Assessment of a Multiplex LAMP Assay (Eazyplex® CSF Direct M) for Rapid Molecular Diagnosis of Bacterial Meningitis: Accuracy and Pitfalls
Abstract
:1. Introduction
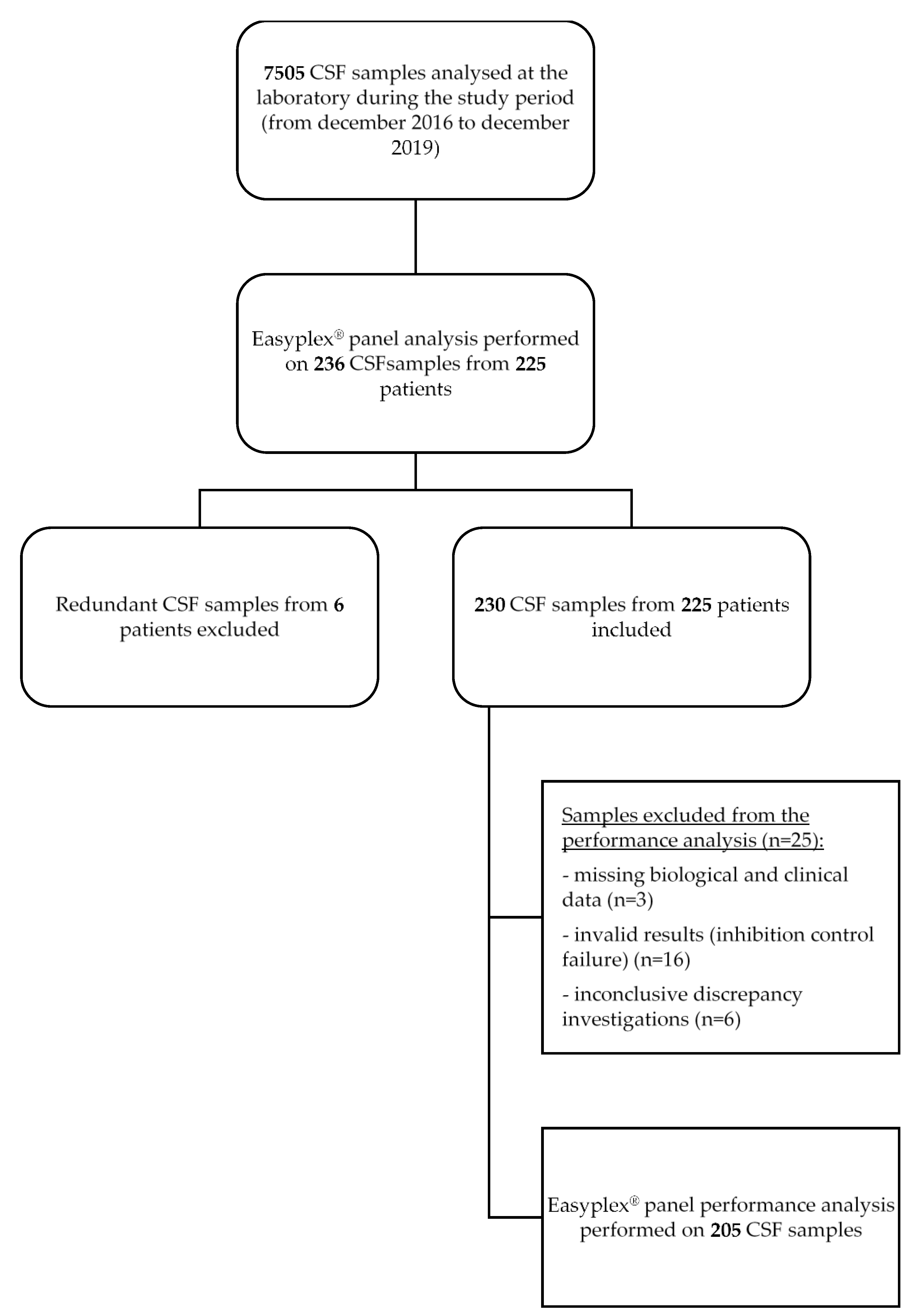
2. Materials and Methods
2.1. Clinical Samples
2.2. Ethics Approval
2.3. Eazyplex® CSF Direct M Testing
2.4. Routine Testing
2.5. Data Analysis and Discrepancy Investigation
2.6. Eazyplex® Panel Performance Analysis
2.7. Statistical Analysis
3. Results
3.1. Population and Sample Description
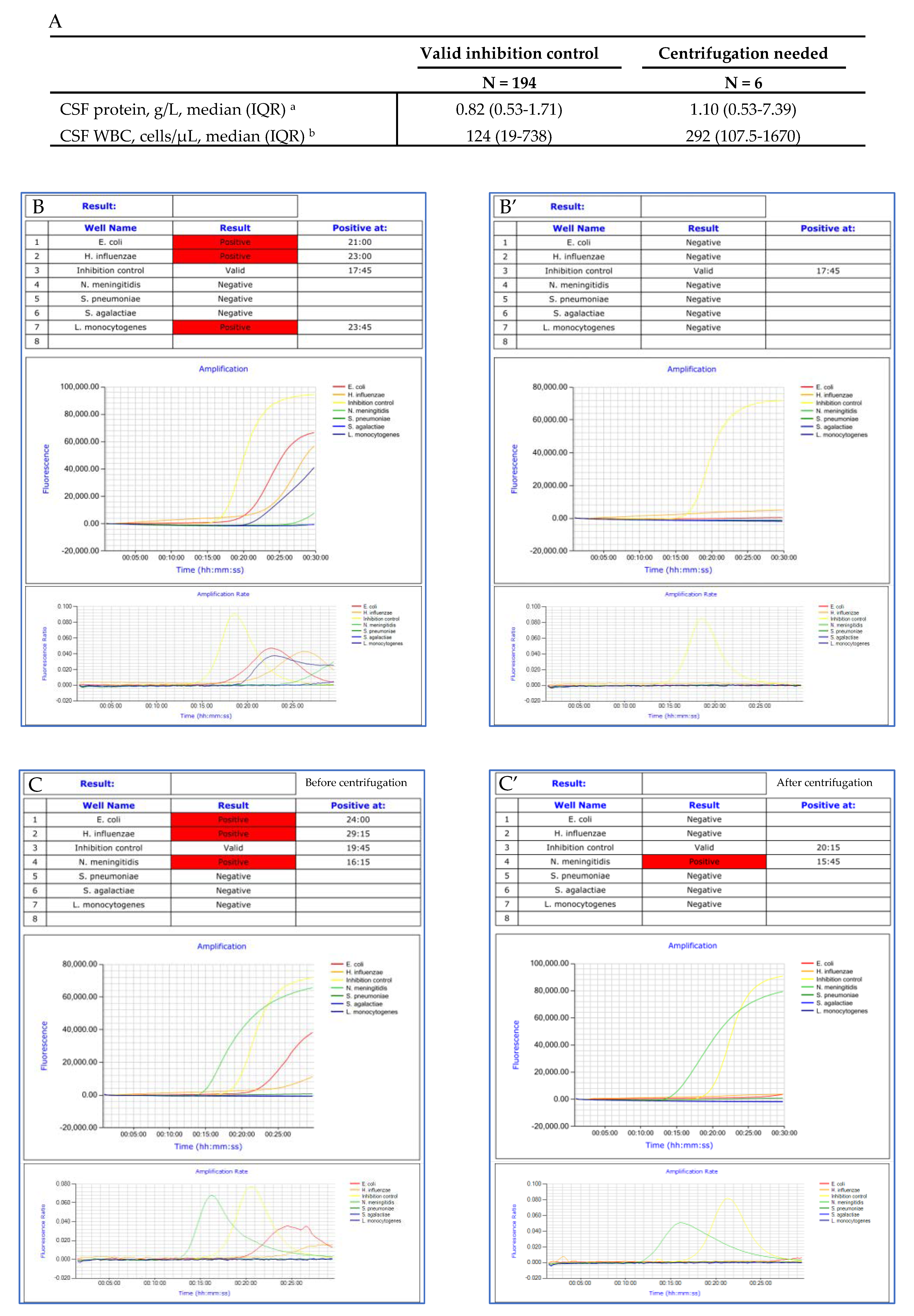
3.2. Impact of CSF Protein Concentration, WBC and RBC Counts on LAMP Inhibition
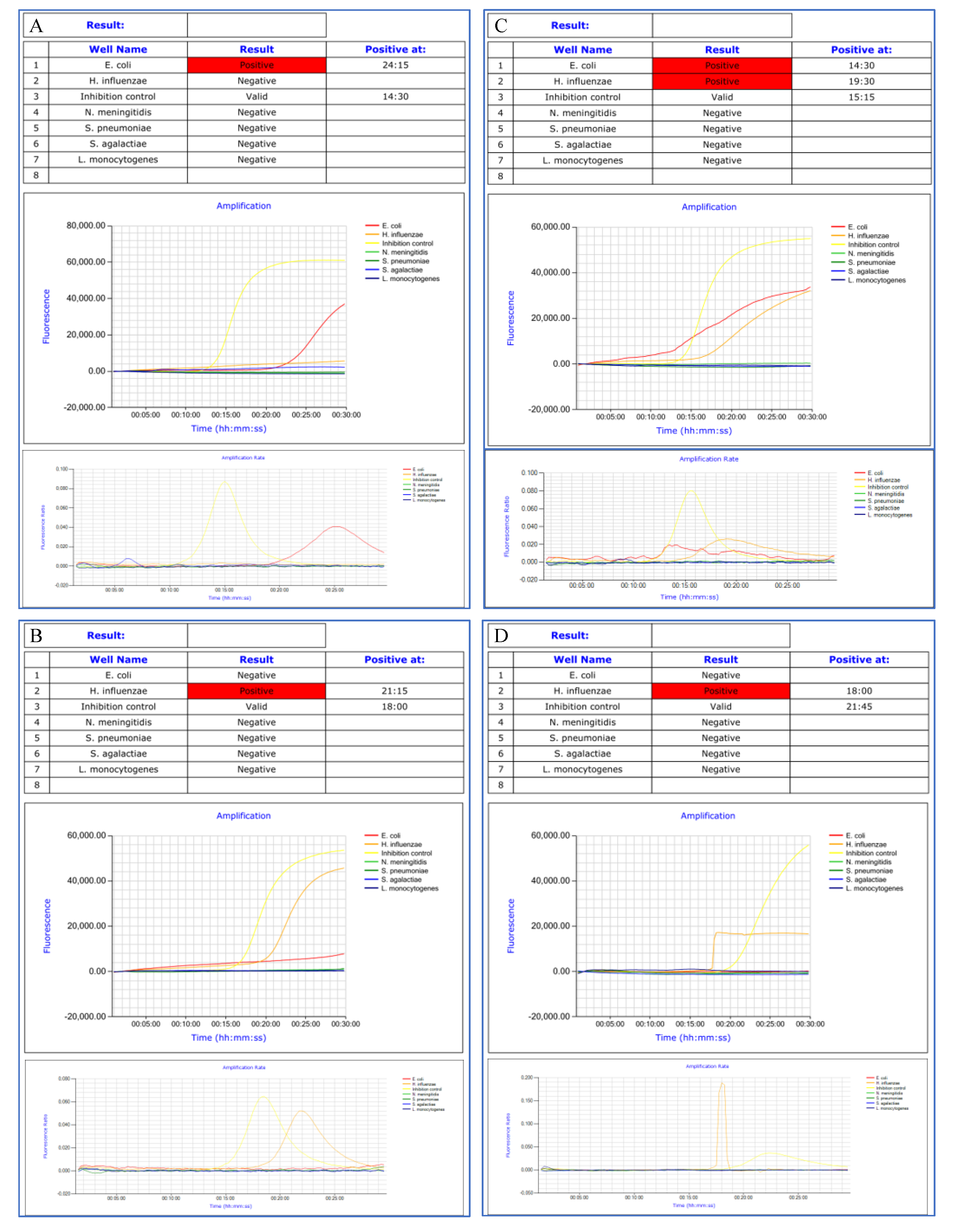
3.3. Amplification Curves Analysis
3.4. Eazyplex® Panel Results
3.5. Eazyplex® Panel Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lepur, D.; Barsić, B. Community-Acquired Bacterial Meningitis in Adults: Antibiotic Timing in Disease Course and Outcome. Infect. 2007, 35, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Glimåker, M.; Johansson, B.; Grindborg, Ö.; Bottai, M.; Lindquist, L.; Sjölin, J. Adult Bacterial Meningitis: Earlier Treatment and Improved Outcome Following Guideline Revision Promoting Prompt Lumbar Puncture. Clin. Infect. Dis. 2015, 60, 1162–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoen, B.; Varon, E.; de Debroucker, T.; Fantin, B.; Grimprel, E.; Wolff, M.; Duval, X. Management of Acute Community-Acquired Bacterial Meningitis (Excluding Newborns). Long Version with Arguments. Med. Mal. Infect. 2019, 49, 405–441. [Google Scholar] [CrossRef] [PubMed]
- van de Beek, D.; Cabellos, C.; Dzupova, O.; Esposito, S.; Klein, M.; Kloek, A.T.; Leib, S.L.; Mourvillier, B.; Ostergaard, C.; Pagliano, P.; et al. ESCMID Guideline: Diagnosis and Treatment of Acute Bacterial Meningitis. Clin. Microbiol. Infect. 2016, 22 (Suppl. 3), S37–S62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feagins, A.R.; Ronveaux, O.; Taha, M.-K.; Caugant, D.A.; Smith, V.; Fernandez, K.; Glennie, L.; Fox, L.M.; Wang, X. Next Generation Rapid Diagnostic Tests for Meningitis Diagnosis. J. Infect. 2020, 81, 712–718. [Google Scholar] [CrossRef] [PubMed]
- D’Inzeo, T.; Menchinelli, G.; De Angelis, G.; Fiori, B.; Liotti, F.M.; Morandotti, G.A.; Sanguinetti, M.; Posteraro, B.; Spanu, T. Implementation of the Eazyplex® CSF Direct Panel Assay for Rapid Laboratory Diagnosis of Bacterial Meningitis: 32-Month Experience at a Tertiary Care University Hospital. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Tunkel, A.R.; Hartman, B.J.; Kaplan, S.L.; Kaufman, B.A.; Roos, K.L.; Scheld, W.M.; Whitley, R.J. Practice Guidelines for the Management of Bacterial Meningitis. Clin. Infect. Dis. 2004, 39, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, A.J.; Holmberg, K.M.; Daly, J.A.; Leber, A.L.; Dien Bard, J.; Korgenski, E.K.; Bourzac, K.M.; Kanack, K.J. Retrospective Evaluation of Infants Aged 1 to 60 Days with Residual Cerebrospinal Fluid (CSF) Tested Using the FilmArray Meningitis/Encephalitis (ME) Panel. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudet, A.; Pantel, A.; Carles, M.-J.; Boclé, H.; Charachon, S.; Enault, C.; Stéphan, R.; Cadot, L.; Lavigne, J.-P.; Marchandin, H. A Review of a 13-Month Period of FilmArray Meningitis/Encephalitis Panel Implementation as a First-Line Diagnosis Tool at a University Hospital. PLoS ONE 2019, 14, e0223887. [Google Scholar] [CrossRef] [PubMed]
- Liesman, R.M.; Strasburg, A.P.; Heitman, A.K.; Theel, E.S.; Patel, R.; Binnicker, M.J. Evaluation of a Commercial Multiplex Molecular Panel for Diagnosis of Infectious Meningitis and Encephalitis. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leber, A.L.; Everhart, K.; Balada-Llasat, J.-M.; Cullison, J.; Daly, J.; Holt, S.; Lephart, P.; Salimnia, H.; Schreckenberger, P.C.; DesJarlais, S.; et al. Multicenter Evaluation of BioFire FilmArray Meningitis/Encephalitis Panel for Detection of Bacteria, Viruses, and Yeast in Cerebrospinal Fluid Specimens. J. Clin. Microbiol. 2016, 54, 2251–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Chen, S.-Y.; Chien, J.-Y.; Lee, T.-F.; Chen, J.-M.; Hsueh, P.-R. Usefulness of the FilmArray Meningitis/Encephalitis (M/E) Panel for the Diagnosis of Infectious Meningitis and Encephalitis in Taiwan. J. Microbiol. Immunol. Infect. 2019, 52, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Tansarli, G.S.; Chapin, K.C. Diagnostic Test Accuracy of the BioFire® FilmArray® Meningitis/Encephalitis Panel: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2020, 26, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Nigrovic, L.E.; Malley, R.; Macias, C.G.; Kanegaye, J.T.; Moro-Sutherland, D.M.; Schremmer, R.D.; Schwab, S.H.; Agrawal, D.; Mansour, K.M.; Bennett, J.E.; et al. Effect of Antibiotic Pretreatment on Cerebrospinal Fluid Profiles of Children with Bacterial Meningitis. Pediatrics 2008, 122, 726–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohr, V.; Rasmussen, N.; Hansen, B.; Kjersem, H.; Jessen, O.; Johnsen, N.; Kristensen, H.S. 875 Cases of Bacterial Meningitis: Diagnostic Procedures and the Impact of Preadmission Antibiotic Therapy. Part III of a Three-Part Series. J. Infect. 1983, 7, 193–202. [Google Scholar] [CrossRef]
- Srinivasan, L.; Pisapia, J.M.; Shah, S.S.; Halpern, C.H.; Harris, M.C. Can Broad-Range 16S Ribosomal Ribonucleic Acid Gene Polymerase Chain Reactions Improve the Diagnosis of Bacterial Meningitis? A Systematic Review and Meta-Analysis. Ann. Emerg. Med. 2012, 60, 609–620.e2. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-Mediated Isothermal Amplification of DNA. Nucleic. Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seki, M.; Kilgore, P.E.; Kim, E.J.; Ohnishi, M.; Hayakawa, S.; Kim, D.W. Loop-Mediated Isothermal Amplification Methods for Diagnosis of Bacterial Meningitis. Front. Pediatr. 2018, 6, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| All Samples | Results by Age Groups | |||||
|---|---|---|---|---|---|---|
| N = 230 | ≤2 Months | 3–11 Months | 1–15 Years | 16–64 Years | ≥65 Years | |
| N = 24 | N = 6 | N = 29 | N = 117 | N = 54 | ||
| Positive results, number (% of total) | 32 (13.9) | 3 (12.5) | 3 (50) | 8 (27.6) | 14 (12) | 4 (7.4) |
| N. meningitidis, number (% of positive samples) | 16 (50) | 0 (0) | 2 (66.7) | 5 (62.5) | 8 (57.2) | 1 (25) |
| S. pneumoniae, number (% of positive samples) | 9 (28) | 0 (0) | 1 (33.3) | 2 (25) | 3 (21.5) | 3 (75) |
| E. coli, number (% of positive samples) | 2 (6.3) | 1 (33.3) | 0 (0) | 0 (0) | 1 (7.1) | 0 (0) |
| S. agalactiae, number (% of positive samples) | 2 (6.3) | 1 (33.3) | 0 (0) | 0 (0) | 1 (7.1) | 0 (0) |
| H. influenzae, number (% of positive samples) | 2 (6.3) | 0 (0) | 0 (0) | 1 (12.5) | 1 (7.1) | 0 (0) |
| L. monocytogenes, number (% of positive samples) | 1 (3.1) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Negative results, number (% of total) | 182 (79.1) | 18 (75) | 3 (50) | 20 (69) | 93 (79.5) | 48 (88.9) |
| Invalid results, number (% of total) | 16 (7) | 3 (12.5) | 0 (0) | 1 (3.4) | 10 (8.5) | 2 (3.7) |
| Target | PPA | NPA | PPV | NPV | ||||
|---|---|---|---|---|---|---|---|---|
| TP/(TP + FN) | % | TN/(TN + FP) | % | TP/(TP + FP) | % | TN/(TN + FN) | % | |
| N. meningitidis | 16/17 | 94.1 | 170/170 | 100 | 16/16 | 100 | 170/171 | 99.4 |
| S. pneumoniae | 9/9 | 100 | 170/170 | 100 | 9/9 | 100 | 170/170 | 100 |
| E. coli | 2/4 | 50 | 170/170 | 100 | 2/2 | 100 | 170/172 | 98.8 |
| S. agalactiae | 2/2 | 100 | 170/170 | 100 | 2/2 | 100 | 170/170 | 100 |
| H. influenzae | 2/2 | 100 | 170/170 | 100 | 2/2 | 100 | 170/170 | 100 |
| L. monocytogenes | 1/1 | 100 | 170/170 | 100 | 1/1 | 100 | 170/170 | 100 |
| Overall | 32/35 | 91.4 | 170/170 | 100 | 32/32 | 100 | 170/173 | 98.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leroy, A.-G.; Persyn, E.; Gibaud, S.-A.; Crémet, L.; Le Turnier, P.; Benhamida, M.; Launay, E.; Guillouzouic, A.; Bémer, P.; Corvec, S.; et al. Assessment of a Multiplex LAMP Assay (Eazyplex® CSF Direct M) for Rapid Molecular Diagnosis of Bacterial Meningitis: Accuracy and Pitfalls. Microorganisms 2021, 9, 1859. https://doi.org/10.3390/microorganisms9091859
Leroy A-G, Persyn E, Gibaud S-A, Crémet L, Le Turnier P, Benhamida M, Launay E, Guillouzouic A, Bémer P, Corvec S, et al. Assessment of a Multiplex LAMP Assay (Eazyplex® CSF Direct M) for Rapid Molecular Diagnosis of Bacterial Meningitis: Accuracy and Pitfalls. Microorganisms. 2021; 9(9):1859. https://doi.org/10.3390/microorganisms9091859
Chicago/Turabian StyleLeroy, Anne-Gaëlle, Elise Persyn, Sophie-Anne Gibaud, Lise Crémet, Paul Le Turnier, Myriam Benhamida, Elise Launay, Aurélie Guillouzouic, Pascale Bémer, Stéphane Corvec, and et al. 2021. "Assessment of a Multiplex LAMP Assay (Eazyplex® CSF Direct M) for Rapid Molecular Diagnosis of Bacterial Meningitis: Accuracy and Pitfalls" Microorganisms 9, no. 9: 1859. https://doi.org/10.3390/microorganisms9091859
APA StyleLeroy, A.-G., Persyn, E., Gibaud, S.-A., Crémet, L., Le Turnier, P., Benhamida, M., Launay, E., Guillouzouic, A., Bémer, P., Corvec, S., & on behalf of the Western French Study Group on Early Bacterial Meningitis. (2021). Assessment of a Multiplex LAMP Assay (Eazyplex® CSF Direct M) for Rapid Molecular Diagnosis of Bacterial Meningitis: Accuracy and Pitfalls. Microorganisms, 9(9), 1859. https://doi.org/10.3390/microorganisms9091859






