 4 | Müller, N. et al., 2014 [61] | Comparison of repeated subgingival air-polishing with a new erythritol powder containing 0.3% chlorhexidine with conventional ultrasonic debridement over 12 months. | Human | Randomized, parallel arm clinical trial with fifty patients on SPT. | Fifty patients were treated with subgingival air-polishing (test side) or ultrasonic debridement (control side) and were monitored at an interval of 3-month intervals up to 12 months. | Non-significant difference in clinical parameters was seen between the study groups. Test group showed significantly lesser count of A. actinomycetemcomitas at 12 months. |
| 5 | Reinhardt, B. et al., 2019 [62] | Comparison of periodontal pathogens of red complex after supragingival debridement (SD) with adjunctive full mouth (FM-GPAP) in periodontal healthy individuals. | Human | Randomized, split mouth study design with eighty-seven. | Subjects with 87 medically and periodontally healthy intraoral carriers of red complex bacteria were randomly assigned to receive SD with adjunctive FM-GPAP (test) or SD alone (control). Microbiological samples were obtained at baseline, and two, five, and nine days following intervention. | The count of red complex bacteria was significantly less in the test group in comparision to the control group following treatment and at day 9.However, the values were similar to baseline values when observed at 6 and 12 weeks. |
| 6 | Jentsch, H.F. et al., 2020 [63] | Comparison of adjunctive use of EPAPduring subgingival instrumentation (SI) with conventional NSPT. | Human | Randomized clinical trial with forty-two patients with moderate to severe periodontitis. | Patients were randomly assigned to control and test group receiving two different approaches of non-surgical periodontal therapy by SI, where test group additionally received EPAP. Clinical parameters, biomarkers and microorganism were measured at baseline, three and six months after SI. | Clinical parameters showed significant improvement at 2 and 6 months. However, test group showed more sites with PD ≥ 5 mm after six months. Significant reduction in the T. forsythia counts and T. denticola along with lesser values of matrix metalloprotienases -8 in the test group. |
| 7 | Hägi, T.T. et al., 2015 [64] | Clinical efficacy of low abrasive EPAP over a period of 6 months in patients undergoing SPT. | Human | Randomized clinical trial with forty chronic periodontitis patients. |
Patients were randomly assigned to control (SRP) and test group (subgingival EPAP). Clinical parameters were evaluated at baseline, 3, and 6 month intervals. Site considered for evaluation had BOP with PPD of ≥ 4 mm | A significant reduction of BOP, PPD and increase of CAL was observed between groups at 3 month intervals, but no significant difference at 6 months. No major change in periodontal pathogens recorded. |
| 8 | Tsang, Y.C. et al., 2018 [65] | Evaluation of GPAP as NSPT in subjects with chronic periodontitis. | Human | Randomized, split mouth study design with twenty-seven chronic periodontitis patients. | Patients received SRP and GPAP (test group) or SRP and air flushing with water (control group) at sites with PPD of ≥5 mm. Clinical parameters, gingival crevicular fluid(GCF) volumes, and the concentrations of interleukin-1β (IL-1β)and interleukin-1ra(IL-1ra) in GCF were measured at baseline and one, three, and six months after the intervention. | Significant improvements were recorded in clinical parameters in both groups. No significant difference in GCF levels of IL-1β and IL-1ra) were seen between the groups. |
| 9 | Kargas, K. et al., 2015 [66] | To evaluate the efficiency of subgingival GPAP during SPT. | Human | Randomized, split mouth study design with twenty-five chronic periodontitis patients. | Patients were randomly allocated to group receiving SRP with hand instruments, GPAP, subgingival ultrasonic debridement (UD), and no subgingival treatment (NT). Clinical parameters were recorded at baseline, three, and sixmonths. Subgingival samples were taken for microbiological analysis. | Clinically and microbiologicaly GPAP has no additional benefits over SRP or subgingival ultrasonic scaling. |
| 10 | Flemmig, T.F. et al., 2012 [67] | Comparison of supragingivally (GPAP) with conventional SRP in patients with in moderate-to-deep periodontal pockets. | Human | Randomized clinical trial with thirty patients with chronic periodontitis. | Patients were randomly allocated to received (FM- GPAP) or(SRP) followed by coronal polishing Patients rinsed with 0.12% chlorhexidine gluconate after debridement, and twice daily, for 2 weeks. | Test group showed significantly lesser total viable bacterial counts in chronic periodontitis patients when compared to SRP immediately after debridement and at the tenth day. |
| 11 | Wennström, J.L. et al., 2011 [68] | Comparison of subgingival air polishing (AP) compared with UD during SPT. | Human | Randomized, split mouth study design with twenty patients on SPT | Patients were randomly assigned two different subgingival debridement treatment groups—GPAP specially designed nozzle (test) and ultrasonic instrumentation (control). Clinical parameters and microbiological were recorded at baseline, fourteen, and sixtydays. | Results: both treatment procedures resulted in significant reductions in clinical parameters—BOP, PPD and relative attachment level at 2 months. Perceived treatment discomfort was less for AP than UD. |
| 12 | Solderer, A. et al. 2020 [76] | Comparison of mechanical debridement with/without air polishing on the healing of induced peri-implantitis. | Dogs | Non-randomized, animal study with forty-eight mandibular implants. | Depending on the study group, specific surgical cleaning approach is adopted along with augmentation procedure.
Histological measurements of the relative bone gain; depth of the defect, remaining bone, and soft tissue was measured. | Non-significant partial regeneration was observed in all treatment approaches. However, pre-treatment with air polishing showed less inflammation. |
| 13 | Menini et al., 2019 [77] | Comparison of the cleaning efficacy of GPAP against two different professional oral hygiene techniques on implants supporting full-arch fixed prostheses. | Human | Randomized, split mouth study design with thirty patients with a total of 32 implant fixed full arch rehabilitations in the maxilla and/or mandible (134 implants). | Patients randomly assigned by following a splitmouth method: all the patients received glycine air polishing (G) in one side of the arch (n = 32), and sodium bicarbonate air polishing (B) (n = 16) or manual scaling with carbon-fiber curette (C) (n = 16) was performed in the opposite side. After the hygiene procedures, plaque index and spontaneous bleeding were recorded. | Plaque index reduction was significantly more for group treated with GPAP and sodium bicarbonate air polishing compared to manual scaling. Group treated with sodium bicarbonate were having maximum spontaneous bleeding as compared to other groups. It was concluded that the professional oral hygiene on implants using GPAP showed better patient acceptance and cleaning. |
| 14 | Siena et al., 2015 [78] | Comparative evaluation of professional oral hygiene with or without the adjunct of GPAP for the treatment of peri-implant mucositis | Human | Non-randomized clinical trial on 30 patients with peri implant mucositis | 30 patients were allocated into two groups. first group received professional oral hygiene manoeuvres (POH) while in the test group, received the GPAP.PPD, bleeding index (BI) and plaque index (PI) were measured at baseline, three, and six months. | The present reports showed that both techniques were useful for the treatment of peri-implant mucositis. In the test group (with glycine powder), a significant reduction inprobing depth was observed. |
| 15 | Lupi, S.M. et al. 2017 [79] | The study evaluated the efficacy of maintenance treatment with glycine powder on the periodontal health of peri-implant tissues. | Human | Single-masked, randomized clinical intervention trial on 46 patients with partial or total edentulism with 88 implants. | 46 patients with 88 implants were randomly assigned into two groups treated with either an air abrasive with the (GPAP) or to a manual debridement and chlorhexidine administration treatment group (MDA). Clinical data were collected at 0, 3, and 6 month intervals. PI, BOP, PPD, CAL, and bleeding score (BS) were analyzed. | Within the limits of the study, treatment with glycine seems appropriate in the maintenance of peri-implant health and more effective than the traditional treatment with plastic curette and chlorhexidine. |
| 16 | John, G. et al., 2015 [80] | Evaluation of the effectiveness of an air-abrasive device (AAD) for non-surgical treatment of peri-implantitis. | Human | Prospective, parallel grouped, randomized controlled clinical trial on twenty five patients with initial to moderate peri-implantitis. | 25 patients, with initial to moderate peri-implantitis in one implant, underwent an oral hygiene program and were randomly treated using either AAD (amino acid glycine powder) or mechanical debridement using carbon curettes and MDA. Clinical parameters were measured at baseline and tweleve months. | The present study has indicated that both treatment procedures resulted in comparable but limited CAL gains at 12 months. Furthermore, it could be detected that AAD was associated with significantly higher BOP decrease than MDA. Thus, AAD seems to be better than MDA. |
| 17 | Ji, Y.J. et al., 2102 [81] | This pilot clinical trial evaluated the effect of GPAP as an adjunct in treating peri-implant mucositis. | Human | Randomized clinical trial with twenty-four patients with peri-implant mucositis. | Twenty-four peri-implant mucositis patients were randomly assigned to test (12 subjects with 17 implants) and control (12 subjects with 16 implants) groups. In the test group, the sites with PPD of 4 mm were additionally treated by GPAP for 5 sec. Clinical parameters were measured at 1-week, one-month, and three-month recall visits. | At the 3-month visit, there was no significant difference existing between two groups in probing depth. This pilot clinical trial suggests that NSPT may be beneficial for treatment of peri-implant mucositis. However, adjunctive GPAP treatment seems to have a minimal beneficial effect. |
| 18 | Al Ghazal, L. et al., 2017 [82] | Comparing the two different methods of debridement for improving peri-implant soft tissue health for a follow up period of 12 months. | Human | Randomized, single blinded, parallel group clinical trial with twenty patients (25 implants. | 20 patients with no signs of pathologic bone loss around implants (25 implants) were selected. Patients were scheduled to be reviewed at 0, 3, 6, 9, and 12 months. Nine patients (15 implants) were randomly allocated to a test group (Air-FlowVR Perio, EMS) (AFP) and control group comprised of nine patients (10 implants) which were treated with titanium curettes (TC). Peri-implant GCF samples were analyzed to quantitatively measure the concentration of six interleukins. | The present study showed that both the treatment methods were effective in reducing perimplant inflammation with no difference in clinical parameter such as BOP. The present study showed a significant relationship between IL-6 and BOP. |
| 19 | Sahm, H. et al., 2011 [83] | To evaluate the effectiveness of an AAD for NST of peri-implantitis. | Human | Prospective, parallel group designed, randomized controlled clinical study with 30 patients of initial to moderate peri- implantitis. | Thirty patients, each of whom displayed at least one implant with initial to moderate peri-implantitis, were enrolled in an oral hygiene program (OHP) and randomly instrumented using either (1) AAD or (2) mechanical debridement using carbon curets and MDA. Clinical parameters were measured at baseline, 3, and 6 months after treatment [e.g., BOP, PPD, CAL]. | The present study concluded that both treatment procedures resulted in comparable but limited CAL gains at 6 months, and OHP+AAD was associated with significantly higher BOP reductions than OHP+MDA. |
| 20 | Persson, G.R.,et al., 2011 [84] | Clinical and microbiological NST of peri-implantitis lesions using either an erbium-doped: yttrium, aluminum, and garnet (Er:YAG) laser or an air-abrasive subgingival polishing method. | Human | Non-randomized clinical trial with 42 patients with peri-implantitis. | 42 patients with peri-implantitis were treated at one time with an Er:YAG laser or an air-abrasive device. Baseline and 6-month intraoral radiographs were assessed with a software program. The checkerboard DNA–DNA hybridization method was used to assess 74 bacterial species from the site with the deepest probing depth (PD) at the implant. | Non-significant probing depth reduction was seen in both the groups. No baseline differences in bacterial counts between groups were found. In the air-abrasive group, Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus anaerobius were found at lower counts at 1 month after therapy. Six-month data demonstrated that both methods failed to reduce bacterial counts. |
| 21 | Hentenaar, D.F. et al., 2021 [85] | Comparison of erythritol air polishing with piezoelectric ultrasonic scaling in the non-surgical treatment of peri-implantitis. | Human | Randomized clinical trial with eight patients of peri-implantitis having 139 implants. | 80 patients (n = 139 implants) with peri-implantitis PPD ≥5 mm, marginal bone loss (MBL) ≥2 mm as compared to bone level at implant placement, bleeding, and/or suppuration on probing (BOP/SOP)) were randomly allocated to EPAP or ultrasonic treatment. Clinical outcome and pain/discomfort VAS were measure at 0, 3,6,9,12 months. | Three months after therapy, no significant difference in mean BOP, plaque score, PPD, MBL between the EPAP and ultrasonic group. Pain/discomfort was low in both groups. EPAP seems as effective as piezoelectric ultrasonic scaling in the NST of peri-implantitis. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrivastava, D.; Natoli, V.; Srivastava, K.C.; Alzoubi, I.A.; Nagy, A.I.; Hamza, M.O.; Al-Johani, K.; Alam, M.K.; Khurshid, Z. Novel Approach to Dental Biofilm Management through Guided Biofilm Therapy (GBT): A Review. Microorganisms 2021, 9, 1966. https://doi.org/10.3390/microorganisms9091966
Shrivastava D, Natoli V, Srivastava KC, Alzoubi IA, Nagy AI, Hamza MO, Al-Johani K, Alam MK, Khurshid Z. Novel Approach to Dental Biofilm Management through Guided Biofilm Therapy (GBT): A Review. Microorganisms. 2021; 9(9):1966. https://doi.org/10.3390/microorganisms9091966
Chicago/Turabian StyleShrivastava, Deepti, Valentino Natoli, Kumar Chandan Srivastava, Ibrahim A Alzoubi, Ahmed Ismail Nagy, May Othman Hamza, Khalid Al-Johani, Mohammad Khursheed Alam, and Zohaib Khurshid. 2021. "Novel Approach to Dental Biofilm Management through Guided Biofilm Therapy (GBT): A Review" Microorganisms 9, no. 9: 1966. https://doi.org/10.3390/microorganisms9091966
APA StyleShrivastava, D., Natoli, V., Srivastava, K. C., Alzoubi, I. A., Nagy, A. I., Hamza, M. O., Al-Johani, K., Alam, M. K., & Khurshid, Z. (2021). Novel Approach to Dental Biofilm Management through Guided Biofilm Therapy (GBT): A Review. Microorganisms, 9(9), 1966. https://doi.org/10.3390/microorganisms9091966