Human Infections by Wohlfahrtiimonas chitiniclastica: A Mini-Review and the First Report of a Burn Wound Infection after Accidental Myiasis in Central Europe
Abstract
:1. Introduction
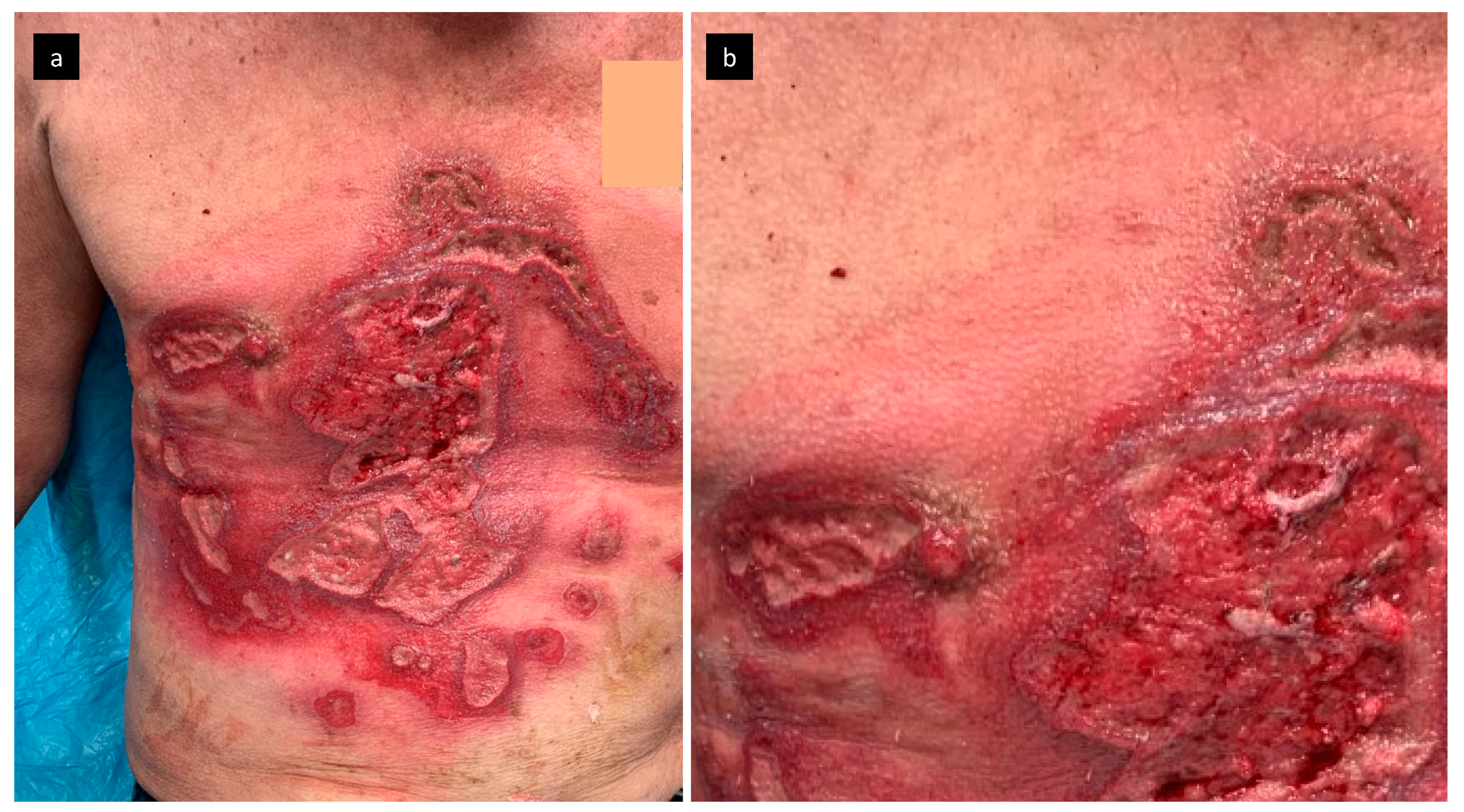
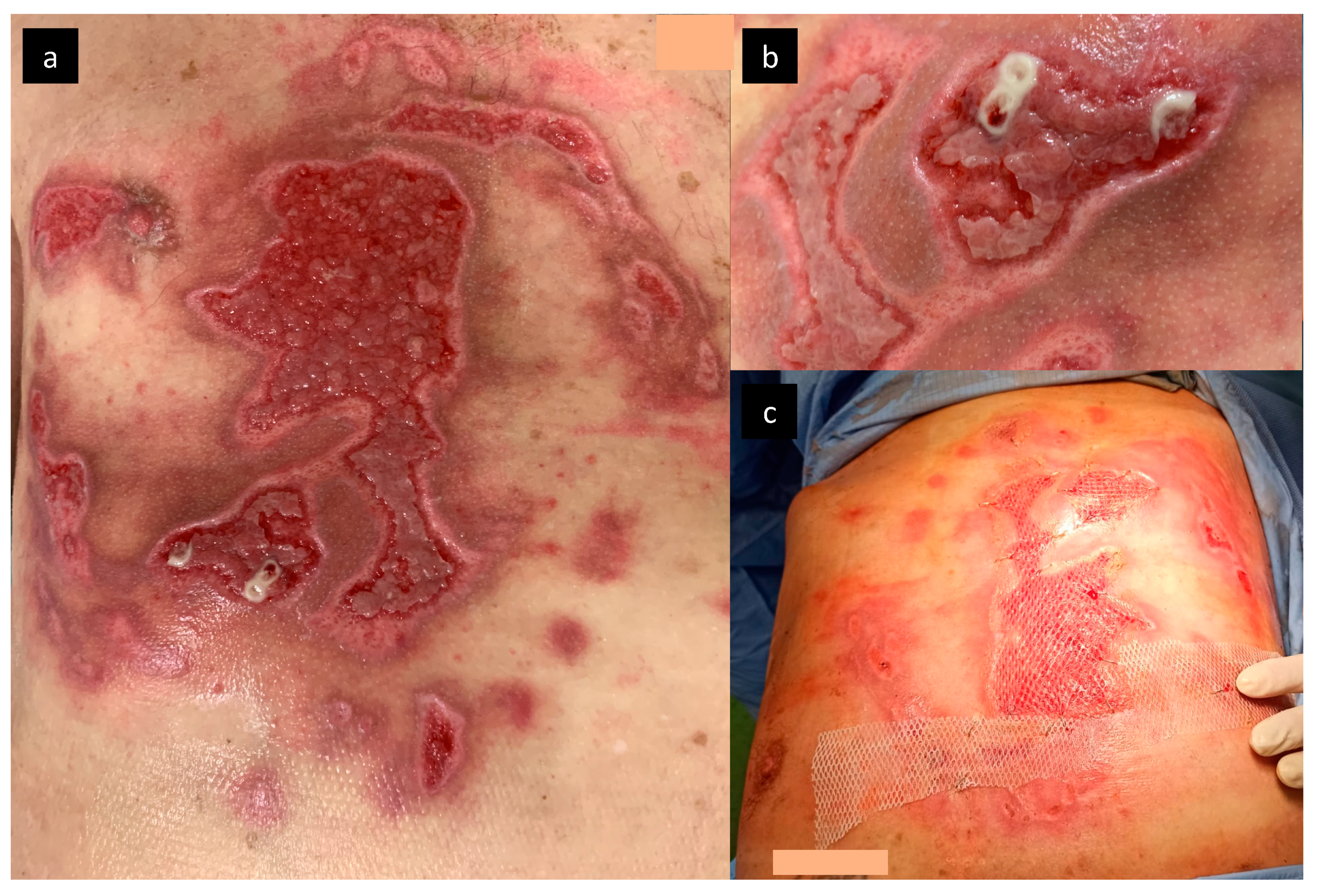
2. Case Report
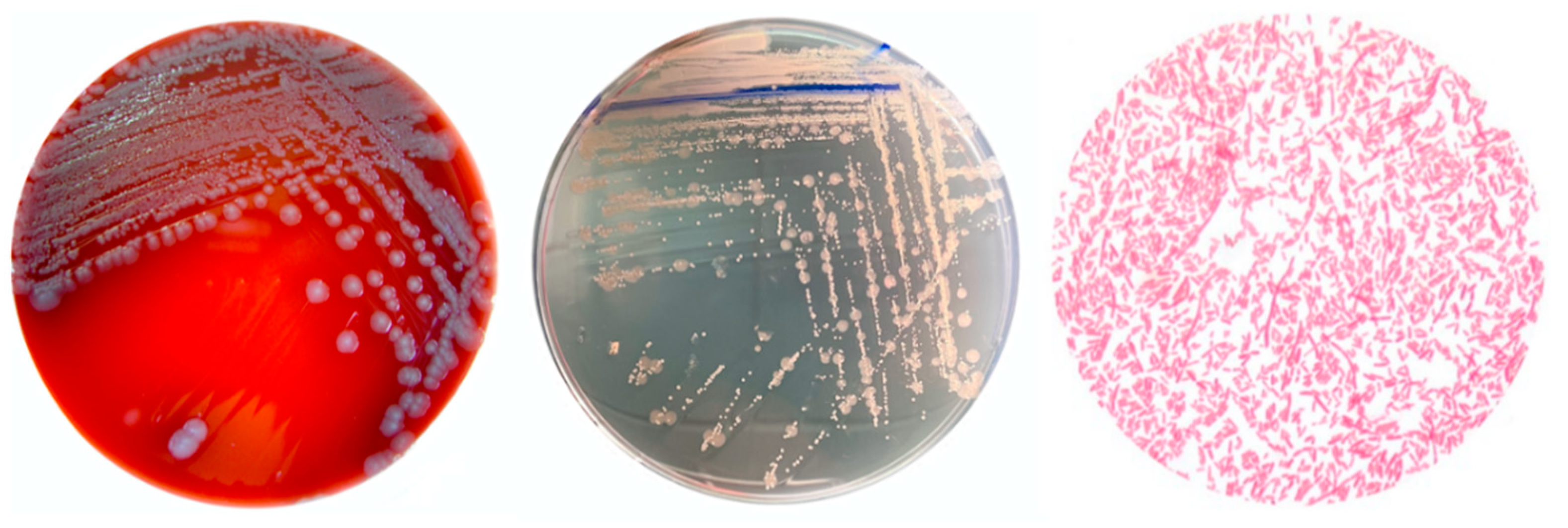
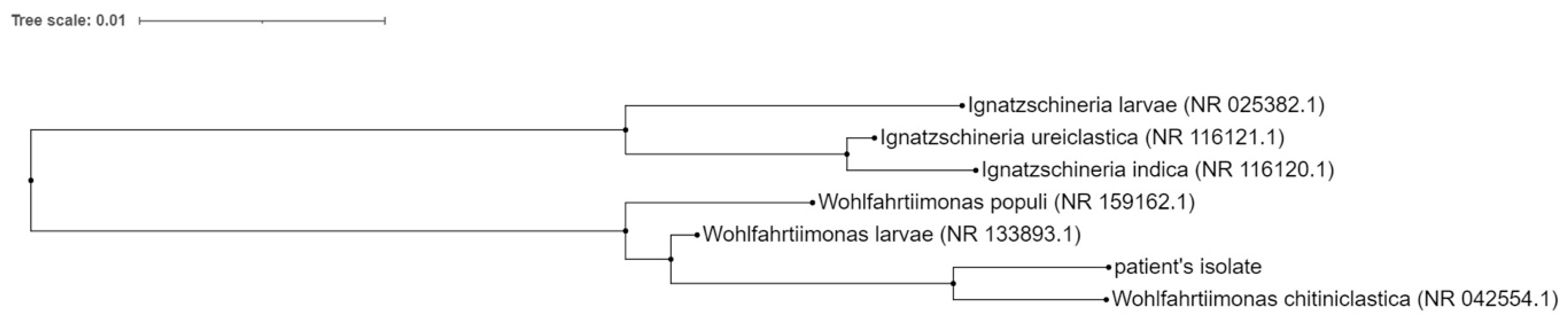
2.1. Pathogen Identification
2.2. Antimicrobial Susceptibility Profile of W. chitiniclastica
3. Review and Discussion
- Larvae should be removed from the wound (cutaneous myiasis); non-invasive removal of larvae is possible by occlusion or suffocation using petroleum jelly, beeswax, or liquid paraffin. Over the course of several hours, the larvae either leave the wound or die and can be removed more easily from the wound [33]. Surgical removal of larvae requires repeated debridement with an application of antiseptic agents. In the case of cavitary myiasis, surgical debridement, incision or even excision of the infected tissue may be necessary [6]. The larvae should be also preserved for potential future identification;
- Microbiological analysis focusing on the bacteria typically associated with accidental myiasis should be performed (e.g., Proteus mirabilis, Ignatzschineria indica, Providencia rettgeri, Morganella morganii, and Staphylococcus aureus). For the identification of rare pathogens, especially W. chitiniclastica, MALDI-TOF MS or 16S rRNA sequencing are recommended [17,22,24] because the use of the VITEK 2 system often leads to misclassification: for example, W. chitiniclastica was incorrectly classified as Acinetobacter lwoffii, Comamonas testosteroni or Rhizobium radiobacter [18,20,25]. As no data are available on the performance of other systems, a study evaluating the performance of other methods with respect to the identification of W. chitiniclastica can be valuable for clinical practice;
- The choice of antimicrobial therapy should be tailored to the overall microbiological findings; management should also be based on the severity of the infectious complication.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tóth, E.M.; Schumann, P.; Borsodi, A.; Kéki, Z.; Kovács, A.L.; Márialigeti, K. Wohlfahrtiimonas chitiniclastica gen. nov., sp. nov., a new gammaproteobacterium isolated from Wohlfahrtia magnifica (Diptera: Sarcophagidae). Int. J. Syst. Evol. Microbiol. 2008, 58, 976–981. [Google Scholar] [CrossRef] [Green Version]
- Rebaudet, S.; Genot, S.; Renvoise, A.; Fournier, P.-E.; Stein, A. Wohlfahrtiimonas chitiniclasticaBacteremia in Homeless Woman. Emerg. Infect. Dis. 2009, 15, 985–987. [Google Scholar] [CrossRef]
- Lipový, B.; Brychta, P.; Řihová, H.; Hanslianová, M.; Loskotová, A.; Jarkovský, J.; Kaloudová, Y.; Suchánek, I. Prevalence of Infectious Complications in Burn Patients Requiring Intensive Care: Data from a Pan-European Study. Epidemiol. Microbiol. Imunol. 2016, 65, 25–32. [Google Scholar]
- U.S. National Library of Medicine: National Center for Biotechnology Information. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome (accessed on 20 August 2021).
- Hope, F.W. On Insects and Thein Larva Occasionally Found in the Human Body, 2nd ed.; Transactions Royal Entomological Society: London, UK, 1840; pp. 256–271. [Google Scholar]
- Gour, S.; Ramesh, G.; Kumar, V.; Thapliyal, G.K.; Nagarajappa, R. Cavitary myiasis and its management. J. Exp. Ther. Oncol. 2018, 12, 211–216. [Google Scholar] [PubMed]
- Bazaliński, D.; Kózka, M.; Karnas, M.; Więch, P. Effectiveness of Chronic Wound Debridement with the Use of Larvae of Lucilia Sericata. J. Clin. Med. 2019, 8, 1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourgi, J.; Said, J.-M.; Yaakoub, C.; Atallah, B.; Al Akkary, N.; Sleiman, Z.; Ghanimé, G. Bacterial infection profile and predictors among patients admitted to a burn care center: A retrospective study. Burns 2020, 46, 1968–1976. [Google Scholar] [CrossRef]
- Norbury, W.; Herndon, D.N.; Tanksley, J.; Jeschke, M.G.; Finnerty, C.C. Infection in Burns. Surg. Infect. 2016, 17, 250–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn Wound Infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [Green Version]
- Suraiya, S.; Zuraina, N.; Ahmad, F.; Rahman, Z.A. Fatal Wohlfahrtiimonas chitiniclastica Bacteremia in an Immunocompromised Patient. Clin. Microbiol. Newsl. 2017, 39, 172–173. [Google Scholar] [CrossRef]
- Gupta, A.K.; Nayduch, D.; Verma, P.; Shah, B.; Ghate, H.V.; Patole, M.S.; Shouche, Y.S. Phylogenetic characterization of bacteria in the gut of house flies (Musca domestica L.). FEMS Microbiol. Ecol. 2012, 79, 581–593. [Google Scholar] [CrossRef]
- Matos, J.; Faria, A.R.; Assef, A.P.D.C.; de Freitas-Almeida, Â.C.; Albano, R.M.; Queiroz, M.L.P. Draft Genome Sequence of a Wohlfahrtiimonas chitiniclastica Strain Isolated from Frozen Chicken in Rio De Janeiro, Brazil. Microbiol. Resour. Announc. 2019, 8, e00352-19. [Google Scholar] [CrossRef] [Green Version]
- Sanyal, S.K.; Mou, T.J.; Chakrabarty, R.P.; Hoque, S.; Hossain, M.A.; Sultana, M. Diversity of arsenite oxidase gene and arsenotrophic bacteria in arsenic affected Bangladesh soils. AMB Express 2016, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Gao, Y.; Wang, G.-S.; Li, L.-B.; Li, L.-L.; Zhao, X.-M.; Du, Y.-J.; Liu, Y.-Q. Identification of Wohlfahrtiimonas chitiniclastica isolated from an infected cow with hoof fetlow, China. Infect. Genet. Evol. 2016, 41, 174–176. [Google Scholar] [CrossRef]
- Sulakova, H.; Gregor, F.; Ježek, J.; Tkoč, M. Nová Invaze Do Našich Obcí a Měst: Koutule Clogmia Albipunctata a Problematika Myiáz. Živa 2014, 1, 29–32. [Google Scholar]
- Almuzara, M.N.; Palombarani, S.; Tuduri, A.; Figueroa, S.; Gianecini, R.A.; Sabater, L.; Ramirez, M.S.; Vay, C.A. First Case of Fulminant Sepsis Due to Wohlfahrtiimonas chitiniclastica. J. Clin. Microbiol. 2011, 49, 2333–2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kõljalg, S.; Telling, K.; Huik, K.; Murruste, M.; Saarevet, V.; Pauskar, M.; Lutsar, I. First report of Wohlfahrtiimonas chitiniclastica from soft tissue and bone infection at an unusually high northern latitude. Folia Microbiol. 2015, 60, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Suryalatha, K.; John, J.; Thomas, S. Wohlfahrtiimonas chitiniclastica-associated osteomyelitis: A rare case report. Future Microbiol. 2015, 10, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- de Dios, A.; Jacob, S.; Tayal, A.; Fisher, M.A.; Dingle, T.C.; Hamula, C.L. First Report of Wohlfahrtiimonas Chitiniclastica Isolation from a Patient with Cellulitis in the United States. J. Clin. Microbiol. 2015, 53, 3942–3944. [Google Scholar] [CrossRef] [Green Version]
- Campisi, L.; Mahobia, N.; Clayton, J.J. Wohlfahrtiimonas chitiniclasticaBacteremia Associated with Myiasis, United Kingdom. Emerg. Infect. Dis. 2015, 21, 1068–1069. [Google Scholar] [CrossRef]
- Nogi, M.; Bankowski, M.J.; Pien, F.D. Wohlfahrtiimonas chitiniclastica Infections in 2 Elderly Patients, Hawaii, USA. Emerg. Infect. Dis. 2016, 22, 567–568. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, R.; Fortuin, F.; Newton-Foot, M.; Singh, S. First report of Wohlfahrtiimonas chitiniclastica bacteraemia in South Africa. S. Afr. Med. J. 2016, 106, 1062. [Google Scholar] [CrossRef] [Green Version]
- Schröttner, P.; Rudolph, W.W.; Damme, U.; Lotz, C.; Jacobs, E.; Gunzer, F. Wohlfahrtiimonas chitiniclastica: Current insights into an emerging human pathogen. Epidemiol. Infect. 2017, 145, 1292–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavez, J.A.; Alexander, A.J.; Balada-Llasat, J.M.; Pancholi, P. A case of Wohlfahrtiimonas chitiniclastica bacteremia in continental United States. JMM Case Rep. 2017, 4, e005134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lysaght, T.B.; Wooster, M.E.; Jenkins, P.C.; Koniaris, L.G. Myiasis-induced sepsis: A rare case report of Wohlfahrtiimonas chitiniclastica and Ignatzschineria indica bacteremia in the continental United States. Medicine 2018, 97, e13627. [Google Scholar] [CrossRef] [PubMed]
- Katanami, Y.; Kutsuna, S.; Nagashima, M.; Takaya, S.; Yamamoto, K.; Takeshita, N.; Hayakawa, K.; Kato, Y.; Kanagawa, S.; Ohmagari, N. Wohlfahrtiimonas chitiniclastica Bacteremia Hospitalized Homeless Man with Squamous Cell Carcinoma. Emerg. Infect. Dis. 2018, 24, 1746–1748. [Google Scholar] [CrossRef] [Green Version]
- Bonwitt, J.H.; Tran, M.; Dykstra, E.A.; Eckmann, K.; Bell, M.E.; Leadon, M.; Sixberry, M.; Glover, W.A. Fly Reservoir Associated with Wohlfahrtiimonas Bacteremia in a Human. Emerg. Infect. Dis. 2018, 24, 370–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connelly, K.L.; Freeman, E.; Smibert, O.C.; Lin, B. Wohlfahrtiimonas chitiniclastica bloodstream infection due to a maggot-infested wound in a 54-year-old male. J. Glob. Infect. Dis. 2019, 11, 125–126. [Google Scholar] [CrossRef]
- Fenwick, A.J.; Arora, V.; Ribes, J.A. Wohlfahrtiimonas chitiniclastica: Two Clinical Cases and a Review of the Literature. Clin. Microbiol. Newsl. 2019, 41, 33–38. [Google Scholar] [CrossRef]
- Snyder, S.; Singh, P.; Goldman, J. Emerging pathogens: A case of Wohlfahrtiimonas chitiniclastica and Ignatzschineria indica bacteremia. IDCases 2020, 19, e00723. [Google Scholar] [CrossRef]
- Bueide, P.; Hunt, J.; Bande, D.; Guerrero, D.M. Maggot Wound Therapy Associated with Wohlfahrtiimonas chitiniclastica Blood Infection. Cureus 2021, 13, e12471. [Google Scholar] [CrossRef]
- Geary, M.J.; Russell, R.C.; Hudson, B.J.; Hardy, A. Exotic myiasis with Lund’s fly (Cordylobia rodhaini). Med. J. Aust. 1999, 171, 654–655. [Google Scholar] [CrossRef] [PubMed]

| Antibiotic | MIC Value (mg/L) | Interpretation |
|---|---|---|
| Amoxicillin/clavulanic acid | <2.0 | S |
| Piperacillin/tazobactam | <4.0 | S |
| Cefotaxime | 1.0 | S |
| Ceftazidime | <0.5 | S |
| Cefepime | <0.5 | S |
| Ciprofloxacin | 0.25 | S |
| Levofloxacin | 0.5 | S |
| Ofloxacin | 0.25 | S |
| Imipenem | <1.0 | S |
| Meropenem | <0.5 | S |
| Year [Ref.] | Country | Sex/age Years | Culture Site | Anatomic Localization | Associated Myiasis | Antibiotics | Outcome | Associated Bacteria |
|---|---|---|---|---|---|---|---|---|
| 2009 [2] | France | F/60 | Blood culture | Scalp(head) | Yes | ceftriaxon | Survived | - |
| 2011 [17] | Argentina | M/70 | Blood culture | Inguinal regions | No | ciprofloxacin + ampicillin-sulbactam and later ceftazidime + amikacin | Died | |
| 2014 [18] | Estonia | M/64 | Resected bone | Right foot | No | amoxicillin-clavulonate | Survived | MYOD |
| 2015 [19] | India | M/43 | Ulcer swab | Right lower limb ulcer | No | cefoperazone-sulbactam followed by cefpodoxime (outpatient care) | Survived | |
| 2015 [20] | USA | M/26 | Ulcer swab | Right leg | No | cefpodoxime | Survived | PRVU, KLPN, MSSA |
| 2015 [21] | UK | F/82 | Blood culture | Excoriations of head, face, and neck | Yes | cefuroxime, metronidazole, clarithromycin, topical chloramphenicol, fucidic acid followed by flucloxacillin (outpatient care) | Survived | PRMI, PRRE, MSSA |
| 2016 [22] | USA | F/69 M/72 | F/Ulcer swab M/blood culture | F/Sacral decubitus M/Right foot and umbilical wound | F/No M/Yes | F/ceftaroline fosamil, meropenem, amoxicillin-clavulonate (outpatient care) M/piperacilline-tazobactam, vancomycin, clindamycin | F/Survived M/Died | F/Blood culture: ANSU Urine: PRMI Decubitus: MSSA, AEspp., STspp., BAFR M/ESCO |
| 2016 [23] | South Africa | M/17 | Blood culture | Right shoulder wound | No | ceftriaxone | Survived | |
| 2017 [24] | Germany | F/78 M/43 M/71 M/79 | F/78 Swabs from ulcers M/43 Swabs from ulcers M/71 Swabs from ulcers M/79 Swab from ulcer | F/78 Leg ulcer M/43 Leg ulcer M/71 Leg ulcer M/79 Leg ulcer | No No No No | F/78 no antibiotic treatment M/43 no antibiotic treatment M/71 no antibiotic treatment M/79 cefuroxime followed by levofloxacin and clindamycin | F/78 Survived M/43 Survived M/71 Survived M/79 Survived | F/78 PRMI, MSSA, SEMA, MOMO M/43 PRMI M/71 PRMI, PRST, PSAE M/79: ESCO |
| 2017 [25] | USA | F/41 | Blood culture and decubitus swab | Ischial decubitus or lower extremity bilateral excoriations | No | vancomycin, cefepim, metronidazole | Died due to other disease | Blood culture: PRMI Swab: MYIN, ENFA ENFA, BAspp. |
| 2017 [11] | Malaysia | F/47 | Blood culture | - | No | cefoperazone | Died due to other disease | |
| 2018 [26] | USA | M/37 | Blood culture | Left lower extremity ulcer | Yes | piperacilline-tazobactam, vancomycin, clindamycin later cefepime | Survived | PRST, IGIN |
| 2018 [27] | Japan | M/75 | Blood culture | Left shoulder lesion | Yes | vancomycin, cefepime, metronidazole | Survived | MOMO, STspp., BAspp., PRMI |
| 2018 [28] | USA | M/57 | Blood culture | Right ankle gangrene | Yes | Not stated | Died | PRAC, CNS |
| 2019 [29] | Australia | M/54 | Blood culture | Right foot wound | Yes | piperacilline-tazobactam + meropenem, ciprofloxacin (outpatient care) | Survived | MOMO |
| 2019 [30] | USA | M/63 F/87 | M/blood culture F/swab | M/Right foot ulcer F/Left lower extremity wound | Yes Yes | M/vancomycin+ piperacilin/tazobactam F/unknown | M/Died F/unknown | M/PRRE F/- |
| 2020 [31] | USA | M/82 | Blood culture | Right lower extremity | Yes | vancomycin and cefepim, later daptomycin and ceftriaxon | Survived | IGIN, MRSA |
| 2021 [32] | USA | M/70 | Blood culture | Ulcer of left temporal region | Yes | levofloxacin | Survived | MSSA, PRMI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hladík, M.; Lipovy, B.; Kaloudova, Y.; Hanslianova, M.; Vitkova, I.; Deissova, T.; Kempny, T.; Svoboda, M.; Kala, Z.; Brychta, P.; et al. Human Infections by Wohlfahrtiimonas chitiniclastica: A Mini-Review and the First Report of a Burn Wound Infection after Accidental Myiasis in Central Europe. Microorganisms 2021, 9, 1934. https://doi.org/10.3390/microorganisms9091934
Hladík M, Lipovy B, Kaloudova Y, Hanslianova M, Vitkova I, Deissova T, Kempny T, Svoboda M, Kala Z, Brychta P, et al. Human Infections by Wohlfahrtiimonas chitiniclastica: A Mini-Review and the First Report of a Burn Wound Infection after Accidental Myiasis in Central Europe. Microorganisms. 2021; 9(9):1934. https://doi.org/10.3390/microorganisms9091934
Chicago/Turabian StyleHladík, Martin, Bretislav Lipovy, Yvona Kaloudova, Marketa Hanslianova, Ivana Vitkova, Tereza Deissova, Tomas Kempny, Martin Svoboda, Zdenek Kala, Pavel Brychta, and et al. 2021. "Human Infections by Wohlfahrtiimonas chitiniclastica: A Mini-Review and the First Report of a Burn Wound Infection after Accidental Myiasis in Central Europe" Microorganisms 9, no. 9: 1934. https://doi.org/10.3390/microorganisms9091934
APA StyleHladík, M., Lipovy, B., Kaloudova, Y., Hanslianova, M., Vitkova, I., Deissova, T., Kempny, T., Svoboda, M., Kala, Z., Brychta, P., & Borilova Linhartova, P. (2021). Human Infections by Wohlfahrtiimonas chitiniclastica: A Mini-Review and the First Report of a Burn Wound Infection after Accidental Myiasis in Central Europe. Microorganisms, 9(9), 1934. https://doi.org/10.3390/microorganisms9091934






