Specific Changes in the Mammalian Gut Microbiome as a Biomarker for Oxytocin-Induced Behavioral Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Housing and Samples Collection
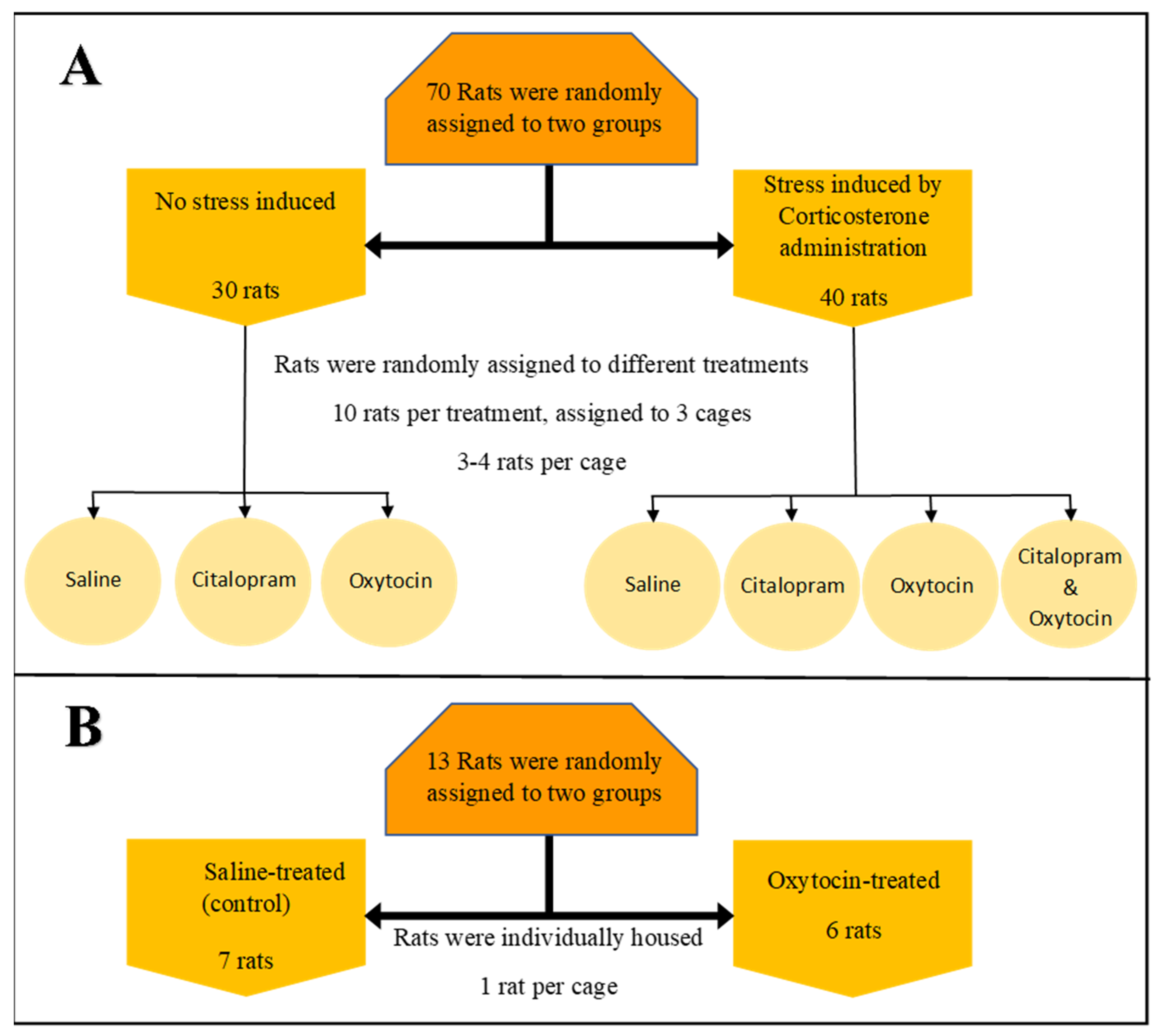
2.1.1. Multidrug Environment: Experimental Setup—Experiment 1
2.1.2. Single Drug Environment: Experimental Setup—Experiment 2
2.2. DNA Extraction and Microbiota Analysis
2.3. Behavioral Data
3. Results
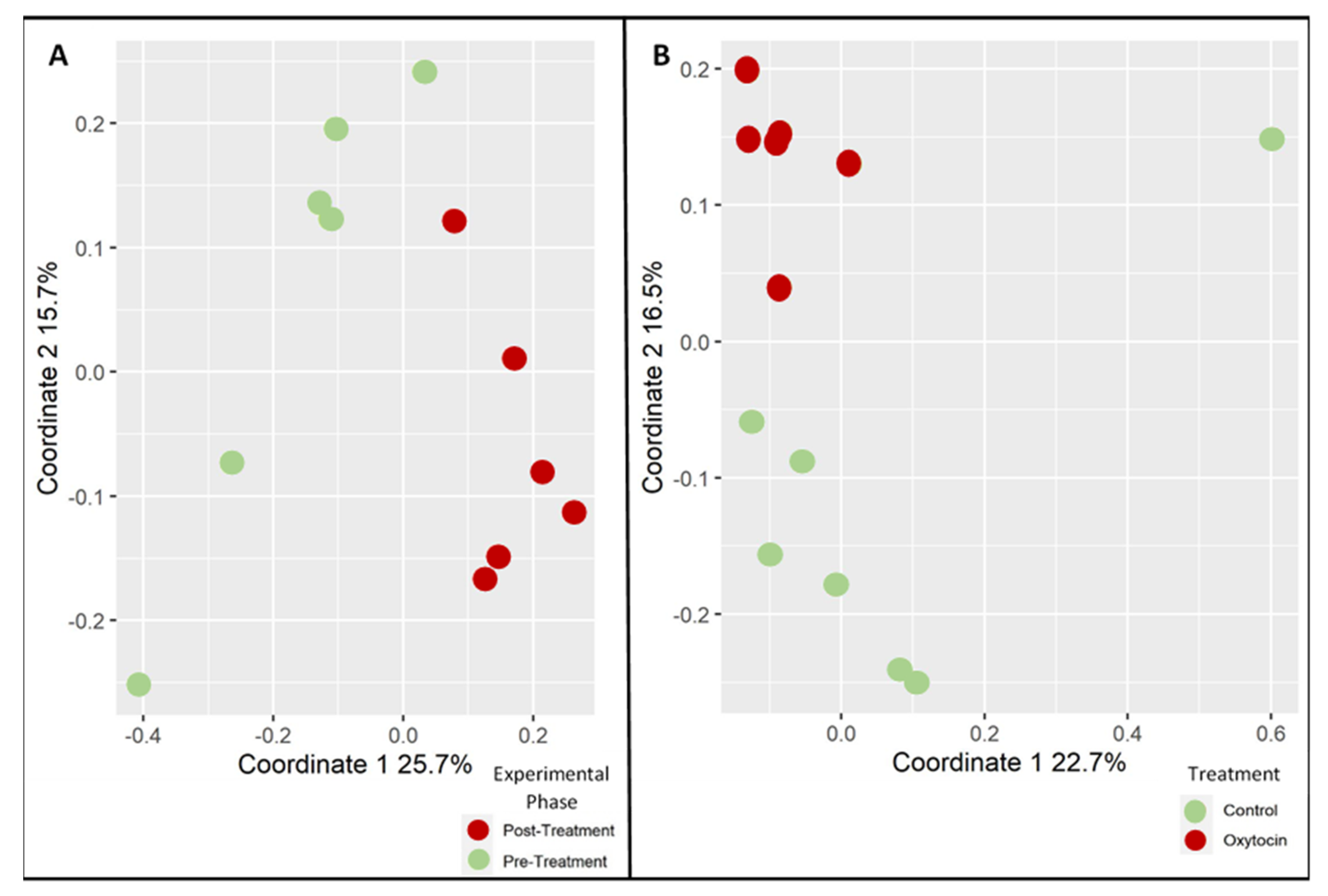
3.1. Oxytocin Has a Strong Impact on the Composition of Rat Fecal Microbiota
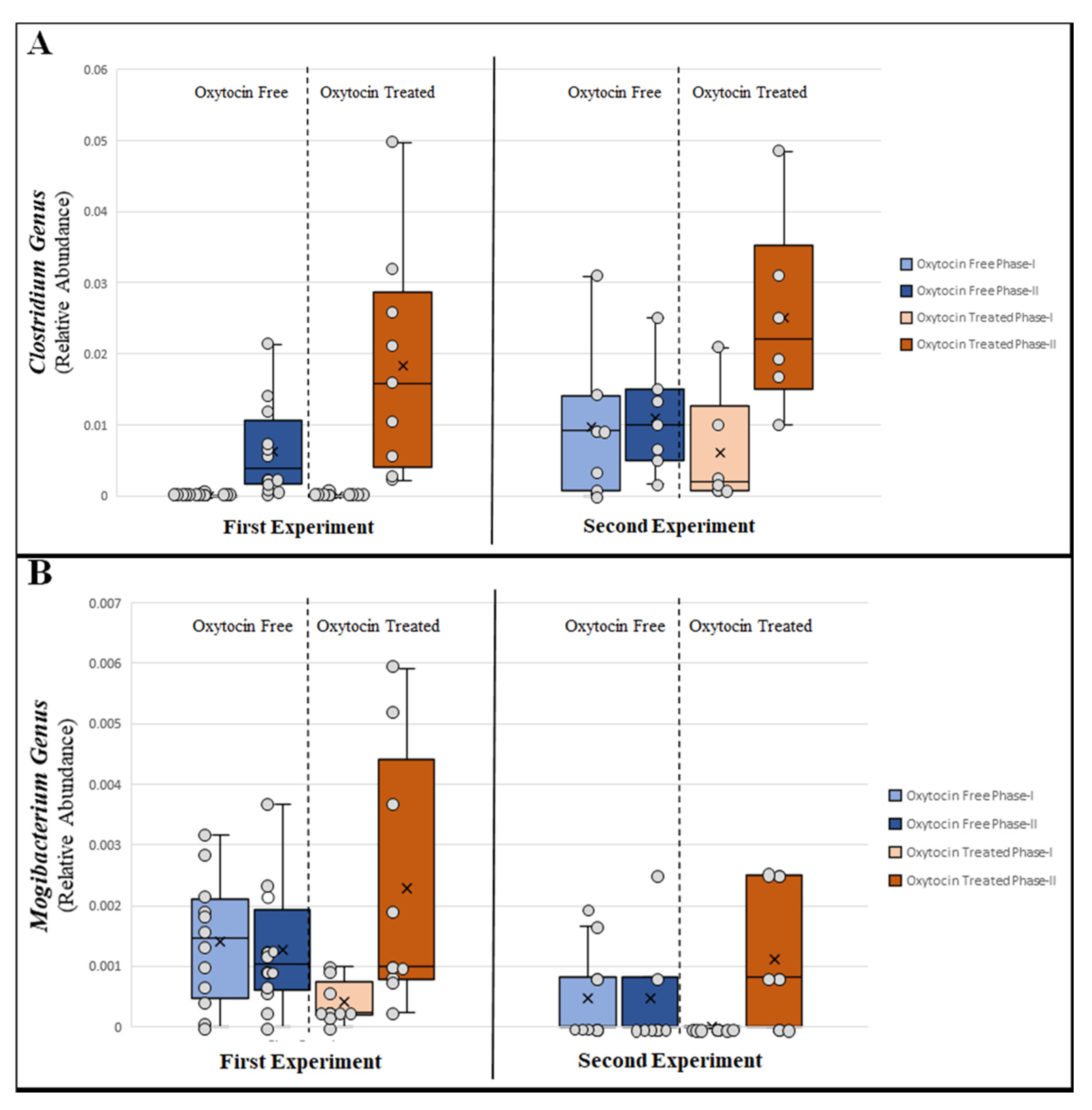
3.2. Changes in Relative Abundance of Specific Bacterial Taxa
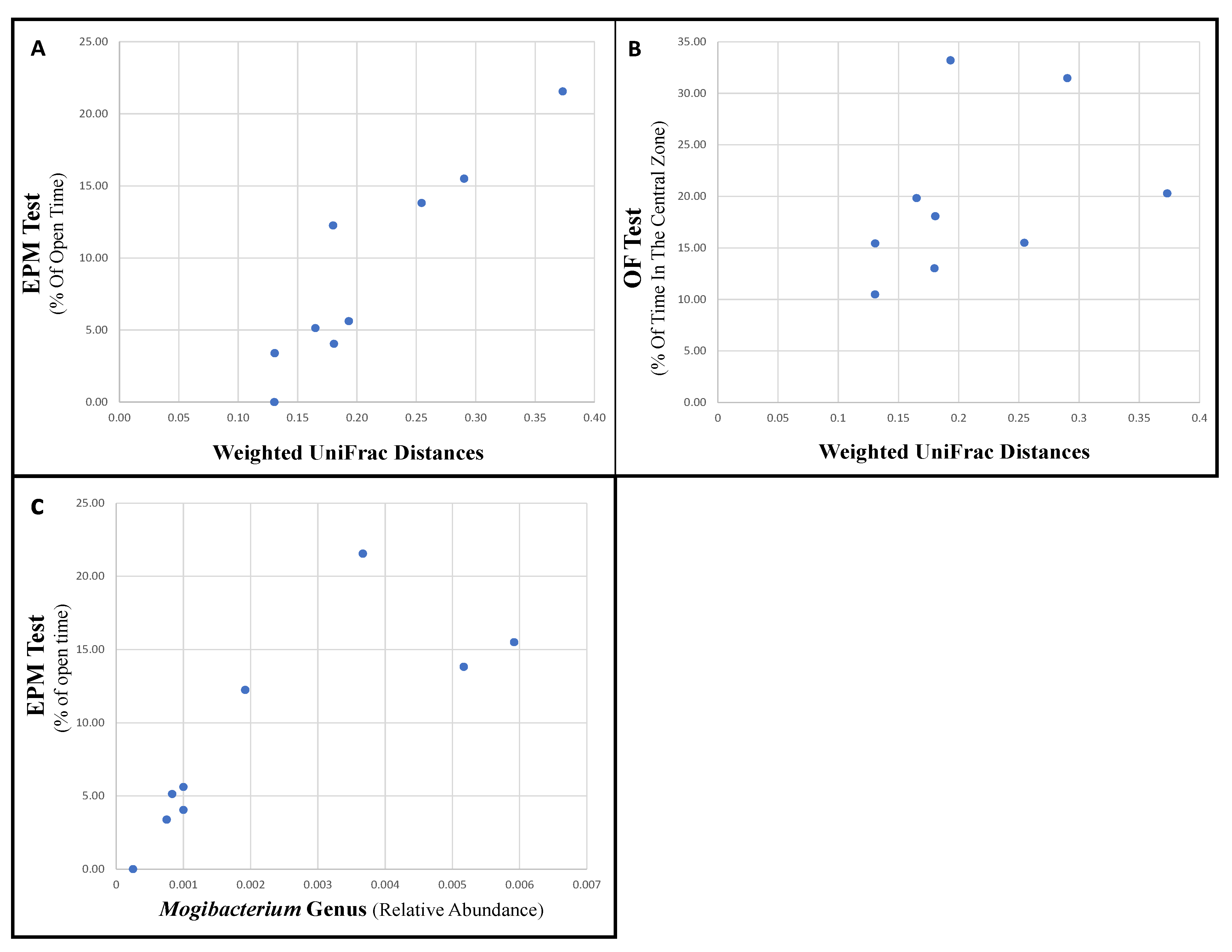
3.3. Specific Bacteria Are Associated with Changes in Behavior
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philpott, H.L.; Nandurkar, S.; Lubel, J.; Gibson, P.R. Republished: Drug-induced gastrointestinal disorders. Postgrad. Med. J. 2014, 90, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.L.; Nihalani, N.; Jindal, S.; Virk, S.; Jones, N. Psychiatric medication-induced obesity: A review. Obes. Rev. 2004, 5, 115–121. [Google Scholar] [CrossRef]
- Every-Palmer, S.; Inns, S.J.; Grant, E.; Ellis, P.M. Effects of Clozapine on the Gut: Cross-Sectional Study of Delayed Gastric Emptying and Small and Large Intestinal Dysmotility. CNS Drugs 2018, 33, 81–91. [Google Scholar] [CrossRef]
- DiMatteo, M.R.; Giordani, P.J.; Lepper, H.S.; Croghan, T.W. Patient Adherence and Medical Treatment Outcomes. Med. Care 2002, 40, 794–811. [Google Scholar] [CrossRef]
- Morris, L.S.; Schulz, R.M. Patient compliance-an overview. J. Clin. Pharm. Ther. 1992, 17, 283–295. [Google Scholar] [CrossRef]
- Lavsa, S.M.; Holzworth, A.; Ansani, N.T. Selection of a validated scale for measuring medication adherence. J. Am. Pharm. Assoc. 2011, 51, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Bickart, K.C.; Wright, C.I.; Dautoff, R.J.; Dickerson, B.C.; Barrett, L.F. Amygdala volume and social network size in humans. Nat. Neurosci. 2010, 14, 163–164. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.S.C.; Hatfield, R.A.; Suls, J.M.; Chamberlain, M.J. Psychological and Physical Stress Induce Differential Effects on Human Colonic Motility. Am. J. Gastroenterol. 1998, 93, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Taché, Y.; Martinez, V.; Million, M.; Wang, L. III. Stress-related alterations of gut motor function: Role of brain corticotropin-releasing factor receptors. Am. J. Physiol. Liver Physiol. 2001, 280, G173–G177. [Google Scholar] [CrossRef] [Green Version]
- Enck, P.; Holtmann, G. Stress and gastrointestinal motility in animals: A review of the literature. Neurogastroenterol. Motil. 2008, 4, 83–90. [Google Scholar] [CrossRef]
- Taché, Y.; Martinez, V.; Million, M.; Rivier, J. Corticotropin-Releasing Factor and the Brain-Gut Motor Response to Stress. Can. J. Gastroenterol. 1999, 13, 18A–25A. [Google Scholar] [CrossRef] [Green Version]
- Ochi, M.; Tominaga, K.; Tanaka, F.; Tanigawa, T.; Shiba, M.; Watanabe, T.; Fujiwara, Y.; Oshitani, N.; Higuchi, K.; Arakawa, T. Effect of chronic stress on gastric emptying and plasma ghrelin levels in rats. Life Sci. 2008, 82, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roager, H.M.; Hansen, L.B.S.; Bahl, M.I.; Frandsen, H.L.; Carvalho, V.; Gøbel, R.J.; Dalgaard, M.D.; Plichta, D.R.; Sparholt, M.H.; Vestergaard, H.; et al. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat. Microbiol. 2016, 1, 16093. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016, 65, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Galley, J.D.; Mackos, A.R.; Varaljay, V.A.; Bailey, M.T. Stressor exposure has prolonged effects on colonic microbial community structure in Citrobacter rodentium-challenged mice. Sci. Rep. 2017, 7, 45012. [Google Scholar] [CrossRef] [Green Version]
- Gautam, A.; Kumar, R.; Chakraborty, N.; Muhie, S.; Hoke, A.; Hammamieh, R.; Jett, M. Altered fecal microbiota composition in all male aggressor-exposed rodent model simulating features of post-traumatic stress disorder. J. Neurosci. Res. 2018, 96, 1311–1323. [Google Scholar] [CrossRef]
- Werbner, M.; Barsheshet, Y.; Werbner, N.; Zigdon, M.; Averbuch, I.; Ziv, O.; Brant, B.; Elliott, E.; Gelberg, S.; Titelbaum, M.; et al. Social-Stress-Responsive Microbiota Induces Stimulation of Self-Reactive Effector T Helper Cells. mSystems 2019, 4, e00292-18. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, J.A.; Ptacek, T.S.; Carter, S.J.; Liu, N.; Kumar, R.; Hyndman, L.; Lefkowitz, E.; Morrow, C.D.; Rogers, L.Q. Gut microbiota composition associated with alterations in cardiorespiratory fitness and psychosocial outcomes among breast cancer survivors. Support. Care Cancer 2017, 25, 1563–1570. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, C.; Dong, X.; Hu, T.; Wang, L.; Zhao, W.; Zhu, S.; Li, G.; Hu, Y.; Gao, Q.; et al. Chronic alcohol exposure induced gut microbiota dysbiosis and its correlations with neuropsychic behaviors and brain BDNF/Gabra1 changes in mice. BioFactors 2019, 45, 187–199. [Google Scholar] [CrossRef]
- Chen, J.; Toyomasu, Y.; Hayashi, Y.; Linden, D.R.; Szurszewski, J.H.; Nelson, H.; Farrugia, G.; Kashyap, P.C.; Chia, N.; Ordog, T. Altered gut microbiota in female mice with persistent low body weights following removal of post-weaning chronic dietary restriction. Genome Med. 2016, 8, 103. [Google Scholar] [CrossRef] [Green Version]
- Lyte, M.; Chapel, A.; Lyte, J.M.; Ai, Y.; Proctor, A.; Jane, J.-L.; Phillips, G.J. Resistant Starch Alters the Microbiota-Gut Brain Axis: Implications for Dietary Modulation of Behavior. PLoS ONE 2016, 11, e0146406. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-W.; Park, Y.-M.; Holscher, H.; Padilla, J.; Scroggins, R.J.; Welly, R.; Britton, S.L.; Koch, L.G.; Vieira-Potter, V.J.; Swanson, K. Physical Activity Differentially Affects the Cecal Microbiota of Ovariectomized Female Rats Selectively Bred for High and Low Aerobic Capacity. PLoS ONE 2015, 10, e0136150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tetel, M.J.; De Vries, G.J.; Melcangi, R.C.; Panzica, G.; O’Mahony, S.M. Steroids, stress and the gut microbiome-brain axis. J. Neuroendocr. 2018, 30, e12548. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lee, S.K.; Zhang, D.; Frenette, P.S. The Gut Microbiome Regulates Psychological-Stress-Induced Inflammation. Immunity 2020, 53, 417–428.e4. [Google Scholar] [CrossRef]
- Liu, R. The microbiome as a novel paradigm in studying stress and mental health. Am. Psychol. 2017, 72, 655–667. [Google Scholar] [CrossRef]
- Bastiaanssen, T.F.S.; Cussotto, S.; Claesson, M.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Gutted! Unraveling the Role of the Microbiome in Major Depressive Disorder. Harv. Rev. Psychiatry 2020, 28, 26–39. [Google Scholar] [CrossRef]
- Bastiaanssen, T.F.S.; Cowan, C.S.M.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Making Sense of … the Microbiome in Psychiatry. Int. J. Neuropsychopharmacol. 2019, 22, 37–52. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Xu, M.; Wang, C.; Krolick, K.N.; Shi, H.; Zhu, J. Difference in post-stress recovery of the gut microbiome and its altered metabolism after chronic adolescent stress in rats. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bharwani, A.; Mian, M.F.; Foster, J.A.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology 2016, 63, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef]
- Marin, I.A.; Goertz, J.; Ren, T.; Rich, S.S.; Onengut-Gumuscu, S.; Farber, E.; Wu, M.; Overall, C.C.; Kipnis, J.; Gaultier, A. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 2017, 7, srep43859. [Google Scholar] [CrossRef] [PubMed]
- Papalini, S.; Michels, F.; Kohn, N.; Wegman, J.; van Hemert, S.; Roelofs, K.; Arias-Vasquez, A.; Aarts, E. Stress matters: Randomized controlled trial on the effect of probiotics on neurocognition. Neurobiol. Stress 2019, 10, 100141. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemmings, S.M.; Malan-Muller, S.; Heuvel, L.L.V.D.; Demmitt, B.; Stanislawski, M.; Smith, D.; Bohr, A.D.; Stamper, C.E.; Hyde, E.R.; Morton, J.T.; et al. The Microbiome in Posttraumatic Stress Disorder and Trauma-Exposed Controls: An Exploratory Study. Psychosom. Med. 2017, 79, 936–946. [Google Scholar] [CrossRef]
- Carvalho, F.A.; Koren, O.; Goodrich, J.K.; Johansson, M.E.V.; Nalbantoglu, I.; Aitken, J.D.; Su, Y.; Chassaing, B.; Walters, W.A.; González, A.; et al. Transient Inability to Manage Proteobacteria Promotes Chronic Gut Inflammation in TLR5-Deficient Mice. Cell Host Microbe 2012, 12, 139–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodrich, J.K.; Di Rienzi, S.; Poole, A.; Koren, O.; Walters, W.A.; Caporaso, J.G.; Knight, R.; Ley, R.E. Conducting a Microbiome Study. Cell 2014, 158, 250–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastiaanssen, T.F.; Gururajan, A.; van de Wouw, M.; Moloney, G.M.; Ritz, N.L.; Long-Smith, C.M.; Wiley, N.C.; Murphy, A.B.; Lyte, J.M.; Fouhy, F.; et al. Volatility as a Concept to Understand the Impact of Stress on the Microbiome. Psychoneuroendocrinology 2021, 124, 105047. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, Y.; Kurokawa, S.; Ishii, D.; Miyaho, K.; Ishii, C.; Sanada, K.; Fukuda, S.; Mimura, M.; Kishimoto, T. Effects of Psychotropics on the Microbiome in Patients With Depression and Anxiety: Considerations in a Naturalistic Clinical Setting. Int. J. Neuropsychopharmacol. 2021, 24, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Donner, N.C.; Montoya, C.D.; Wacker, A.J.; Lowry, C.A. Chronic, non-invasive corticosterone delivery disrupts the circadian pattern of hypothalamic-pituitary-adrenal (HPA) axis activity and induces anxiety- and depression-like behaviors in rats. Neurosci Meet Plan. Diego CA Soc. Neurosci. 2010. [Google Scholar]
- Willner, P. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology 1997, 134, 319–329. [Google Scholar] [CrossRef]
- Montgomery, S.A.; Loft, H.; Sánchez, C.; Reines, E.H.; Papp, M. Escitalopram (S-Enantiomer of Citalopram): Clinical Efficacy and Onset of Action Predicted from a Rat Model. Pharmacol. Toxicol. 2008, 88, 282–286. [Google Scholar] [CrossRef]
- Dewan, M.J.; Anand, V.S. Evaluating the Tolerability of the Newer Antidepressants. J. Nerv. Ment. Dis. 1999, 187, 96–101. [Google Scholar] [CrossRef]
- Janssen, P.; Vos, R.; Tack, J. The influence of citalopram on interdigestive gastrointestinal motility in man. Aliment. Pharmacol. Ther. 2010, 32, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Neumann, I.D.; Slattery, D.A. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biol. Psychiatry 2016, 79, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donner, N.C.; Montoya, C.D.; Lukkes, J.L.; Lowry, C.A. Chronic non-invasive corticosterone administration abolishes the diurnal pattern of tph2 expression. Psychoneuroendocrinology 2012, 37, 645–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Stanić, D.; Plećaš-Solarović, B.; Mirković, D.; Jovanović, P.; Dronjak, S.; Marković, B.; Đorđević, T.; Ignjatović, S.; Pešić, V. Oxytocin in corticosterone-induced chronic stress model: Focus on adrenal gland function. Psychoneuroendocrinology 2017, 80, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.E.; Chen, H.-C.; Chou, H.-C.L.; Chen, I.-M.; Lee, M.-S.; Chuang, L.-C.; Liu, Y.-W.; Lu, M.-L.; Chen, C.-H.; Wu, C.-S.; et al. Exploration of microbiota targets for major depressive disorder and mood related traits. J. Psychiatr. Res. 2019, 111, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, G.; Lu, W.; Huang, S.; Zhang, Z. Dominant and Subordinate Relationship Formed by Repeated Social Encounters Alters Gut Microbiota in Greater Long-Tailed Hamsters. Microb. Ecol. 2019, 79, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Lukic, I.; Getselter, D.; Ziv, O.; Oron, O.; Reuveni, E.; Koren, O.; Elliott, E. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl. Psychiatry 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Jiang, P.; Lin, L.; Jiang, J.; Yu, B.; Rao, J.; Liu, H.; Wei, W.; Qiao, Y. Oral treatment with Lactobacillus reuteri attenuates depressive-like behaviors and serotonin metabolism alterations induced by chronic social defeat stress. J. Psychiatr. Res. 2020, 122, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.; Borre, Y.; Brien, C.O.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Gray, M. Geodiversity: Valuing and conserving abiotic nature. Wiley Publ. 2013, 17, 5324. [Google Scholar] [CrossRef]
- Kashyap, P.C.; Chia, N.; Nelson, H.; Segal, E.; Elinav, E. Microbiome at the Frontier of Personalized Medicine. Mayo Clin. Proc. 2017, 92, 1855–1864. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.S. Oxytocin Pathways and the Evolution of Human Behavior. Annu. Rev. Psychol. 2014, 65, 17–39. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.-Q.; Luo, Z.-C.; Xu, H.; Fraser, W.D. The Effect of Early Oxytocin Augmentation in Labor. Obstet. Gynecol. 2009, 114, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Naja, W.J.; Aoun, M.P. Oxytocin and Anxiety Disorders: Translational and Therapeutic Aspects. Curr. Psychiatry Rep. 2017, 19, 67. [Google Scholar] [CrossRef] [PubMed]
- Bartz, J.A.; Zaki, J.; Bolger, N.; Ochsner, K.N. Social effects of oxytocin in humans: Context and person matter. Trends Cogn. Sci. 2011, 15, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Feng, M.; Wang, C.; Ye, Y.; Wang, P.S.; Liu, C. Oxytocin receptor expressed on the smooth muscle mediates the excitatory effect of oxytocin on gastric motility in rats. Neurogastroenterol. Motil. 2009, 21, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xi, T.-F.; Li, Y.-X.; Wang, H.-H.; Qin, Y.; Zhang, J.-P.; Cai, W.-T.; Huang, M.-T.; Shen, J.-Q.; Fan, X.-M.; et al. Oxytocin decreases colonic motility of cold water stressed ratsviaoxytocin receptors. World J. Gastroenterol. 2014, 20, 10886–10894. [Google Scholar] [CrossRef]
- Wu, C.-L.; Doong, M.-L.; Wang, P.S. Involvement of cholecystokinin receptor in the inhibition of gastrointestinal motility by oxytocin in ovariectomized rats. Eur. J. Pharmacol. 2008, 580, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, B.; Truedsson, M.; Djerf, P.; Sundler, F. Oxytocin is expressed throughout the human gastrointestinal tract. Regul. Pept. 2006, 135, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Welch, M.G.; Tamir, H.; Gross, K.J.; Chen, J.; Anwar, M.; Gershon, M.D. Expression and developmental regulation of oxytocin (OT) and oxytocin receptors (OTR) in the enteric nervous system (ENS) and intestinal epithelium. J. Comp. Neurol. 2009, 512, 256–270. [Google Scholar] [CrossRef] [Green Version]
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’Sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.; Dinan, T.; Cryan, J. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 2015, 48, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dangoor, I.; Stanić, D.; Reshef, L.; Pešić, V.; Gophna, U. Specific Changes in the Mammalian Gut Microbiome as a Biomarker for Oxytocin-Induced Behavioral Changes. Microorganisms 2021, 9, 1938. https://doi.org/10.3390/microorganisms9091938
Dangoor I, Stanić D, Reshef L, Pešić V, Gophna U. Specific Changes in the Mammalian Gut Microbiome as a Biomarker for Oxytocin-Induced Behavioral Changes. Microorganisms. 2021; 9(9):1938. https://doi.org/10.3390/microorganisms9091938
Chicago/Turabian StyleDangoor, Itzhak, Dušanka Stanić, Leah Reshef, Vesna Pešić, and Uri Gophna. 2021. "Specific Changes in the Mammalian Gut Microbiome as a Biomarker for Oxytocin-Induced Behavioral Changes" Microorganisms 9, no. 9: 1938. https://doi.org/10.3390/microorganisms9091938
APA StyleDangoor, I., Stanić, D., Reshef, L., Pešić, V., & Gophna, U. (2021). Specific Changes in the Mammalian Gut Microbiome as a Biomarker for Oxytocin-Induced Behavioral Changes. Microorganisms, 9(9), 1938. https://doi.org/10.3390/microorganisms9091938






