Microbiota Dynamics of Mechanically Separated Organic Fraction of Municipal Solid Waste during Composting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composting Material
2.2. Experimental Setup
2.3. Determination of the Composted Mass Parameters
2.4. Heat Release
2.5. Profiling of Prokaryotic and Fungal Communities Based on 16S rRNA GENE and Internal Transcribed Spacer (ITS)
2.6. Statistical Data Analysis
3. Results
3.1. Primary Composting Parameters
3.1.1. Temperature Regime and Thermal Energy
3.1.2. Gaseous Emissions
3.1.3. Physicochemical Substrate Parameters
3.1.4. Stability Indicators
3.2. Microbial Community Composition
3.2.1. Fungal Community
3.2.2. Prokaryotic Community
3.2.3. Potential Abilities of the Prokaryotic Community
3.3. Microbial Biodiversity
3.4. Relationship between Physicochemical Processes and Microbial Dynamics
4. Discussion
4.1. Microbial Processes during the Substrate Preparation and Heating
4.2. Microbial Processes in the High-Temperature Stage
4.3. Microbial Processes at the Cooling and Maturation Stage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Seruga, P.; Krzywonos, M.; Seruga, A.; Niedźwiecki, Ł.; Pawlak-Kruczek, H.; Urbanowska, A. Anaerobic Digestion Performance: Separate Collected vs. Mechanical Segregated Organic Fractions of Municipal Solid Waste as Feedstock. Energies 2020, 13, 3768. [Google Scholar] [CrossRef]
- Vaverková, M.D.; Elbl, J.; Voběrková, S.; Koda, E.; Adamcová, D.; Mariusz Gusiatin, Z.; Al Rahman, A.; Radziemska, M.; Mazur, Z. Composting versus mechanical–biological treatment: Does it really make a difference in the final product parameters and maturity. Waste Manag. 2020, 106, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ashkiki, A.R.; Felske, C.; McCartney, D. Impacts of seasonal variation and operating parameters on double-stage trommel performance. Waste Manag. 2019, 86, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Di Lonardo, M.C.; Lombardi, F.; Gavasci, R. Quality evaluation and improvement of mechanically–biologically treated municipal solid waste in view of a possible recovery. Int. J. Environ. Sci. Technol. 2015, 12, 3243–3254. [Google Scholar] [CrossRef] [Green Version]
- López-Gómez, J.P.; Latorre-Sánchez, M.; Unger, P.; Schneider, R.; Coll Lozano, C.; Venus, J. Assessing the organic fraction of municipal solid wastes for the production of lactic acid. Biochem. Eng. J. 2019, 150, 107251. [Google Scholar] [CrossRef]
- Graça, J.; Murphy, B.; Pentlavalli, P.; Allen, C.C.R.; Bird, E.; Gaffney, M.; Duggan, T.; Kelleher, B. Bacterium consortium drives compost stability and degradation of organic contaminants in in-vessel composting process of the mechanically separated organic fraction of municipal solid waste (MS-OFMSW). Bioresour. Technol. Rep. 2021, 13, 100621. [Google Scholar] [CrossRef]
- Jurado, M.M.; Camelo-Castillo, A.J.; Suárez-Estrella, F.; López, M.J.; López-González, J.A.; Estrella-González, M.J.; Síles-Castellano, A.B.; Moreno, J. Integral approach using bacterial microbiome to stabilize municipal solid waste. J. Environ. Manag. 2020, 265, 110528. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, R.F.; Shann, J.F. Microbial Community Changes during the Composting of Municipal Solid Waste. Microb. Ecol. 1997, 1, 78–85. [Google Scholar] [CrossRef]
- Farrell, M.; Griffith, G.W.; Hobbs, P.J.; Perkins, W.T.; Jones, D.L. Microbial diversity and activity are increased by compost amendment of metal-contaminated soil. FEMS Microbiol. Ecol. 2009, 71, 94–105. [Google Scholar] [CrossRef] [Green Version]
- Beffa, T.; Blanc, M.; Marilley, L.; Fischer, J.L.; Lyon, P.-F.; Aragno, M. Taxonomic and Metabolic Microbial Diversity during Composting. In The Science of Composting; De Bertoldi, M., Sequi, P., Lemmes, B., Papi, T., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 149–161. [Google Scholar] [CrossRef]
- Martínez-Valdez, F.J.; Martínez-Ramírez, C.; Martínez-Montiel, L.; Favela-Torres, E.; Soto-Cruz, N.O.; Ramírez-Vives, F.; Saucedo-Castañeda, G. Rapid mineralisation of the Organic Fraction of Municipal Solid Waste. Bioresour. Technol. 2015, 180, 112–118. [Google Scholar] [CrossRef]
- Barrena, R.; Pagans, E.; Faltys, G.; Sánchez, A. Effect of inoculation dosing on the composting of source-selected organic fraction of municipal solid wastes. J. Chem. Technol. Biotechnol. 2006, 81, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Awasthi, M.K.; Pandey, A.K.; Bundela, P.S.; Khan, J. Co-composting of organic fraction of municipal solid waste mixed with different bulking waste: Characterization of physicochemical parameters and microbial enzymatic dynamic. Bioresour. Technol. 2015, 182, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Ryckeboer, J.; Mergaert, J.; Coosemans, J.; Deprins, K.; Swings, J. Microbiological aspects of biowaste during composting in a monitored compost bin. J. Appl. Microbiol. 2003, 94, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacorte, S.; Bono-Blay, F.; Cortina, M. Sample Homogenization. Compr. Sampl. Sample Prep. 2012, 1, 65–84. [Google Scholar] [CrossRef]
- Mironov, V.V.; Bochkova, E.A.; Gannesen, A.V.; Vanteeva, A.V.; Russkova, Y.I.; Nozhevnikova, A.N. Dynamics of Biological Processes during Composting of Anaerobically Digested Wastewater Sludge. Microbiology 2020, 89, 470–482. [Google Scholar] [CrossRef]
- Mironov, V.; Vanteeva, A.; Merkel, A. Microbiological Activity during Co-Composting of Food and Agricultural Waste for Soil Amendment. Agronomy 2021, 11, 928. [Google Scholar] [CrossRef]
- Zorpas, A.A.; Loizidou, M. Sawdust and natural zeolite as a bulking agent for improving quality of a composting prod-uct from anaerobically stabilized sewage sludge. Bioresour. Technol. 2008, 99, 7545–7552. [Google Scholar] [CrossRef]
- Navarro, A.; Cegarra, J.; Roig, A.; Garcia, D. Relationships between organic matter and carbon contents of organic wastes. Bioresour. Technol. 1993, 44, 203–207. [Google Scholar] [CrossRef]
- Arrigoni, J.P.; Paladino, G.; Garibaldi, L.A.; Laos, F. Inside the small-scale composting of kitchen and garden wastes: Thermal performance and stratification effect in vertical compost bins. Waste Manag. 2018, 76, 284–293. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V.; Guida, M. Compost from organic solid waste: Quality assessment and European regulations for its sustainable use. Resour. Conserv. Recycl. 2015, 94, 72–79. [Google Scholar] [CrossRef]
- Palaniveloo, K.; Amran, M.A.; Norhashim, N.A.; Mohamad-Fauzi, N.; Peng-Hui, F.; Hui-Wen, L.; Kai-Lin, Y.; Jiale, L.; Chian-Yee, M.G.; Jing-Yi, L.; et al. Food Waste Composting and Microbial Community Structure Profiling. Processes 2020, 8, 723. [Google Scholar] [CrossRef]
- Castaldi, P.; Garau, G.; Deiana, P.; Melis, P. Evolution of carbon compounds during municipal solid waste composting: Suitability of chemical and biochemical parameters in defining the stability and maturity of the end product. Dyn. Soil Dyn. Plant 2009, 3, 17–31. [Google Scholar]
- Haug, R.T. The Practical Handbook of Compost Engineering; Routledge: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Irvine, G.; Lamont, E.R.; Antizar-Ladislao, B. Energy from Waste: Reuse of Compost Heat as a Source of Renewable Energy. Int. J. Chem. Eng. 2010, 2010, e627930. [Google Scholar] [CrossRef] [Green Version]
- Manjunatha, G.S.; Chavan, D.; Lakshmikanthan, P.; Singh, L.; Kumar, S.; Kumar, R. Specific heat and thermal conductivity of municipal solid waste and its effect on landfill fires. Waste Manag. 2020, 116, 120–130. [Google Scholar] [CrossRef]
- Hanson, J.L.; Yeşiller, N.; Onnen, M.T.; Liu, W.-L.; Oettle, N.K.; Marinos, J.A. Development of numerical model for predicting heat generation and temperatures in MSW landfills. Waste Manag. Landfill Process. 2013, 33, 1993–2000. [Google Scholar] [CrossRef] [Green Version]
- Faitli, J.; Magyar, T.; Erdélyi, A.; Murányi, A. Characterization of thermal properties of municipal solid waste landfills. Waste Manag. 2015, 36, 213–221. [Google Scholar] [CrossRef]
- Kallistova, A.; Merkel, A.; Kanapatskiy, T.; Boltyanskaya, Y.; Tarnovetskii, I.; Perevalova, A.; Kevbrin, V.; Samylina, O.; Pimenov, N. Methanogenesis in the Lake Elton saline aquatic system. Extremophiles 2020, 24, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Efimova, D.; Tyakht, A.; Popenko, A.; Vasilyev, A.; Altukhov, I.; Dovidchenko, N.; Odintsova, V.; Klimenko, N.; Loshkarev, R.; Pashkova, M.; et al. Knomics-Biota—A system for exploratory analysis of human gut microbiota data. BioData Min. 2018, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- KEGG PATHWAY Database. Available online: https://www.genome.jp/kegg/pathway.html (accessed on 3 September 2021).
- Syranidou, E.; Karkanorachaki, K.; Amorotti, F.; Repouskou, E.; Kroll, K.; Kolvenbach, B.; Corvini, P.F.-X.; Fava, F.; Kalogerakis, N. Development of tailored indigenous marine consortia for the degradation of naturally weathered polyethylene films. PLoS ONE 2017, 12, e0183984. [Google Scholar] [CrossRef] [Green Version]
- ClustVis: A Web Tool for Visualizing Clustering of Multivariate Data. Available online: https://biit.cs.ut.ee/clustvis/ (accessed on 3 September 2021).
- Chao, A.; Lee, S.-M. Estimating the Number of Classes via Sample Coverage. J. Am. Stat. Assoc. 1992, 87, 210–217. [Google Scholar] [CrossRef]
- Strode, P.; Brokaw, A. Using biointeractive resources to teach Mathematics and Statistics in Biology. P Strode A Brokaw 2015, 2015, 39. [Google Scholar]
- Wong, J.W.C.; Karthikeyan, O.P.; Selvam, A. Biological nutrient transformation during composting of pig manure and paper waste. Environ. Technol. 2017, 38, 754–761. [Google Scholar] [CrossRef]
- Wang, X.; Cui, H.; Shi, J.; Zhao, X.; Zhao, Y.; Wei, Z. Relationship between bacterial diversity and environmental parameters during composting of different raw materials. Bioresour. Technol. 2015, 198, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, H.; Wang, Z.; Chen, G.; Wang, L. Dynamic changes of the dominant functioning microbial community in the compost of a 90-m3 aerobic solid state fermentor revealed by integrated meta-omics. Bioresour. Technol. 2016, 203, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Estrella-González, M.J.; Suárez-Estrella, F.; Jurado, M.M.; López, M.J.; López-González, J.A.; Siles-Castellano, A.B.; Muñoz-Mérida, A.; Moreno, J. Uncovering new indicators to predict stability, maturity and biodiversity of compost on an industrial scale. Bioresour. Technol. 2020, 313, 123557. [Google Scholar] [CrossRef] [PubMed]
- Insam, H.; de Bertoldi, M. Chapter 3 Microbiology of the composting process. In Waste Management Series, Compost Science and Technology; Diaz, L.F., de Bertoldi, M., Bidlingmaier, W., Stentiford, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 25–48. [Google Scholar] [CrossRef]
- Hashimoto, H.; Iwaasa, T.; Yokotsuka, T. Thermostable Acid Protease Produced by Penicillium duponti K1014, a True Thermophilic Fungus Newly Isolated from Compost. Appl. Microbiol. 1972, 24, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, B.; Hu, Q.; Yin, Z. Effect of inoculation with Penicillium expansum on the microbial community and maturity of compost. Bioresour. Technol. 2011, 102, 11189–11193. [Google Scholar] [CrossRef]
- Tian, X.; Yang, T.; He, J.; Chu, Q.; Jia, X.; Huang, J. Fungal community and cellulose-degrading genes in the composting process of Chinese medicinal herbal residues. Bioresour. Technol. 2017, 241, 374–383. [Google Scholar] [CrossRef]
- Antunes, L.P.; Martins, L.F.; Pereira, R.V.; Thomas, A.M.; Barbosa, D.; Lemos, L.N.; Silva, G.M.M.; Moura, L.M.S.; Epamino, G.W.C.; Digiampietri, L.A.; et al. Microbial community structure and dynamics in thermophilic composting viewed through metagenomics and metatranscriptomics. Sci. Rep. 2016, 6, 38915. [Google Scholar] [CrossRef] [PubMed]
- Bonito, G.; Isikhuemhen, O.S.; Vilgalys, R. Identification of fungi associated with municipal compost using DNA-based techniques. Bioresour. Technol. 2010, 101, 1021–1027. [Google Scholar] [CrossRef]
- Galitskaya, P.; Biktasheva, L.; Saveliev, A.; Grigoryeva, T.; Boulygina, E.; Selivanovskaya, S. Fungal and bacterial successions in the process of co-composting of organic wastes as revealed by 454 pyrosequencing. PLoS ONE 2017, 12, e0186051. [Google Scholar] [CrossRef]
- Partanen, P.; Hultman, J.; Paulin, L.; Auvinen, P.; Romantschuk, M. Bacterial diversity at different stages of the composting process. BMC Microbiol. 2010, 10, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Piazza, S.; Houbraken, J.; Meijer, M.; Cecchi, G.; Kraak, B.; Rosa, E.; Zotti, M. Thermotolerant and Thermophilic Mycobiota in Different Steps of Compost Maturation. Microorganisms 2020, 8, 880. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Bai, Y.; Li, X.; Ye, J.; Liao, H.; Cui, P.; Yu, Z.; Zhou, S. Linking microbial community structure with molecular composition of dissolved organic matter during an industrial-scale composting. J. Hazard. Mater. 2021, 405, 124281. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Gao, J.; Liang, D.; Zhu, Y.; Li, B.; Jin, H. Thermal pretreatment enhances the degradation and humification of lignocellulose by stimulating thermophilic bacteria during dairy manure composting. Bioresour. Technol. 2021, 319, 124149. [Google Scholar] [CrossRef]
- Yu, Z.; Tang, J.; Liao, H.; Liu, X.; Zhou, P.; Chen, Z.; Rensing, C.; Zhou, S. The distinctive microbial community improves composting efficiency in a full-scale hyperthermophilic composting plant. Bioresour. Technol. 2018, 265, 146–154. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, C.; Zhang, L.; Ma, Y.; Li, J.; Li, G.; Luo, W. Bacterial dynamics and functions for gaseous emissions and humification in response to aeration intensities during kitchen waste composting. Bioresour. Technol. 2021, 337, 125369. [Google Scholar] [CrossRef]
- Lüneberg, K.; Schneider, D.; Siebe, C.; Daniel, R. Drylands soil bacterial community is affected by land use change and different irrigation practices in the Mezquital Valley, Mexico. Sci. Rep. 2018, 8, 1413. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Pérez-Lebeña, E.; Martín-Gil, J.; Sánchez-Báscones, M.; Martín-Ramos, P. Applications of Streptomyces spp. Enhanced Compost in Sustainable Agriculture. In Biology of Composts, Soil Biology; Meghvansi, M.K., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 257–291. [Google Scholar] [CrossRef]
- Devi, P.; Kandasamy, S.; Chendrayan, K.; Uthandi, S. Laccase producing Streptomyces bikiniensis CSC12 isolated from compost. J. Microbiol. Biotechnol. Food Sci. 2016, 6, 794–798. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, P.; Das, P.; Solanki, R.; Kapur, M.K. Potential applications of extracellular enzymes from Streptomyces spp. in various industries. Arch Microbiol. 2020, 202, 1597–1615. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Awasthi, S.K.; Liu, T.; Zhang, Z.; Awasthi, M.K. Evaluation of integrated biochar with bacterial consortium on gaseous emissions mitigation and nutrients sequestration during pig manure composting. Bioresour. Technol. 2019, 291, 121880. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, X.; Huang, Y.; Huang, H. Inoculation with nitrogen turnover bacterial agent appropriately increasing nitrogen and promoting maturity in pig manure composting. Waste Manag. 2015, 39, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, C.; Wang, X.; Hai, L. Increased abundance of nitrogen transforming bacteria by higher C/N ratio reduces the total losses of N and C in chicken manure and corn stover mix composting. Bioresour. Technol. 2020, 297, 122410. [Google Scholar] [CrossRef] [PubMed]

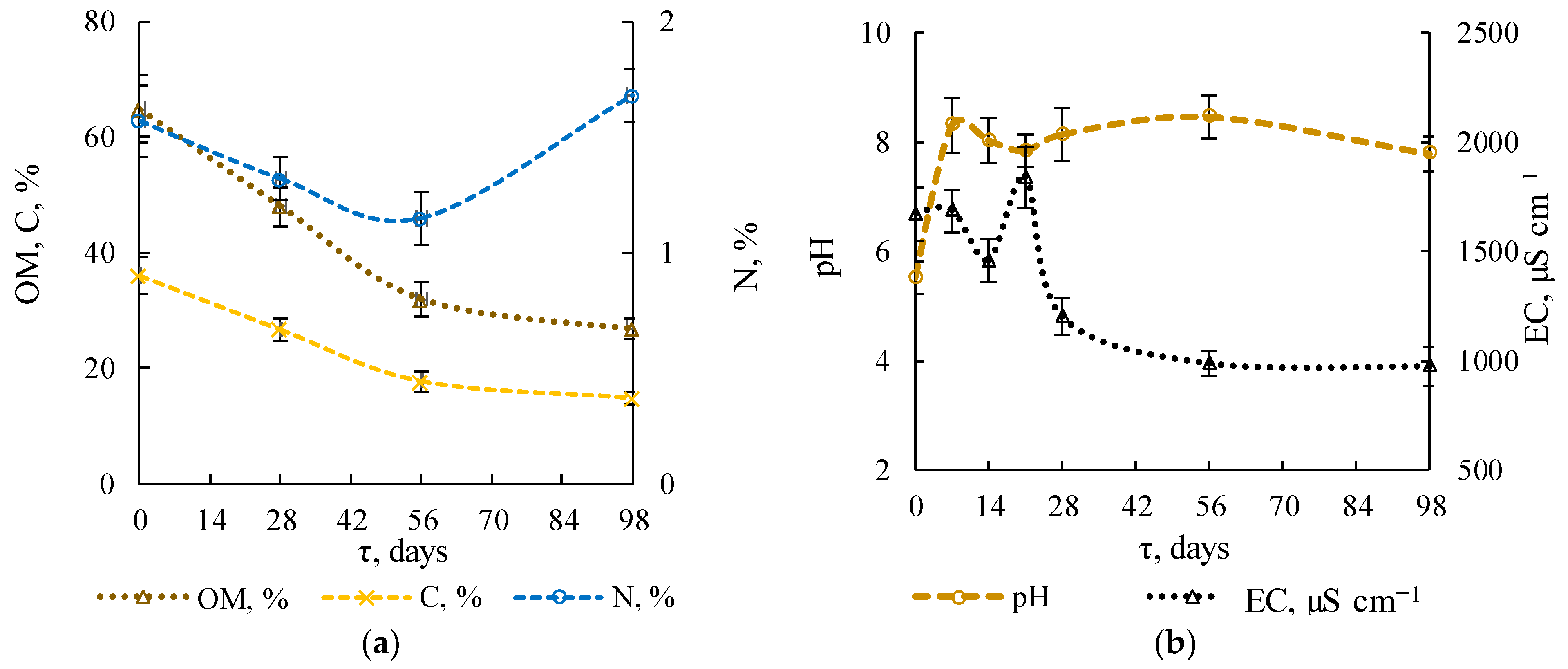
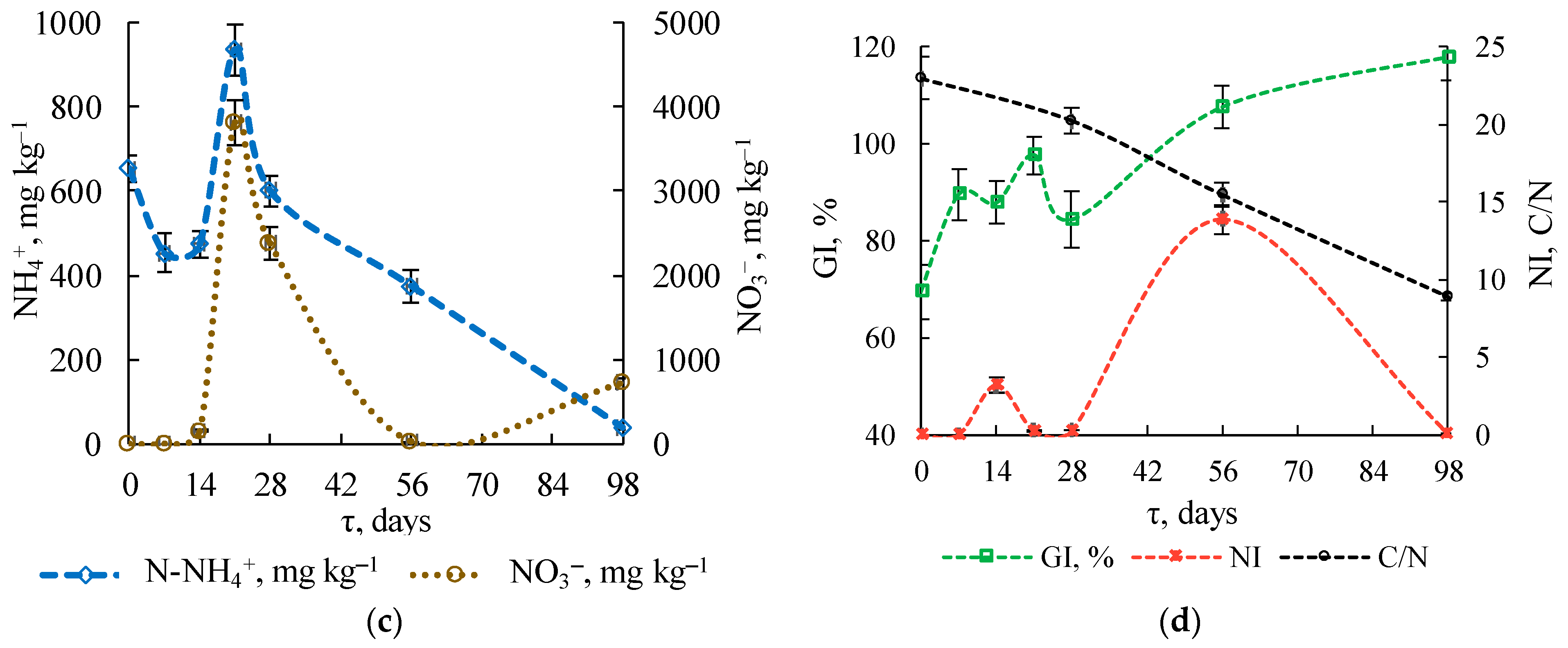

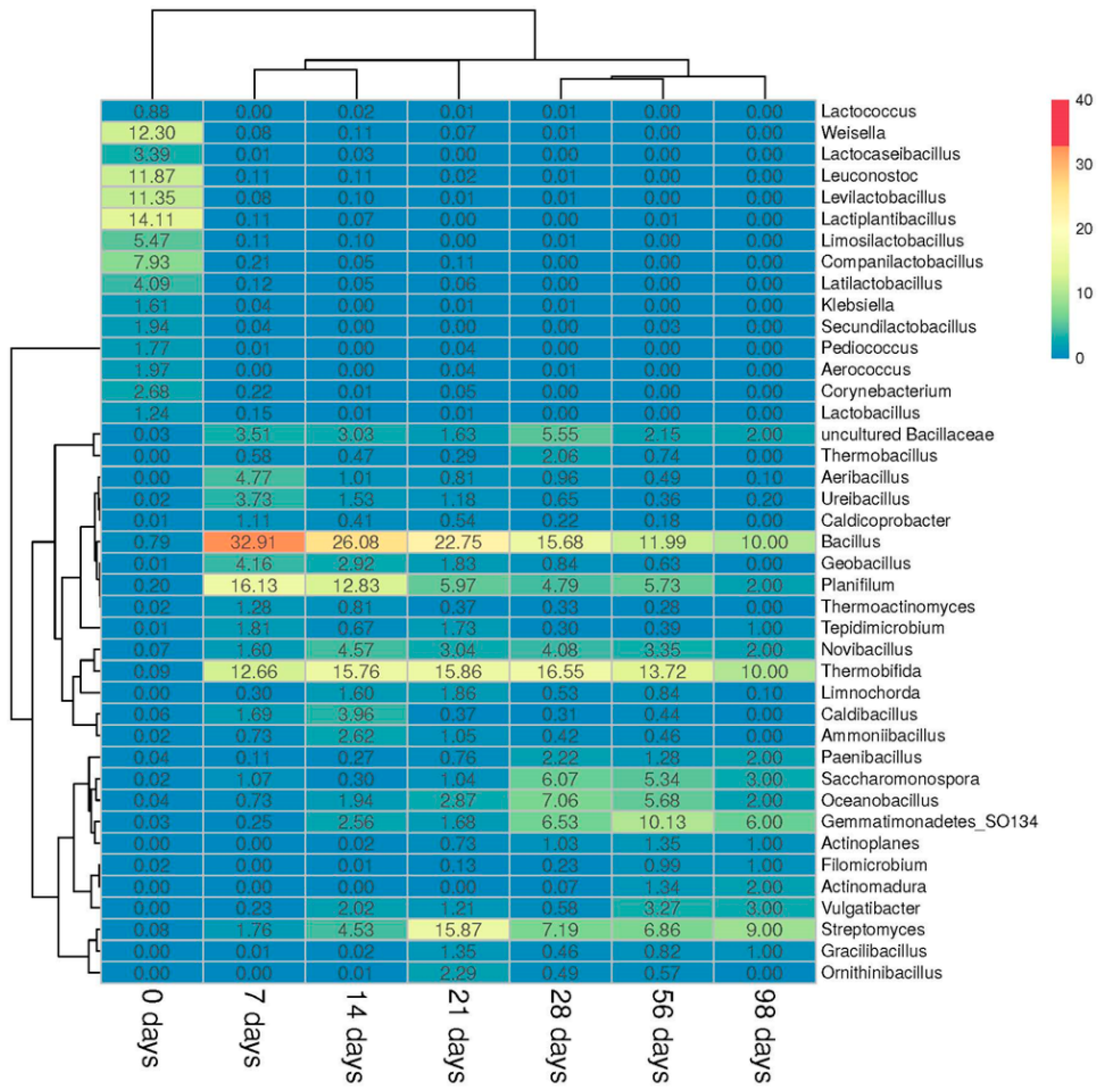
| Parameter | Units | Value * | Optimal Limits |
|---|---|---|---|
| pH | pH units | 5.5 ± 0.3 | 6.5–8.0 [22] |
| Electrical conductivity (EC) | µS cm−1 | 1670 ± 124 | |
| Water content | % | 60.26 ± 3.01 | 50–60% [22] 40–50% [23] |
| Total Kjeldahl nitrogen (N) | % | 1.57 ± 0.16 | |
| NH4+ | mg kg−1 | 653 ± 31 | |
| NO3− | mg kg−1 | 0.0 ± 0.0 | |
| Organic matter (OM) | % | 64.81 ± 5.83 | |
| Total organic carbon (C) | % | 36.0 ± 3.2 | |
| C/N | 22.9 ± 1.4 | 25–30 [11,22,23] | |
| Germination index (GI) | % | 69.5 ± 5.6 |
| Sample, Days | Number of OTUs | Chao1 | Shannon Index (Path) |
|---|---|---|---|
| Prokaryotes | |||
| 0 | 1166 | 3944 | 3.07 |
| 7 | 1324 | 4289 | 2.52 |
| 14 | 1193 | 5143 | 2.70 |
| 21 | 1461 | 5221 | 2.85 |
| 28 | 1146 | 3486 | 3.05 |
| 56 | 1368 | 5700 | 3.38 |
| 98 | 1781 | 7724 | 4.13 |
| Fungi | |||
| 0 | 215 | 266 | 3.15 |
| 7 | 351 | 398 | 4.95 |
| 14 | 145 | 218 | 1.88 |
| 21 | 96 | 176 | 0.28 |
| 28 | 39 | 95 | 0.12 |
| 56 | 75 | 125 | 0.63 |
| 98 | 82 | 151 | 1.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mironov, V.; Vanteeva, A.; Sokolova, D.; Merkel, A.; Nikolaev, Y. Microbiota Dynamics of Mechanically Separated Organic Fraction of Municipal Solid Waste during Composting. Microorganisms 2021, 9, 1877. https://doi.org/10.3390/microorganisms9091877
Mironov V, Vanteeva A, Sokolova D, Merkel A, Nikolaev Y. Microbiota Dynamics of Mechanically Separated Organic Fraction of Municipal Solid Waste during Composting. Microorganisms. 2021; 9(9):1877. https://doi.org/10.3390/microorganisms9091877
Chicago/Turabian StyleMironov, Vladimir, Anna Vanteeva, Diyana Sokolova, Alexander Merkel, and Yury Nikolaev. 2021. "Microbiota Dynamics of Mechanically Separated Organic Fraction of Municipal Solid Waste during Composting" Microorganisms 9, no. 9: 1877. https://doi.org/10.3390/microorganisms9091877
APA StyleMironov, V., Vanteeva, A., Sokolova, D., Merkel, A., & Nikolaev, Y. (2021). Microbiota Dynamics of Mechanically Separated Organic Fraction of Municipal Solid Waste during Composting. Microorganisms, 9(9), 1877. https://doi.org/10.3390/microorganisms9091877






