Bayesian Estimation of the Prevalence and Test Characteristics (Sensitivity and Specificity) of Two Serological Tests (RB and SAT-EDTA) for the Diagnosis of Bovine Brucellosis in Small and Medium Cattle Holders in Ecuador
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study
2.2. Diagnostic Assays
2.3. Statistical Analysis
3. Results
3.1. Apparent Prevalence at Animal Level
3.2. Agreement between Tests
3.3. True Prevalence at Animal Level
3.4. Test Characteristics of RB and SAT-EDTA
3.5. Relationship between Test Results and the Status of the Disease
3.6. Prevalence at Animal Level on Farms with and without Vaccination
3.7. Apparent and True Prevalence at Herd Level
3.8. Risk Factors for Farm Seropositivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
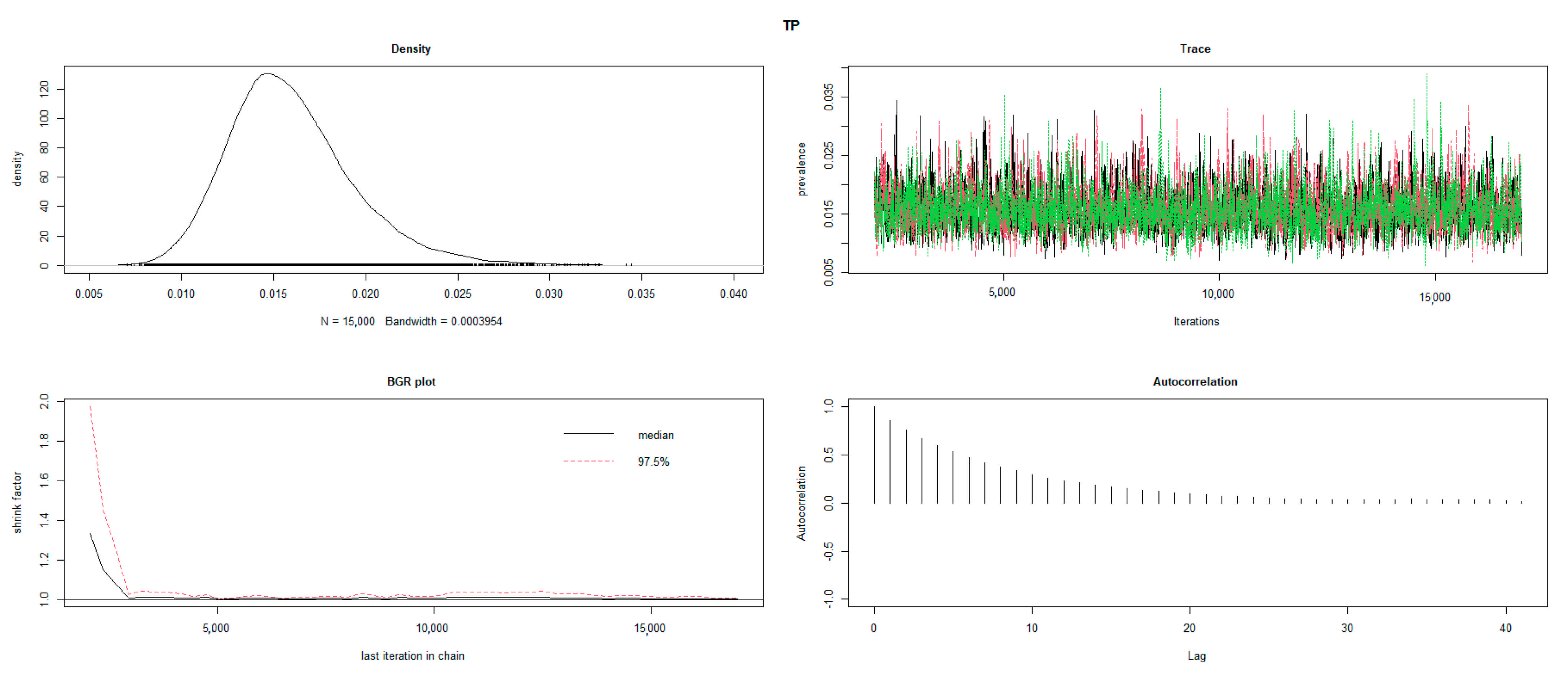
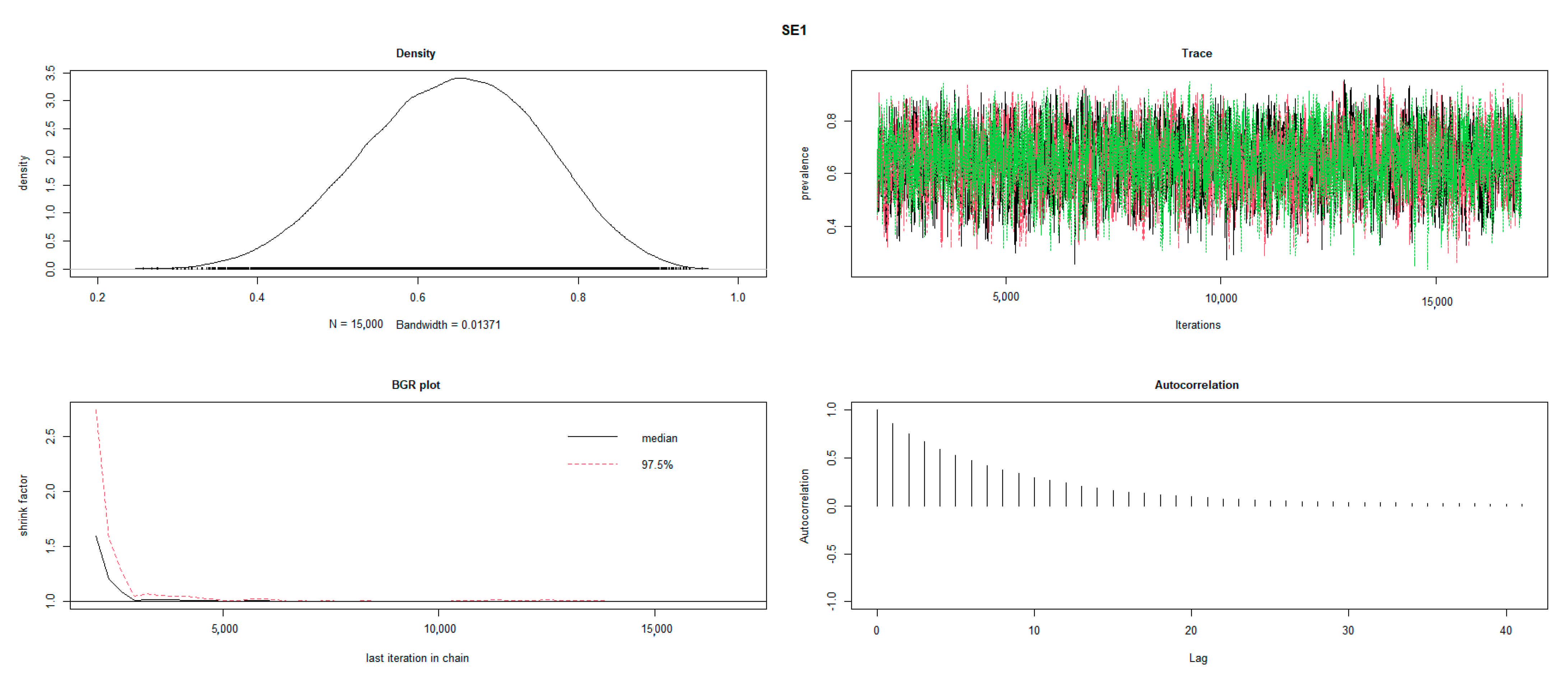


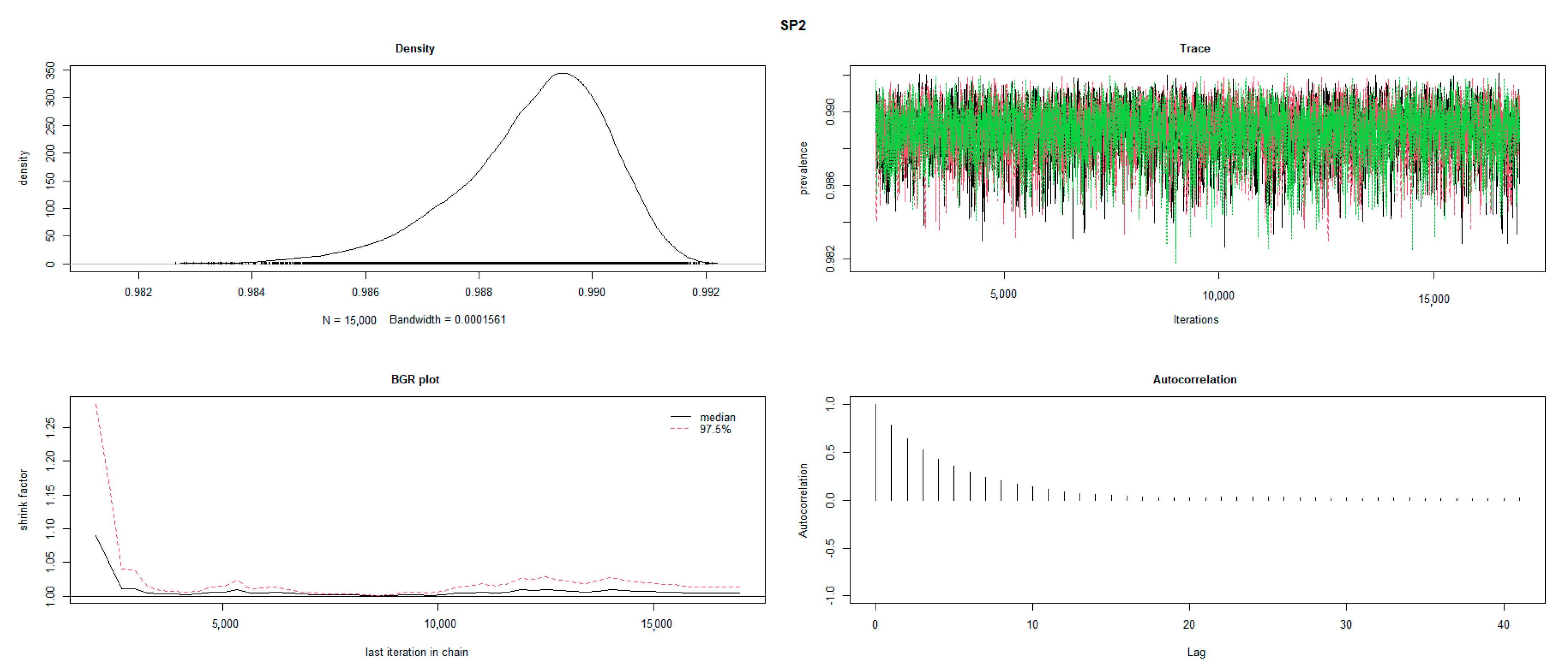
| Region | MODEL 1 | MODEL 2 | REFERENCES | ||||
|---|---|---|---|---|---|---|---|
| Parameter | Value | Distribution | Parameter | Value | Distribution | ||
| Coastal Region | theta [1] | 4.0% | Beta = (9, 193) | TP | 4% | Beta = (9, 193) | [3] |
| theta [2] | 66.4–96.0% | Beta = (16, 6) | SE [1] | 66.4–96.0% | Beta = (16, 6) | [37] | |
| theta [3] | 71.0–99.0% | Uniform = (0.71, 0.99) | SP [1] | 71.0–99.0% | Uniform = (0.71, 0.99) | [37] | |
| theta [4] | NI | Uniform = (0.00, 1.00) | SE [2] | 50.0–99.0% | Uniform = (0.55, 0.99) | [38] | |
| theta [5] | NI | Uniform = (0.00, 1.00) | SP [2] | 90.0–100.0% | Uniform = (0.90, 1.0) | [38] | |
| theta [6] | NI | Uniform = (0.00, 1.00) | a [1] | NI | Uniform = (0.00, 0.25) | - | |
| theta [7] | NI | Uniform = (0.00, 1.00) | b [1] | NI | Uniform = (−0.25, 0.25) | - | |
| Northern Highlands | theta [1] | 2.5% | Beta = (5, 197) | TP | 2.5% | Beta = (5, 197) | [3] |
| theta [2] | 66.4–96.0% | Beta = (16, 6) | SE [1] | 66.4–96.0% | Beta = (16, 6) | [37] | |
| theta [3] | 71–99% | Uniform = (0.71, 0.99) | SP [1] | 71.0–99.0% | Uniform = (0.71, 0.99) | [37] | |
| theta [4] | NI | Uniform = (0.70, 1.00) | SE [2] | 50.0–99.0% | Uniform = (0.55, 0.99) | [38] | |
| theta [5] | NI | Uniform = (0.00, 1.00) | SP [2] | 90.0–100.0% | Uniform = (0.90, 1.00) | [38] | |
| theta [6] | NI | Uniform = (0.00, 1.00) | a [1] | NI | Uniform = (0.00, 0.25) | - | |
| theta [7] | NI | Uniform = (0.00, 1.00) | b [1] | NI | Uniform = (−0.25, 0.25) | - | |
| Southern Highlands | theta [1] | 0.5% | Beta = (1, 201) | TP | 0.5% | Beta = (1, 201) | [3] |
| theta [2] | 66.4–96.0% | Beta = (16, 6) | SE [1] | 66.4–96.0% | Beta = (16, 6) | [37] | |
| theta [3] | 71.0–99.0% | Beta = (20, 2) | SP [1] | 71.0–99.0% | Beta = (20, 2) | [37] | |
| theta [4] | NI | Uniform = (0.40, 1.00) | SE [2] | 30.0%–90.0% | Uniform = (0.30, 0.90) | Supposed | |
| theta [5] | NI | Uniform = (0.00, 0.60) | SP [2] | 90.0–100.0% | Uniform = (0.90, 1.00) | [38] | |
| theta [6] | NI | Uniform = (0.00, 1.00) | a [1] | NI | Uniform = (0.00, 0.25) | - | |
| theta [7] | NI | Uniform = (0.00, 0.60) | b [1] | NI | Uniform = (−0.25, 0.25) | - | |
| Amazon Region | theta [1] | 1% | Beta = (2, 200) | TP | 1% | Beta = (2, 200) | [57] |
| theta [2] | 66.4–96.0% | Beta = (18, 4) | SE [1] | 66.4–96.0% | Beta = (18, 4) | [37] | |
| theta [3] | 71.0–99.0% | Beta = (21, 1) | SP [1] | 71.0–99.0% | Beta = (21, 1) | [37] | |
| theta [4] | NI | Uniform = (0.80, 1.00) | SE [2] | 50.0–99.0% | Uniform = (0.55, 0.99) | [38] | |
| theta [5] | NI | Uniform = (0.00, 0.60) | SP [2] | 90.0–100.0% | Uniform = (0.90, 1.00) | [38] | |
| theta [6] | NI | Uniform = (0.50, 1.00) | a [1] | NI | Uniform = (0.00, 0.25) | - | |
| theta [7] | NI | Uniform = (0.00, 0.60) | b [1] | NI | Uniform = (−0.25, 0.25) | - | |
| REGION | MODEL 1 | REFERENCES | ||
|---|---|---|---|---|
| Parameter | Value | Distribution | ||
| With and without vaccination | theta [1] | 3% | Beta = (7, 195) | [3] |
| theta [2] | 66.4–96.0% | Beta = (17, 5) | [37] | |
| theta [3] | 71.0–99.0% | Beta = (20, 2) | [37] | |
| theta [4] | NI | Uniform = (0.00, 1.00) | - | |
| theta [5] | NI | Uniform = (0.00, 1.00) | - | |
| theta [6] | NI | Uniform = (0.00, 1.00) | - | |
| theta [7] | NI | Uniform = (0.00, 1.00) | - | |
| Region | Parameter | Model 1 (%) | Model 2 (%) |
|---|---|---|---|
| Mean (CrI) | Mean (CrI) | ||
| Coastal Region | True prevalence | 2.5% (1.3–3.8%) | 2.5% (1.5–3.5%) |
| 72.4 (51.4–89.5%) | 72.6% (58.2–87.5%) | ||
| 98.5 (97.7–99.0%) | 98.5% (97.8–99.0%) | ||
| 69.6 (47.8–88.6%) | 70.1% (56.3–86.9%) | ||
| 98.6 (97.7–99.1%) | 98.6% (97.9–99.1%) | ||
| cov_a | 0.150 (0.043–0.226) | 0.157 (0.060–0.223) | |
| cov_b | 0.014 (0.010–0.023) | 0.013 (0.008–0.020) | |
| BGR | 1.00 | 1.01 | |
| Bayes-p | 0.52 | 1.00 | |
| DIC | 20.07 | 36.72 | |
| Northern Highlands | True prevalence | 1.0% (0.4–1.7%) | 1.0% (0.4–1.6%) |
| 73.2% (53.2–89.2%) | 72.6% (57.1–87.2%) | ||
| 98.7% (98.2–99.0%) | 98.7% (98.2–99.0%) | ||
| 70.8% (48.5–91.2%) | 70.4% (56.1–89.6%) | ||
| 98.7% (98.3–99.1%) | 98.7% (98.3–99.1%) | ||
| cov_a | 0.121 (0.005–0.215) | 0.128 (0.019–0.214) | |
| cov_b | 0.013 (0.010–0.018) | 0.012 (0.009–0.016) | |
| BGR | 1.0009 | 1.0495 | |
| Bayes-p | 0.54 | 1.00 | |
| DIC | 17.81 | 32.170 | |
| Southern Highlands | Real prevalence | 0.1% (0.0–0.3%) | 0.1% (0.0–0.2%) |
| 73.0% (53.2–88.8%) | 71.7% (52.5–87.2%) | ||
| 99.8% (99.6–99.9%) | 99.8% (99.7–99.9%) | ||
| 59.4% (33.9–85.5%) | 59.7% (32.2–86.7%) | ||
| 99.8% (99.6–99.9%) | 99.8% (99.7–99.9%) | ||
| cov_a | 0.028 (−0.134–0.176) | 0.088 (0.005–0.198) | |
| cov_b | 0.002 (0.000–0.004) | 0.002 (0.001–0.003) | |
| BGR | 1.00 | 1.03 | |
| Bayes-p | 0.66 | 1.00 | |
| DIC | 11.36 | 18.41 | |
| Amazon Region | Real prevalence | 0.8% (0.1–1.8%) | 0.5% (0.1–1.4%) |
| 81.8% (64.0–94.5%) | 78.8% (61.0–92.4%) | ||
| 99.4% (98.5–100.0%) | 99.1% (98.4–99.9%) | ||
| 81.0% (64.2–93.8%) | 80.3% (58.2–96.7%) | ||
| 99.2% (98.2–99.8%) | 98.9% (98.1–99.7%) | ||
| cov_a | 0.060 (0.020–0.109) | 0.073 (0.003–0.179) | |
| cov_b | 0.006 (0.000–0.015) | 0.008 (0.001–0.015) | |
| BGR | 1.0002 | 1.0081 | |
| Bayes-p | 0.66 | 1.00 | |
| DIC | 11.87 | 20.27 |
References
- Spickler, A. Brucelosis; The Center for Food Security & Public Health-Iowa State University: Ames, IA, USA, 2009; pp. 1–15. [Google Scholar]
- European Court of Auditors. Programas de Erradicación, Control y Vigilancia Para Combatir las Enfermedades Animales; Informe Especial no. 06; European Court of Auditors Publications Office: Luxembourg, 2016; ISBN 978-92-872-4483-3. [Google Scholar]
- Torres, H. Programa Nacional de Control de Brucelosis Bovina; Ministerio de Agricultura, Ganadería, Acuacultura y Pesca (MAGAP), Servicio Ecuatoriano de Saniadad Agropecuaria (SESA): Quito, Ecuador, 2008; pp. 3–98. [Google Scholar]
- Carbonero, A.; Guzmán, L.; García-Bocanegra, I.; Borge, C.; Adaszek, L.; Arenas, A.; Saa, L.R. Seroprevalence and risk factors associated with Brucella seropositivity in dairy and mixed cattle herds from Ecuador. Trop. Anim. Health Prod. 2017, 50, 197–203. [Google Scholar] [CrossRef]
- Poulsen, K.P.; Hutchins, F.T.; McNulty, C.M.; Tremblay, M.; Zabala, C.; Barragan, V.; Lopez, L.; Trueba, G.; Bethel, J.W. Brucellosis in dairy cattle and goats in northern ecuador. Am. J. Trop. Med. Hyg. 2014, 90, 712–715. [Google Scholar] [CrossRef] [Green Version]
- PNSA; MAG. Programa Nacional de Sanidad Animal del Ministerio de Agricultura y Ganadería; PNSA; MAG: Quito, Ecuador, 1979; pp. 1–72. [Google Scholar]
- Godfroid, J.; Nielsen, K.; Saegerman, C. Diagnosis of brucellosis in livestock and wildlife. Croat. Med. J. 2010, 51, 296–305. [Google Scholar] [CrossRef] [Green Version]
- Yagupsky, P. Detection of brucellae in blood cultures. J. Clin. Microbiol. 1999, 37, 3437–3442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yagupsky, P.; Morata, P.; Colmenero, J.D. Laboratory diagnosis of human brucellosis. Clin. Microbiol. Rev. 2019, 33. [Google Scholar] [CrossRef]
- Lucero, N.E.; Ayala, S.M.; Escobar, G.I.; Jacob, N.R. Brucella isolated in humans and animals in Latin America from 1968 to 2006. Epidemiol. Infect. 2007, 136, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Sbriglio, J.L.; Sbriglio, D.H.; Sainz, B.S. Una patología generalmente subdiagnosticada en Humanos y que impacta negativamente en la producción pecuaria y desarrollo de nuestros países. Rev. Bioanálisis 2007, 5, 18–22. [Google Scholar]
- OIE. Brucelosis (Brucella abortus, melitensis y suis). In Manual Terrestre de la OIE (Capítulo 2.14); OIE: Paris, France, 2016; pp. 1–44. [Google Scholar]
- Adamu, S.G.; Atsanda, N.N.; Tijjani, A.O.; Usur, A.M.; Sule, A.G.; Gulani, I.A. Epidemiological study of bovine brucellosis in three senatorial zones of Bauchi State, Nigeria. Veter. World 2016, 9, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Lukambagire, A.S.; Mendes, Â.J.; Bodenham, R.F.; McGiven, J.A.; Mkenda, N.A.; Mathew, C.; Rubach, M.P.; Sakasaka, P.; Shayo, D.D.; Maro, V.P.; et al. Performance characteristics and costs of serological tests for brucellosis in a pastoralist community of northern Tanzania. Sci. Rep. 2021, 11, 5480. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Nam, H.; Kim, J.; Heo, E.; Cho, Y.S.; Hwang, I.; Kim, J.; Jung, S.; More, S.; Kim, J. Quantitative rose bengal test for diagnosis of bovine brucellosis. J. Immunoass. Immunochem. 2010, 31, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.-H.; Teng, J.-J.; Chao, S.; Chao, H.; Waghela, S.D. Comparison of the rose bengal plate and the complement fixation tests with the tube agglutination test for diagnosis of human brucellosis. Open J. Clin. Diagn. 2017, 07, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Smits, H.L.; Kadri, S.M. Brucellosis in India: A deceptive infectious disease. Indian J. Med. Res. 2005, 122, 375–384. [Google Scholar] [PubMed]
- Ron-Román, J.; Ron-Garrido, L.; Abatih, E.; Celi-Erazo, M.; Vizcaíno-Ordóñez, L.; Calva-Pacheco, J.; González-Andrade, P.; Berkvens, D.; Benítez-Ortíz, W.; Brandt, J.; et al. Bayesian evaluation of three serological tests for detecting antibodies against Brucella spp. among humans in the northwestern part of ecuador. Am. J. Trop. Med. Hyg. 2019, 100, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Cowling, D.W.; Gardner, I.A.; Johnson, W.O. Comparison of methods for estimation of individual-level prevalence based on pooled samples. Prev. Veter. Med. 1999, 39, 211–225. [Google Scholar] [CrossRef]
- Gardner, I.A. The utility of Bayes’ theorem and Bayesian inference in veterinary clinical practice and research. Aust. Veter. J. 2002, 80, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Gyorkos, T.W.; Coupal, L. Bayesian estimation of disease prevalence and the parameters of diagnostic tests in the absence of a gold standard. Am. J. Epidemiol. 1995, 141, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, Z.L. La Brucelosis Bovina y Sus Factores de Riesgo: Evaluación a Nivel Mundial y en Colombia. Ph.D. Thesis, Universitat Autònoma de Barcelona, Barcelona, Spain, 2018. [Google Scholar]
- Diallo, A.; Khounsy, S. Subscripciones. 2013, pp. 1–8. Available online: www.oie.int/boutique (accessed on 11 August 2021).
- Benítez, W.; Ron-Román, J.; Enríquez, S.; Ron-Garrido, L. Proyecto, Encuesta Nacional Sobre Brucelosis, Tuberculosis y Garrapatas. Available online: http://www.uce.edu.ec/home (accessed on 11 August 2021).
- Vizcaino, D.; Estrella, V.; Villareal, V. Instructivo Para los Procesos de Certicación y Recertificación de Predios Libres de Brucelosis y Tuberculosis Bovina. Gest. Manejo Control Enferm. Anim. Programa Nac. Control Brucelosis Tuberc. Bov. 2016, 34, 1–28. [Google Scholar]
- Humphry, R.W.; Cameron, A.; Gunn, G.J. A practical approach to calculate sample size for herd prevalence surveys. Prev. Veter. Med. 2004, 65, 173–188. [Google Scholar] [CrossRef]
- SINAVE. Manual Para la Vigilancia Epidemiológica de la Brucelosis; Secretaria de Salud, Dirección General de Epidemiología: Mexico City, Mexico, 2013; pp. 1–45. [Google Scholar]
- Gardner, I.A.; Stryhn, H.; Lind, P.; Collins, M.T. Conditional dependence between tests affects the diagnosis and surveillance of animal diseases. Prev. Veter. Med. 2000, 45, 107–122. [Google Scholar] [CrossRef]
- Cebul, R.D.; Hershey, J.C.; Williams, S.V. Using multiple tests: Series and parallel approaches. Clin. Lab. Med. 1982, 2, 871–890. [Google Scholar] [CrossRef]
- Avalos, O. Las Pruebas Diagnósticas. Su Aplicación en los Estudios Epidemiológicos. Nefrología 2000, 5, 1–5. [Google Scholar]
- León, E.A.; Duffy, S.J. Pruebas Diagnósticas: Principios y Métodos para su Evaluación e Interpretación. Temas Zoonosis 2006, 3, 4–16. [Google Scholar]
- Berkvens, D.; Speybroeck, N.; Praet, N.; Adel, A.; Lesaffre, E. Estimating disease prevalence in a bayesian framework using probabilistic constraints. Epidemiology 2006, 17, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Branscum, A.; Gardner, I.; Johnson, W. Estimation of diagnostic-test sensitivity and specificity through Bayesian modeling. Prev. Veter. Med. 2005, 68, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Devleesschauwer, B.; Torgerson, P.R.; Charlier, J.; Levecke, B.; Praet, N.; Dorny, P.; Berkvens, D.; Speybroeck, N. Package ‘prevalence’. Compr. R Arch. Netw. 2013. [Google Scholar] [CrossRef]
- R Foundation for Statistical Computing. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Lunn, D.J.; Thomas, A.; Best, N.; Spiegelhalter, D.; Lunn, D.J.; Thomas, A.; Best, N.; Spiegelhalter, D. WinBUGS—A Bayesian modelling framework: Concepts, structure, and extensibility 2000. Stat. Comput. 2000, 10, 325–337. [Google Scholar] [CrossRef]
- Gall, D.; Nielsen, K. Serological diagnosis of bovine brucellosis: A review of test performance and cost comparison. Rev. Sci. Tech. l’OIE 2004, 23, 939–1002. [Google Scholar] [CrossRef]
- Abernethy, D.; Menzies, F.; McCullough, S.; McDowell, S.; Burns, K.; Watt, R.; Gordon, A.; Greiner, M.; Pfeiffer, D. Field trial of six serological tests for bovine brucellosis. Veter. J. 2012, 191, 364–370. [Google Scholar] [CrossRef]
- Revelle, W. Psych: Procedures for Personality and Psychological Research; Northwestern University: Evanston, IL, USA, 2020. [Google Scholar]
- Cicchetti, D.V.; Feinstein, A.R. High agreement but low kappa: II. Resolving the paradoxes. J. Clin. Epidemiol. 1990, 43, 551–558. [Google Scholar] [CrossRef]
- Uebersax, J. Raw Agreement Indices; SAS program for multiple binary ratings. Available online: john-uebersax.com (accessed on 11 August 2021).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Guzmán-Hernández, R.L.; Contreras-Rodríguez, A.; Ávila-Calderón, E.D.; Morales-García, M.R. Brucelosis: Zoonosis de importancia en México. Rev. Chil. Infectología 2016, 33, 656–662. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, K.; Yu, W.L. Serological Diagnosis of Brucellosis. Prilozi 2010, 31, 65–89. [Google Scholar] [PubMed]
- Sanogo, M.; Thys, E.; Achi, Y.L.; Fretin, D.; Michel, P.; Abatih, E.; Berkvens, D.; Saegerman, C. Bayesian estimation of the true prevalence, sensitivity and specificity of the Rose Bengal and indirect ELISA tests in the diagnosis of bovine brucellosis. Veter. J. 2013, 195, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Gelman, A. Prior distributions for variance parameters in hierarchical models (comment on article by browne and draper). Bayesian Anal. 2006, 1, 515–534. [Google Scholar] [CrossRef]
- Shi, D.; Tong, X. The impact of prior information on Bayesian latent basis growth model estimation. SAGE Open 2017, 7. [Google Scholar] [CrossRef]
- Sanogo, M.; Abatih, E.; Saegerman, C. Bayesian versus frequentist methods for estimating true prevalence of disease and diagnostic test performance. Veter. J. 2014, 202, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Holt, H.R.; Eltholth, M.M.; Hegazy, Y.M.; El-Tras, W.F.; Tayel, A.A.; Guitian, J. Brucella spp. infection in large ruminants in an endemic area of Egypt: Cross-sectional study investigating seroprevalence, risk factors and livestock owner’s knowledge, attitudes and practices (KAPs). BMC Public Health 2011, 11, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saegerman, C.; De Waele, L.; Gilson, D.; Godfroid, J.; Thiange, P.; Michel, P.; Limbourg, B.; Vo, T.-O.; Limet, J.; Letesson, J.-J.; et al. Evaluation of three serum i-ELISAs using monoclonal antibodies and protein G as peroxidase conjugate for the diagnosis of bovine brucellosis. Veter. Microbiol. 2004, 100, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Escrig-Sos, J.; Martinez-Ramos, D.; Miralles-Tena, J.M. Pruebas diagnósticas: Nociones básicas para su correcta interpretación y uso. Cirugía Española 2006, 79, 267–273. [Google Scholar] [CrossRef]
- Talavera, J.O.; Wacher-Rodarte, N.H.; Rivas-Ruiz, R. Investigación clínica II. Estudios de proceso (prueba diagnóstica). Rev. Méd. Inst. Mex. Seguro Soc. 2011, 49, 163–170. [Google Scholar]
- Greiner, M.; Gardner, I. Epidemiologic issues in the validation of veterinary diagnostic tests. Prev. Veter. Med. 2000, 45, 3–22. [Google Scholar] [CrossRef]
- Ponce, J. Acuerdo Ministerial No 394; Ministerio de Agricultura, Ganadería, Acuacultura y Pesca: Quito, Ecuador, 2013; pp. 1–10.
- INIAP. La Ganadería de Carne En Ecuador. Publ. Misc. Dep. Econ. Agrícola Ecuad. 1975, 2, 1–28.
- Cárdenas, L.; Peña, M.; Melo, O.; Casal, J. Risk factors for new bovine brucellosis infections in Colombian herds. BMC Veter. Res. 2019, 15, 81. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo, V.; Yépez, C. Determinación de Seroprevalencia de Brucelosis Bovina en la Provincia de Pastaza y Posibles Factores de Riesgo Asociados con la Enfermedad. Master’s Thesis, Universidad Central del Ecuador, Quito, Ecuador, 2013; p. 83. [Google Scholar]
- Blasco, J.M. Estado Actual de la Brucelosis en España. Salud Anim. 2002, 12, 22–34. [Google Scholar]
- Omer, M.K.; Assefaw, T.; Skjerve, E.; Tekleghiorghis, T.; Woldehiwet, Z. Prevalence of antibodies to Brucella spp. and risk factors related to high-risk occupational groups in Eritrea. Epidemiol. Infect. 2002, 129, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Salguero, A. Brucelosis en Bovinos de las Provincias de Carchi, Esmeraldas e Imbabura y Análisis de Factores de Riesgo. Master’s Thesis, Universidad Central del Ecuador, Quito, Ecuador, 2014. [Google Scholar]
- Calderón-Rangel, A.; Angulo-Maza, L.A.; Tique-Salleg, V.P.; Rodríguez-Rodríguez, V.C.; Ensuncho-Hoyos, C.F. Seroprevalencia de brucelosis bovina en dos localidades del Caribe colombiano. Orinoquia 2015, 19, 203. [Google Scholar] [CrossRef]
- Berhe, G.; Belihu, K.; Asfaw, Y. Seroepidemiological investigation of bovine brucellosis in the extensive cattle production system of Tigray region of Ethiopia. Int. J. Appl. Res. Vet. Med. 2007, 5, 65. [Google Scholar]
- Ibrahim, N.; Belihu, K.; Lobago, F.; Bekana, M. Sero-prevalence of bovine brucellosis and its risk factors in Jimma zone of Oromia Region, South-western Ethiopia. Trop. Anim. Health Prod. 2009, 42, 35–40. [Google Scholar] [CrossRef]
- De Alencar Mota, A.L.A.; Ferreira, F.; Neto, J.S.F.; Dias, R.A.; Amaku, M.; Grisi-Filho, J.H.H.; Telles, E.O.; Gonçalves, V.S.P. Large-scale study of herd-level risk factors for bovine brucellosis in Brazil. Acta Trop. 2016, 164, 226–232. [Google Scholar] [CrossRef]
- Awah-Ndukum, J.; Mouiche, M.M.M.; Bayang, H.N.; Ngwa, V.N.; Assana, E.; Feussom, K.J.M.; Manchang, T.K.; Zoli, P.A. Seroprevalence and associated risk factors of brucellosis among indigenous cattle in the Adamawa and north regions of Cameroon. Veter. Med. Int. 2018, 2018, 3468596. [Google Scholar] [CrossRef] [Green Version]
- Miyashiro, S.; Scarcelli, E.; Piatti, R.M.; Campos, F.R.; Vialta, A.; Keid, L.B.; Dias, R.A.; Genovez, M.E. Detection of brucella abortus DNA in illegal cheese from São Paulo and Minas Gerais and differentiation of B19 vaccinal strain by means of the polymerase chain reaction (PCR). Braz. J. Microbiol. 2007, 38, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Gerbier, G.; Garin-Bastuji, B.; Pouillot, R.; Véry, P.; Cau, C.; Berr, V.; Dufour, B.; Moutou, F. False positive serological reactions in bovine brucellosis: Evidence of the role of Yersinia enterocolitica serotype O:9 in a field trial. Veter. Res. 1997, 28, 375–383. [Google Scholar]
- Kaltungo, B.Y.; Saidu, S.N.A.; Sackey, A.K.B.; Kazeem, H.M. A review on diagnostic techniques for brucellosis. Afr. J. Biotechnol. 2014, 13, 1–10. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, X.J.; Zhao, R.Q.; Huang, R.H.; Wang, Y.H.; Yin, C.P.; Shen, Q.; Schinckel, A.P. Effects of tail docking and teeth clipping on the physiological responses, wounds, behavior, growth, and backfat depth of pigs1. J. Anim. Sci. 2013, 91, 4908–4916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, Z.I.; Pascual, D.W. Brucellosis vaccines for livestock. Veter. Immunol. Immunopathol. 2016, 181, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Ron-Román, J.; Berkvens, D.; Barzallo-Rivadeneira, D.; Angulo-Cruz, A.; González-Andrade, P.; Minda-Aluisa, E.; Benítez-Ortíz, W.; Brandt, J.; Rodríguez-Hidalgo, R.; Saegerman, C. The unexpected discovery of Brucella abortus Buck 19 vaccine in goats from Ecuador underlines the importance of biosecurity measures. Trop. Anim. Health Prod. 2017, 49, 569–574. [Google Scholar] [CrossRef] [Green Version]
- Young, E.J. Serologic diagnosis of human brucellosis: Analysis of 214 cases by agglutination tests and review of the literature. Clin. Infect. Dis. 1991, 13, 359–372. [Google Scholar] [CrossRef]
- Terán, M.; Maribel, E. Prevalencia Aparente de Brucelosis Bovina a Través de ELISA Indirecto en 48 Fincas de los Cantones rio Verde y Quinindé, Provincia de Esmeraldas; Universidad San Francisco de Ecuador: Quito, Ecuador, 2018. [Google Scholar]
- Pacheco, W.A.; Genovez, M.E.; Pozzi, C.R.; Silva, L.M.P.; Azevedo, S.S.; Did, C.C.; Piatti, R.M.; Pinheiro, E.S.; Castro, V.; Miyashiro, S.; et al. Excretion of brucella abortus vaccine B19 strain during a reproductive cycle in dairy cows. Braz. J. Microbiol. 2012, 43, 594–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zambrano, D.; Pérez, M. Estudio Epidemiológico de Brucelosis Bovina en la Provincia de Manabí, Ecuador. Ph.D. Thesis, Universidad Agraria de la Habana, San José de las Lajas, Cuba, 2016; p. 164. [Google Scholar]
- Getachew, T.; Sintayehu, G.; Getenet, M.; Fasil, A. Bayesian estimation of sensitivity and specificity of rose bengal, complement fixation, and indirect ELISA tests for the diagnosis of bovine brucellosis in Ethiopia. Veter. Med. Int. 2016, 2016, 8032753. [Google Scholar] [CrossRef] [Green Version]
- Consumers Health Agriculture and Food Executive Agency. Eradication Programme for Bovine Brucellosis; European Commission; Health and Consumers Directorate-General: London, UK, 2012; p. 64.
- Aranís, C.; Oporto, J.; Espinoza, M.; Riedel, I.; Pérez, C.; García, P. Utilidad de la determinación de anticuerpos IgG e IgM por ELISA e inmunocaptura en una serie clínica de brucelosis humana. Rev. Chil. Infectología 2008, 25, 116–121. [Google Scholar] [CrossRef]
- Rahman, A.A.; Saegerman, C.; Berkvens, D.; Fretin, D.; Gani, O.; Ershaduzzaman; Ahmed, M.U.; Emmanuel, A. Bayesian estimation of true prevalence, sensitivity and specificity of indirect ELISA, Rose Bengal Test and Slow Agglutination Test for the diagnosis of brucellosis in sheep and goats in Bangladesh. Prev. Veter. Med. 2013, 110, 242–252. [Google Scholar] [CrossRef]
- Dendukuri, N.; Joseph, L. Bayesian approaches to modeling the conditional dependence between multiple diagnostic tests. Biom. 2001, 57, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Landis, J.R.; Koch, G.G. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977, 33, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Matope, G.; Muma, J.; Toft, N.; Gori, E.; Lund, A.; Nielsen, K.; Skjerve, E. Evaluation of sensitivity and specificity of RBT, c-ELISA and fluorescence polarisation assay for diagnosis of brucellosis in cattle using latent class analysis. Veter. Immunol. Immunopathol. 2011, 141, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, E.A.; Uzal, F.A.; Nielsen, K.H. Comparación de cuatro técnicas de ELISA en la evaluación de la respuesta serológica de terneras vacunadas con Brucella abortus cepa. Arch. Med. Vet. 1995, 27, 51–57. [Google Scholar]
- Fosgate, G.T.; Adesiyun, A.A.; Hird, D.W.; Johnson, W.O.; Hietala, S.K.; Schurig, G.G.; Ryan, J. Comparison of serologic tests for detection of Brucella infections in cattle and water buffalo (Bubalus bubalis). Am. J. Veter. Res. 2002, 63, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- McGiven, J.; Tucker, J.; Perrett, L.; Stack, J.; Brew, S.; MacMillan, A. Validation of FPA and cELISA for the detection of antibodies to Brucella abortus in cattle sera and comparison to SAT, CFT, and iELISA. J. Immunol. Methods 2003, 278, 171–178. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Rutjes, A.W.S.; Khan, K.S.; Coomarasamy, A.; Bossuyt, P.M. A review of solutions for diagnostic accuracy studies with an imperfect or missing reference standard. J. Clin. Epidemiol. 2009, 62, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Vilar, M.J.; Ranta, J.; Virtanen, S.; Korkeala, H. Bayesian estimation of the true prevalence and of the diagnostic test sensitivity and specificity of enteropathogenic yersiniain finnish pig serum samples. BioMed Res. Int. 2015, 2015, 931542. [Google Scholar] [CrossRef] [Green Version]

| Parameter | Sample | Positive ** | ||||
|---|---|---|---|---|---|---|
| N° | % | Mean * | N° | % | Mean * | |
| COASTAL REGION (Animals) | 9355 | 42.28 | 13.08 | 310 | 65.13 | 2.33 |
| COASTAL REGION (APUs) | 715 | 26.16 | - | 133 | 61.29 | - |
| Large Farms | 165 | 6.03 | - | 61 | 28.11 | - |
| Medium Farms | 325 | 11.89 | - | 57 | 26.27 | - |
| Small Farms | 212 | 7.76 | - | 12 | 5.53 | - |
| Not reported | 13 | 0.48 | - | 3 | 1.38 | - |
| NORTHERN HIGHLANDS (Animals) | 6880 | 31.09 | 5.6 | 139 | 29.2 | 1.96 |
| NORTHERN HIGHLANDS (APUs) | 1229 | 44.97 | - | 71 | 32.72 | - |
| Large Farms | 52 | 1.91 | - | 11 | 5.07 | - |
| Medium Farms | 175 | 6.4 | - | 23 | 10.6 | - |
| Small Farms | 985 | 36.04 | - | 36 | 16.59 | - |
| Not reported | 17 | 0.62 | - | 1 | 0.46 | - |
| SOUTHERN HIGHLANDS (Animals) | 4767 | 21.54 | 7.13 | 11 | 2.31 | 1.38 |
| SOUTHERN HIGHLANDS (APUs) | 669 | 24.48 | - | 8 | 3.69 | - |
| Large Farms | 30 | 1.1 | - | 0 | 0 | - |
| Medium Farms | 175 | 6.4 | - | 3 | 1.38 | - |
| Small Farms | 410 | 1.5 | - | 3 | 1.38 | - |
| Not reported | 40 | 1.47 | - | 1 | 0.46 | - |
| AMAZON REGION (Animals) | 1124 | 5.08 | 9.37 | 16 | 3.36 | 3.2 |
| AMAZON REGION (APUs) | 120 | 4.39 | - | 5 | 2.3 | - |
| Large Farms | 3 | 0.11 | - | 1 | 0.46 | - |
| Medium Farms | 59 | 2.16 | - | 1 | 0.46 | - |
| Small Farms | 55 | 2.01 | - | 3 | 1.38 | - |
| Not reported | 3 | 0.11 | - | 0 | 0 | - |
| Total General (Animals) | 22,126 | 100 | 8.1 | 476 | 100 | 2.19 |
| Total General (APUs) | 2733 | 100 | - | 217 | 100 | - |
| MODEL 1 | MODEL 2 | REFERENCES | ||||
|---|---|---|---|---|---|---|
| Parameter | Value | Distribution | Parameter | Value | Distribution | |
| theta [1] | 3% | Beta = (16, 486) | TP | 3% | Beta = (16, 486) | [3] |
| theta [2] | 72% | Beta = (16, 6) | SE [1] | 72% | Beta = (16, 6) | [37] |
| theta [3] | 71–99% | Uniform = (0.71, 0.99) | SP [1] | 71–99% | Uniform = (0.71, 0.99) | [37] |
| theta [4] | NI | Uniform = (0.00, 1.00) | SE [2] | 50–99% | Uniform = (0.50, 0.99) | [2,38] |
| theta [5] | NI | Uniform = (0.00, 1.00) | SP [2] | 90–100% | Uniform = (0.90, 1.00) | [38] |
| theta [6] | NI | Uniform = (0.00, 1.00) | a [1] | NI | Uniform = (0.00, 0.25) | - |
| theta [7] | NI | Uniform = (0.00, 1.00) | b [1] | NI | Uniform = (−0.25, 0.25) | - |
| Region | Apparent Prevalence (95% CI) Animal Level | Apparent Prevalence (95% CI) Farm Level * | True Prevalence (95% CI) Farm Level * | |
|---|---|---|---|---|
| Ecuador | RB | 2.10 (1.72–2.56) | 7.9 (7.0–9.0) | 12.2 (7.8–17.9) |
| SAT-EDTA | 2.03 (1.66–2.49) | |||
| Parallel interpretation | 2.20 (1.81–2.67) | |||
| Coastal Region | RB | 3.29 (2.56–4.22) | 18.6 (15.9–21.7) | 28.2 (15.7–39.8) |
| SAT-EDTA | 3.15 (2.44–4.06) | |||
| Parallel interpretation | 3.43 (2.69–4.36) | |||
| Northern Highlands | RB | 1.97 (1.40–2.78) | 5.8 (4.6–7.3) | 5.5 (2.2–9.2) |
| SAT-EDTA | 1.91 (1.33–2.74) | |||
| Parallel interpretation | 2.03 (1.45–2.84) | |||
| Southern Highlands | RB | 0.23 (0.11–0.49) | 1.2 (0.6–2.4) | 0.7 (0.6–2.1) |
| SAT-EDTA | 0.21 (0.09–0.47) | |||
| Parallel interpretation | 0.23 (0.11–0.49) | |||
| Amazon Region | RB | 1.17 (0.30–4.57) | 4.2 (1.5–9.9) | 7.2 (0.9–15.6) |
| SAT-EDTA | 1.44 (0.45–4.68) | |||
| Parallel interpretation | 1.44 (0.45–4.68) | |||
| Total | |||
|---|---|---|---|
| 419 | 36 | 455 | |
| 21 | 21,650 | 21,671 | |
| Total | 440 | 21,686 | 22,126 |
| Parameter | With and without Vaccination | With Vaccination | Without Vaccination | |
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| Mean (95% CrI) | Mean (95% CrI) | Mean (95% CrI) | Mean (95% CrI) | |
| TP | 1.6% (1.0–2.3%) | 1.5% (1.0–2.0%) | 2.6% (1.0–4.5%) | 1.4% (0.7–2.2%) |
| 64.5% (43.1–84.9%) | 65.2% (51.9–82.6%) | 79.4% (60.4–92.7%) | 77.0% (57.0–91.9%) | |
| 98.9% (98.6–99.0%) | 98.9% (98.6–99.0%) | 98.1% (96.7–99.7%) | 99.6% (99.0–99.9%) | |
| 62.2% (40.6–84.2%) | 63.0% (50.8–81.9%) | 80.8% (60.0–95.7%) | 77.8% (56.7–94.5%) | |
| 98.9% (98.6–99.1%) | 98.9% (98.6–99.1%) | 98.1% (96.6–99.7%) | 99.5% (98.9–99.9%) | |
| cov_a | 0.176 (0.074–0.234) | 0.179 (0.090–0.232) | 0.108 (0.006–0.207) | 0.123 (0.014–0.220) |
| cov_b | 0.011 (0.010–0.014) | 0.010 (0.009–0.013) | 0.018 (0.003–0.032) | 0.004 (0.000–0.010) |
| BGR | 1.00 | 1.01 | 1.01 | 1.00 |
| Bayes-p | 0.55 | 1.00 | 0.54 | 0.52 |
| DIC | 21.66 | 39.63 | 18.18 | 19.84 |
| Risk Factor | N° Farms | Positive Farms | Seropos | Initial Model | Final Model | |||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||||
| Farm size | Large | 264 | 74 | 28.0% | Reference | |||
| Medium | 734 | 84 | 11.4% | 0.462 (0.313–0.681) | <0.001 | 0.457 (0.310–0.671) | <0.001 | |
| Small | 1662 | 54 | 3.2% | 0.176 (0.110–0.281) | <0.001 | 0.170 (0.107–0.271) | <0.001 | |
| Type of production | Beef | 234 | 14 | 6.0% | Reference | |||
| Dairy | 1782 | 112 | 6.3% | 1.215 (0.642–2.302) | 0.550 | 1.283 (0.681–2.415) | 0.441 | |
| Mixed | 648 | 89 | 13.7% | 1.744 (0.919–3.310) | 0.089 | 1.780 (0.939–3.375) | 0.077 | |
| Vaccination | No | 2324 | 133 | 5.7% | Reference | |||
| Yes | 261 | 71 | 27.2% | 3.083 (2.129–4.463) | <0.001 | 1.895 (1.375–2.612) | <0.001 | |
| Veterinary control | No | 1922 | 128 | 6.7% | Reference | |||
| Yes | 751 | 84 | 11.2% | 1.183 (0.840–1.67) | 0.336 | – | ||
| Abortions | No | 1907 | 107 | 5.6% | Reference | |||
| Yes | 730 | 106 | 14.5% | 1.862 (1.346–2.575) | <0.001 | 3.130 (2.172–4.509) | <0.001 | |
| Reproduction system | Insemination | 282 | 36 | 13.6% | Reference | |||
| Mixed | 301 | 25 | 3.4% | 0.548 (0.291–1.033) | 0.063 | – | ||
| Natural breeding | 2040 | 155 | 9.3% | 0.757 (0.474–1.210) | 0.245 | – | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paucar, V.; Ron-Román, J.; Benítez-Ortiz, W.; Celi, M.; Berkvens, D.; Saegerman, C.; Ron-Garrido, L. Bayesian Estimation of the Prevalence and Test Characteristics (Sensitivity and Specificity) of Two Serological Tests (RB and SAT-EDTA) for the Diagnosis of Bovine Brucellosis in Small and Medium Cattle Holders in Ecuador. Microorganisms 2021, 9, 1815. https://doi.org/10.3390/microorganisms9091815
Paucar V, Ron-Román J, Benítez-Ortiz W, Celi M, Berkvens D, Saegerman C, Ron-Garrido L. Bayesian Estimation of the Prevalence and Test Characteristics (Sensitivity and Specificity) of Two Serological Tests (RB and SAT-EDTA) for the Diagnosis of Bovine Brucellosis in Small and Medium Cattle Holders in Ecuador. Microorganisms. 2021; 9(9):1815. https://doi.org/10.3390/microorganisms9091815
Chicago/Turabian StylePaucar, Valeria, Jorge Ron-Román, Washington Benítez-Ortiz, Maritza Celi, Dirk Berkvens, Claude Saegerman, and Lenin Ron-Garrido. 2021. "Bayesian Estimation of the Prevalence and Test Characteristics (Sensitivity and Specificity) of Two Serological Tests (RB and SAT-EDTA) for the Diagnosis of Bovine Brucellosis in Small and Medium Cattle Holders in Ecuador" Microorganisms 9, no. 9: 1815. https://doi.org/10.3390/microorganisms9091815
APA StylePaucar, V., Ron-Román, J., Benítez-Ortiz, W., Celi, M., Berkvens, D., Saegerman, C., & Ron-Garrido, L. (2021). Bayesian Estimation of the Prevalence and Test Characteristics (Sensitivity and Specificity) of Two Serological Tests (RB and SAT-EDTA) for the Diagnosis of Bovine Brucellosis in Small and Medium Cattle Holders in Ecuador. Microorganisms, 9(9), 1815. https://doi.org/10.3390/microorganisms9091815







