Herpes Simplex Virus Re-Activation in Patients with SARS-CoV-2 Pneumonia: A Prospective, Observational Study
Abstract
1. Introduction
2. Methods
2.1. Definitions
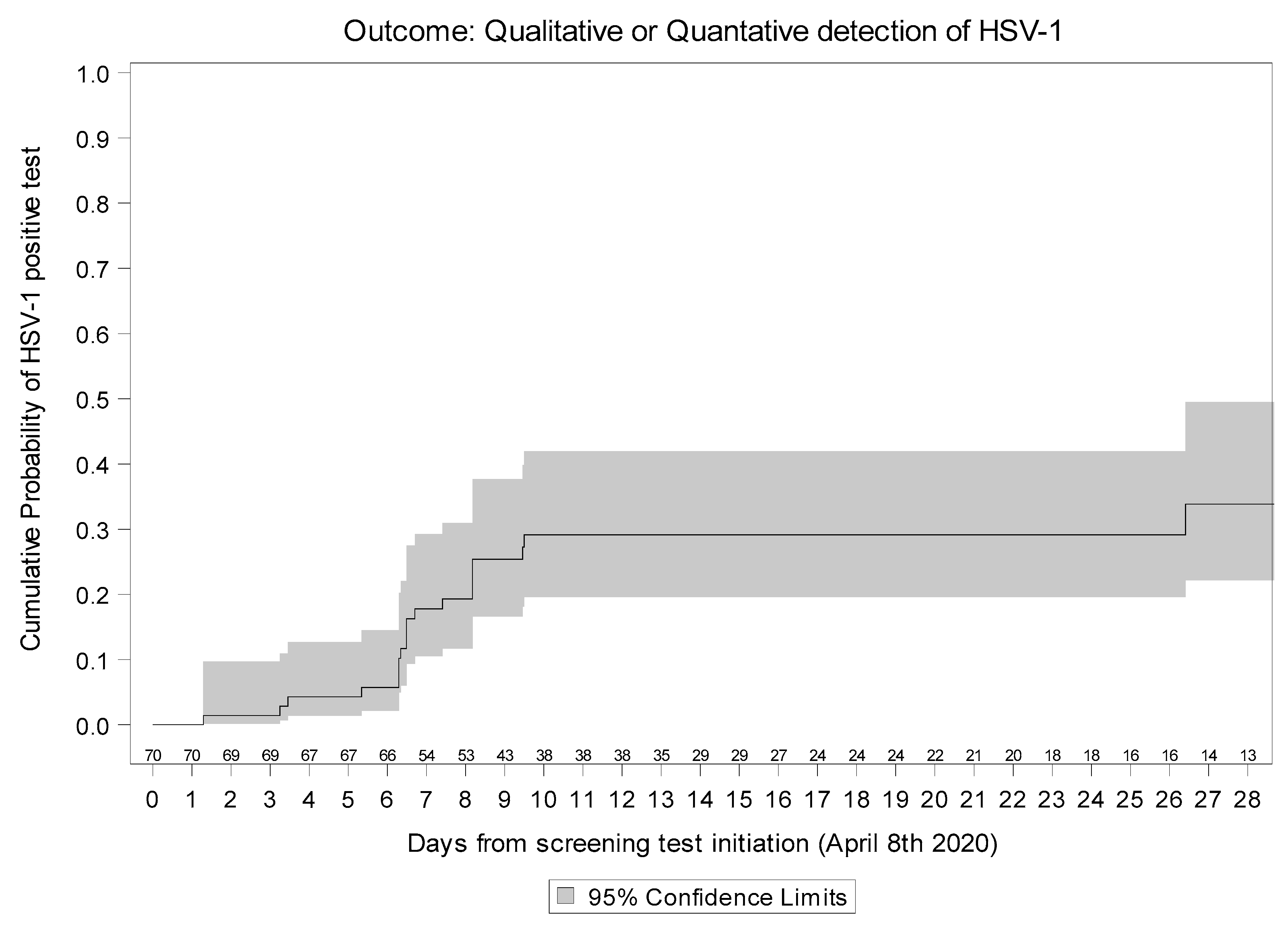
2.2. HSV-1 Re-Activation
2.3. Standard of Care (SOC)
2.4. Tocilizumab Treatment
2.5. Steroid Treatment
2.6. Statistical Analysis
3. Results
4. Discussion
- The role of the virus itself. Indeed, patients with SARS-CoV-2, especially those with severe pneumonia, have a dysregulated immune response at hospitalization and often develop immune suppression characterized by lymphopenia, mainly in CD4 and CD8 T cells after the pro-inflammatory phase [30]. This virus-induced immunosuppression, followed by the administration of immunomodulatory drugs, further blocks the immune response inhibiting antiviral immunity [31]. In our study, we could test retrospectively for HSV-1 PCR at hospital admission in only 14 out of 70 patients. Despite the low number of tested patients, since they were all negative, this could suggest that SARS-CoV-2 by itself seems not to play a role in herpes re-activation.
- 2.
- Tocilizumab. In our cohort, a high percentage of patients received tocilizumab with a dose higher than that used in rheumatoid arthritis [32]. In that setting, no clinically relevant manifestations of HSV-1 were described [33] and Gron et al. reported antiviral prescription in 5% of patients treated with tocilizumab without specifying the clinical reason [34]. Only one case of Herpes zoster meningitis was reported [35]. In the setting of hematological patients after CAR-T-cell infusion no statistically significant differences in risk of HSV-1 infection due to tocilizumab were reported but patients underwent herpes prophylaxis [22].
- 3.
- IMV. It is well known that herpes re-activation is a common finding in patients admitted to ICU. In a pre-COVID study evaluating 201 patients with prolonged (>4 days) IMV, Luyt et al. found that 20% had HSV bronchopneumonitis with cytological and/or histological signs of deep lung infection [36]. In our analysis 57.1% of patients with HSV re-activation underwent IMV, that in the unadjusted analysis was associated with HSV-1 re-activation.
- 4.
- Steroid treatment. Steroids may exacerbate herpetic re-activation of latent virus, especially among patients undergoing other stress-inducing or immunosuppressive therapies such as irradiation or chemotherapy [37]. Case reports have been described in patients with inflammatory bowel disease [38]. Our analysis shows convincing evidence for an association between use of steroids and risk of HSV-1 re-activation. The association was even stronger after controlling for previous use of both tocilizumab and IMV (OR = 5.13, p = 0.016). Interestingly, the effect size was larger when restricting to participants who were treated with high-dose steroids, while there was no association with duration of steroid treatment.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Bacca, E.; Digaetano, M.; Meschiari, M.; Franceschini, E.; Menozzi, M.; Cuomo, G.; Mussini, C. Immunomodulation for the management of severe SARS-CoV2 infections. State of the art and review of the literature. Biochem. Biophys. Res. Commun. 2021, 538, 151–155. [Google Scholar] [CrossRef]
- Huang, I.; Pranata, R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): Systematic review and meta-analysis. J. Intensive Care 2020, 8, 36. [Google Scholar] [CrossRef]
- De Biasi, S.; Tartaro, D.L.; Meschiari, M.; Gibellini, L.; Bellinazzi, C.; Borella, R.; Fidanza, L.; Mattioli, M.; Paolini, A.; Gozzi, L.; et al. Expansion of plasmablasts and loss of memory B cells in peripheral blood from COVID-19 patients with pneumonia. Eur. J. Immunol. 2020, 50, 1283–1294. [Google Scholar] [CrossRef]
- The Recovery Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2020, 384, 693–704. [Google Scholar] [CrossRef]
- Società Italiana di Malattie Infettive e Tropicali (SIMIT). Vademecum per La Cura Delle Persone Con Malattia Da COVI-19, 2nd ed.; Società Italiana di Malattie Infettive e Tropicali: Milano, Italy, 2020. [Google Scholar]
- Guaraldi, G.; Meschiari, M.; Cozzi-Lepri, A.; Milic, J.; Tonelli, R.; Menozzi, M.; Franceschini, E.; Cuomo, G.; Orlando, G.; Borghi, V.; et al. Tocilizumab in patients with severe COVID-19: A retrospective cohort study. Lancet Rheumatol. 2020, 2, e474–e484. [Google Scholar] [CrossRef]
- Klopfenstein, T.; Zayet, S.; Lohse, A.; Balblanc, J.-C.; Badie, J.; Royer, P.-Y.; Toko, L.; Mezher, C.; Kadiane-Oussou, N.J.; Bossert, M.; et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Méd. Mal. Infect. 2020, 50, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Mussini, C.; Falcone, M.; Nozza, S.; Sagnelli, C.; Parrella, R.; Meschiari, M.; Petrosillo, N.; Mastroianni, C.; Cascio, A.; Iaria, C.; et al. Therapeutic strategies for severe COVID-19: A position paper from the Italian Society of Infectious and Tropical Diseases (SIMIT). Clin. Microbiol. Infect. 2021, 27, 389–395. [Google Scholar] [CrossRef] [PubMed]
- NIHR Imperial Biomedical Research Centre. The RECAP (Remote COVID-19 Assessment in Primary Care); Project 2020; Imperial Biomedical Research Centre: London, UK, 2020. [Google Scholar]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Salama, C.; Han, J.; Yau, L.; Reiss, W.G.; Kramer, B.; Neidhart, J.D.; Criner, G.J.; Kaplan-Lewis, E.; Baden, R.; Pandit, L.; et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N. Engl. J. Med. 2021, 384, 20–30. [Google Scholar] [CrossRef]
- Le Balc’H, P.; Pinceaux, K.; Pronier, C.; Seguin, P.; Tadié, J.-M.; Reizine, F. Herpes simplex virus and cytomegalovirus reactivations among severe COVID-19 patients. Crit. Care 2020, 24, 530. [Google Scholar] [CrossRef] [PubMed]
- Luyt, C.-E.; Bréchot, N.; Trouillet, J.-L.; Chastre, J. Antibiotic stewardship in the intensive care unit. Crit. Care 2014, 18, 480. [Google Scholar] [CrossRef] [PubMed]
- Kämmerer, T.; Walch, J.; Flaig, M.; French, L.E. COVID-19-associated herpetic gingivostomatitis. Clin. Exp. Dermatol. 2021, 46, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Marzano, A.V.; Genovese, G.; Fabbrocini, G.; Pigatto, P.; Monfrecola, G.; Piraccini, B.M.; Veraldi, S.; Rubegni, P.; Cusini, M.; Caputo, V.; et al. Varicella-like exanthem as a specific COVID-19–associated skin manifestation: Multicenter case series of 22 patients. J. Am. Acad. Dermatol. 2020, 83, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.A.; de Romão, T.T.; Macedo, Y.S.; Pupe, C.; Nascimento, O.J. M Fellow of the American Academy of Neurology (FAAN) COVID-19 and Herpes Zoster Co-Infection Presenting with Trigeminal Neuropathy. Eur. J. Neurol. 2020, 27, 1748–1750. [Google Scholar] [CrossRef] [PubMed]
- Majtanova, N.; Kriskova, P.; Keri, P.; Fellner, Z.; Majtan, J.; Kolar, P. Herpes Simplex Keratitis in Patients with SARS-CoV-2 Infection: A Series of Five Cases. Medicina 2021, 57, 412. [Google Scholar] [CrossRef]
- Busani, S.; Bedini, A.; Biagioni, E.; Serio, L.; Tonelli, R.; Meschiari, M.; Franceschini, E.; Guaraldi, G.; Cossarizza, A.; Clini, E.; et al. Two fatal cases of acute liver failure due to HSV-1 infection in COVID-19 patients following immunomodulatory therapies. Clin. Infect. Dis. 2020, 73, e252–e255. [Google Scholar] [CrossRef]
- Frobert, E.; Billaud, G.; Casalegno, J.-S.; Eibach, D.; Gonçalves, D.; Robert, J.-M.; Lina, B.; Morfin, F. The clinical interest of HSV1 semi-quantification in bronchoalveolar lavage. J. Clin. Virol. 2013, 58, 265–268. [Google Scholar] [CrossRef]
- Società Italiana di Malattie Infettive e Tropicali (SIMIT); Sezione Regione Lombardia. Vademecum per la Cura Delle Persone Con Malattia da COVI-19 Edizione 2.0. 13 March 2020. Available online: https://www.eahp.eu/sites/default/files/covid19_vademecum_2.0_13_marzo_2020.03_11.pdf (accessed on 10 February 2021).
- Le, R.Q.; Li, L.; Yuan, W.; Shord, S.S.; Nie, L.; Habtemariam, B.A.; Przepiorka, D.; Farrell, A.T.; Pazdur, R. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist 2018, 23, 943–947. [Google Scholar] [CrossRef]
- Meduri, G.U.; Siemieniuk, R.A.C.; Ness, R.A.; Seyler, S.J. Prolonged low-dose methylprednisolone treatment is highly effective in reducing duration of mechanical ventilation and mortality in patients with ARDS. J. Intensiv. Care 2018, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Busani, S.; Tosi, M.; Mighali, P.; Vandelli, P.; D’Amico, R.; Marietta, M.; Forfori, F.; Donati, A.; Cinnella, G.; De Monte, A.; et al. Multi-centre, three arm, randomized controlled trial on the use of methylprednisolone and unfractionated heparin in critically ill ventilated patients with pneumonia from SARS-CoV-2 infection: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 724. [Google Scholar] [CrossRef]
- Martha, J.W.; Wibowo, A.; Pranata, R. Prognostic value of elevated lactate dehydrogenase in patients with COVID-19: A systematic review and meta-analysis. Postgrad. Med. J. 2021. [Google Scholar] [CrossRef]
- Henry, B.M.; Aggarwal, G.; Wong, J.; Benoit, S.; Vikse, J.; Plebani, M.; Lippi, G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am. J. Emerg. Med. 2020, 38, 1722–1726. [Google Scholar] [CrossRef]
- Fernandez-Nieto, D.; Ortega-Quijano, D.; Suarez-Valle, A.; Burgos-Blasco, P.; Jimenez-Cauhe, J.; Fernandez-Guarino, M. Comment on: “To consider varicella-like exanthem associated with COVID-19, virus varicella zoster and virus herpes simplex must be ruled out. Characterization of herpetic lesions in hospitalized COVID-19 patients”. J. Am. Acad. Derm. 2020, 83, e257–e259. [Google Scholar] [CrossRef]
- Ertugrul, G.; Aktas, H. Herpes zoster cases increased during COVID-19 outbreak. Is it possible a relation? J. Derm. Treat. 2020. [Google Scholar] [CrossRef]
- Soffritti, I.; D’Accolti, M.; Fabbri, C.; Passaro, A.; Manfredini, R.; Zuliani, G.; Libanore, M.; Franchi, M.; Contini, C.; Caselli, E. Oral Microbiome Dysbiosis Is Associated with Symptoms Severity and Local Immune/Inflammatory Response in COVID-19 Patients: A Cross-Sectional Study. Front. Microbiol. 2021, 12, 687513. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Ritchie, A.I.; Singanayagam, A. Immunosuppression for hyperinflammation in COVID-19: A double-edged sword? Lancet 2020, 395, 1111. [Google Scholar] [CrossRef]
- Zhang, X.; Georgy, A.; Rowell, L. Pharmacokinetics and pharmacodynamics of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, following single-dose administration by subcutaneous and intravenous routes to healthy subjects. Int. J. Clin. Pharmacol. Ther. 2013, 51, 443–455. [Google Scholar] [CrossRef]
- Balandraud, N.; Texier, G.; Massy, E.; Muis-Pistor, O.; Martin, M.; Auger, I.; Guzian, M.-C.; Guis, S.; Pham, T.; Roudier, J. Long term treatment with abatacept or tocilizumab does not increase Epstein-Barr virus load in patients with rheumatoid arthritis–A three years retrospective study. PLoS ONE 2017, 12, e0171623. [Google Scholar] [CrossRef] [PubMed]
- Grøn, K.L.; Glintborg, B.; Nørgaard, M.; Mehnert, F.; Østergaard, M.; Dreyer, L.; Krogh, N.S.; Hetland, M.L. Overall infection risk in rheumatoid arthritis during treatment with abatacept, rituximab and tocilizumab; an observational cohort study. Rheumatology 2019, 59, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Tsurukawa, S.; Iwanaga, N.; Izumi, Y.; Shirakawa, A.; Kawahara, C.; Shukuwa, T.; Inamoto, M.; Kawakami, A.; Migita, K. Herpes Zoster Meningitis Complicating Combined Tocilizumab and Cyclosporine Therapy for Adult-Onset Still’s Disease. Case Rep. Rheumatol. 2016, 2016, 4232657. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luyt, C.-E.; Combes, A.; Deback, C.; Aubriot-Lorton, M.-H.; Nieszkowska, A.; Trouillet, J.-L.; Capron, F.; Agut, H.; Gibert, C.; Chastre, J. Herpes Simplex Virus Lung Infection in Patients Undergoing Prolonged Mechanical Ventilation. Am. J. Respir. Crit. Care Med. 2007, 175, 935–942. [Google Scholar] [CrossRef]
- Graber, J.; Rosenblum, M.K.; DeAngelis, L.M. Herpes simplex encephalitis in patients with cancer. J. Neuro-Oncol. 2011, 105, 415–421. [Google Scholar] [CrossRef]
- Santos-Antunes, J.; Abreu, C.; Magro, F.; Coelho, R.; Vilas-Boas, F.; Andrade, P.; Lopes, S.; Macedo, G. Disseminated cutaneous herpes simplex infection in a patient with Crohn’s disease under azathioprine and steroids: First case report and literature review. J. Crohn’s Colitis 2014, 8, 326–330. [Google Scholar] [CrossRef]
- Luyt, C.-E.; Forel, J.-M.; Hajage, D.; Jaber, S.; Cayot-Constantin, S.; Rimmelé, T.; Coupez, E.; Lu, Q.; Diallo, M.H.; Penot-Ragon, C.; et al. Acyclovir for Mechanically Ventilated Patients With Herpes Simplex Virus Oropharyngeal Reactivation: A Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 263–272. [Google Scholar] [CrossRef] [PubMed]
| HSV−1 | ||||
|---|---|---|---|---|
| Positive | Negative | p-Value * | Total | |
| N = 21 | N = 49 | N = 70 | ||
| Patients’ Characteristics | ||||
| Age, years Median (IQR) | 72 (66, 76) | 67 (52, 76) | 0.185 | 70 (58, 76) |
| BMI, Kg/m2 (49) | 27.5 (25.6, 32.8) | 26.2 (24.2, 29.9) | 0.150 | 26.7 (24.6, 31.1) |
| Any comorbidity, n (%) | ||||
| Yes | 15 (71.4%) | 28 (57.1%) | 0.264 | 43 (61.4%) |
| Comorbidities, n (%) | ||||
| Diabetes | 6 (28.6%) | 8 (16.3%) | 0.244 | 14 (20.0%) |
| Hypertension | 13 (61.9%) | 25 (51.0%) | 0.406 | 38 (54.3%) |
| Cardiovascular Disease | 3 (14.3%) | 10 (20.4%) | 0.549 | 13 (18.6%) |
| Chronic Kidney Disease | 2 (9.5%) | 4 (8.2%) | 0.853 | 6 (8.6%) |
| Cancer | 0 (0.0%) | 3 (6.1%) | 0.250 | 3 (4.3%) |
| Hepatitis B/C | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Signs and symptoms, n (%) | ||||
| Fever, median (IQR) | 36 (36, 36) | 36 (36, 37) | 0.521 | 36 (36, 36) |
| Cough | 3 (14.3%) | 14 (28.6%) | 0.205 | 17 (24.3%) |
| Myalgia | 0 (0.0%) | 4 (8.2%) | 0.181 | 4 (5.7%) |
| Sputum | 1 (4.8%) | 1 (2.0%) | 0.534 | 2 (2.9%) |
| Headache | 1 (4.8%) | 3 (6.1%) | 0.823 | 4 (5.7%) |
| Haemoptysis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Diarrhea | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Systolic pressure, mmHg median (IQR) | 129 (120, 140) | 110 (101, 130) | 0.027 | 120 (110, 135) |
| Respiratory rate, % median (IQR) | 22 (20, 36) | 22 (20, 27) | 0.490 | 22 (20, 30) |
| BaselinePaO2/FiO2 | 161 (104, 187) | 157 (79, 296) | 0.438 | 159 (80, 285) |
| SOFA Score | 2 (0, 4) | 2 (0, 4) | 0.730 | 2 (0, 4) |
| Markers, Median (IQR) | ||||
| Haemoglobin, g/L | 12.6 (10.3, 13.9) | 12.4 (11.4, 13.5) | 0.934 | 12.5 (10.8, 13.8) |
| White cells, mm3 | 6510 (5170, 8490) | 6180 (5190, 8200) | 0.729 | 6365 (5170, 8490) |
| Total lymphocytes, N | 1791 (570.0, 2519) | 1290 (810.0, 2383) | 0.984 | 1358 (700.0, 2519) |
| Total lymphocytes, % | 27.9 (7.8, 30.9) | 20.3 (8.8, 36.0) | 0.625 | 22.4 (8.6, 33.9) |
| Alanine amino-transferase, U/L | 39.0 (29.0, 69.0) | 48.0 (31.0, 81.0) | 0.513 | 41.5 (29.0, 81.0) |
| Bilirubin, mg/L | 0.6 (0.4, 0.9) | 0.5 (0.4, 0.8) | 0.366 | 0.6 (0.4, 0.8) |
| Calcium, mg/L | 8.4 (8.2, 8.7) | 8.6 (8.2, 8.9) | 0.363 | 8.5 (8.2, 8.9) |
| Creatine Kinase, U/L | 127.0 (64.0, 305.0) | 78.0 (33.0, 180.0) | 0.106 | 97.5 (36.0, 206.0) |
| Chloride, mmol/L | 100.5 (98.0, 104.0) | 100.0 (96.0, 103.0) | 0.454 | 100.0 (97.0, 104.0) |
| Creatinine, mg/L | 0.8 (0.7, 1.0) | 0.9 (0.7, 1.1) | 0.353 | 0.8 (0.7, 1.1) |
| D-dimer, mg/L | 1200 (810.0, 2650) | 900.0 (460.0, 1800) | 0.088 | 1060 (580.0, 2070) |
| Lactate dehydrogenase, U/L | 831.0 (556.0, 998.0) | 609.0 (466.0, 745.0) | 0.022 | 652.0 (473.0, 832.0) |
| C-reactive protein, mg/L | 15.5 (5.0, 22.2) | 7.5 (4.5, 18.9) | 0.405 | 9.0 (4.5, 19.7) |
| Platelets, 109/L | 182.0 (140.0, 244.0) | 180.0 (151.0, 251.0) | 0.888 | 181.0 (149.0, 251.0) |
| Potassium, mmol/L | 3.7 (3.5, 3.9) | 3.8 (3.6, 4.0) | 0.213 | 3.7 (3.5, 4.0) |
| Sodium, mmol/L | 137.0 (134.0, 139.0) | 136.0 (135.0, 139.0) | 0.663 | 136.0 (135.0, 139.0) |
| IL−6, mg/L | 412.8 (241.1, 1252) | 253.4 (78.9, 1418) | 0.392 | 280.5 (92.8, 1349) |
| Ferritin, mg/L | 987.5 (472.5, 1475) | 603.5 (416.0, 1562) | 0.684 | 688.0 (423.0, 1518) |
| Disease Duration | ||||
| Days from symptoms onset to hospitalisation, median (IQR) | 5 (2, 7) | 8 (4, 15) | 0.572 | 7 (3, 12) |
| Days from hospitalisation to intubation, median (IQR) | 4 (2, 7) | 4 (1, 6) | 0.827 | 4 (2, 6) |
| Follow-up, days | 7 (3, 24) | 14 (6, 27) | 0.170 | 13 (6, 25) |
| Intervention, n (%) | 0.027 | |||
| Tocilizumab subcutaneous | 3 (27.3%) | 5 (12.5%) | 8 (15.7%) | |
| Tocilizumab intravenous | 11 (52.4%) | 30 (61.2%) | 41 (58.6%) | |
| Only SOC | 7 (33.3%) | 13 (26.5%) | 20 (28.6%) | |
| Steroids | 16 (76.2%) | 24 (49.0%) | 0.036 | 40 (57.1%) |
| Outcomes | ||||
| Events, n (%) | ||||
| Invasive mechanical ventilation | 12 (57.1%) | 11 (22.4%) | 0.005 | 23 (32.9%) |
| Death-all | 6 (28.6%) | 9 (18.4%) | 0.344 | 15 (21.4%) |
| (A) | ||||
|---|---|---|---|---|
| Unadjusted | Adjusted * | |||
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
| Steroids | ||||
| Yes (any dose) vs. No | 3.33 (1.06, 10.53) | 0.040 | 5.13 (1.36, 19.32) | 0.016 |
| Low dose vs. No | 3.06 (0.90, 10.33) | 0.072 | 4.80 (1.20, 19.26) | 0.027 |
| High dose vs. No | 4.17 (0.91, 19.18) | 0.067 | 6.16 (1.06, 35.74) | 0.043 |
| per day longer exposure | 1.04 (0.89, 1.22) | 0.625 | 1.07 (0.90, 1.26) | 0.461 |
| (B) | ||||
| Unadjusted | Adjusted * | |||
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
| Use of tocilizumab | ||||
| Yes vs. No | 1.87 (0.54, 6.53) | 0.323 | 1.91 (0.36, 10.21) | 0.452 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franceschini, E.; Cozzi-Lepri, A.; Santoro, A.; Bacca, E.; Lancellotti, G.; Menozzi, M.; Gennari, W.; Meschiari, M.; Bedini, A.; Orlando, G.; et al. Herpes Simplex Virus Re-Activation in Patients with SARS-CoV-2 Pneumonia: A Prospective, Observational Study. Microorganisms 2021, 9, 1896. https://doi.org/10.3390/microorganisms9091896
Franceschini E, Cozzi-Lepri A, Santoro A, Bacca E, Lancellotti G, Menozzi M, Gennari W, Meschiari M, Bedini A, Orlando G, et al. Herpes Simplex Virus Re-Activation in Patients with SARS-CoV-2 Pneumonia: A Prospective, Observational Study. Microorganisms. 2021; 9(9):1896. https://doi.org/10.3390/microorganisms9091896
Chicago/Turabian StyleFranceschini, Erica, Alessandro Cozzi-Lepri, Antonella Santoro, Erica Bacca, Guido Lancellotti, Marianna Menozzi, William Gennari, Marianna Meschiari, Andrea Bedini, Gabriella Orlando, and et al. 2021. "Herpes Simplex Virus Re-Activation in Patients with SARS-CoV-2 Pneumonia: A Prospective, Observational Study" Microorganisms 9, no. 9: 1896. https://doi.org/10.3390/microorganisms9091896
APA StyleFranceschini, E., Cozzi-Lepri, A., Santoro, A., Bacca, E., Lancellotti, G., Menozzi, M., Gennari, W., Meschiari, M., Bedini, A., Orlando, G., Puzzolante, C., Digaetano, M., Milic, J., Codeluppi, M., Pecorari, M., Carli, F., Cuomo, G., Alfano, G., Corradi, L., ... Mussini, C. (2021). Herpes Simplex Virus Re-Activation in Patients with SARS-CoV-2 Pneumonia: A Prospective, Observational Study. Microorganisms, 9(9), 1896. https://doi.org/10.3390/microorganisms9091896









