Removal of Phenols in Table Olive Processing Wastewater by Using a Mixed Inoculum of Candida boidinii and Bacillus pumilus: Effects of Inoculation Dynamics, Temperature, pH, and Effluent Age on the Abatement Efficiency
Abstract
:1. Introduction
- (a)
- To evaluate if the way of inoculation (before yeast and then bacterium or viceversa) could affect the removal efficiency.
- (b)
- To study the potentialities of the combination on the bioremediation of TOPW as a function of their age (TOPW treated immediately after their discharge or after a preliminary storage in temporary tanks, as usually done by producers).
2. Materials and Methods
2.1. Microorganisms
2.2. Table Olive Processing Wastewater
2.3. Bioremediation through a Sequential Inoculation of Bacillus pumilus and Candida boidinii
2.4. Microbiological and Chemical Analyses
2.5. Statistical Analyses
3. Results
3.1. Qualitative Effect of Sequential Inoculation, Sampling Time and Combination of pH/Temperature/Dilution
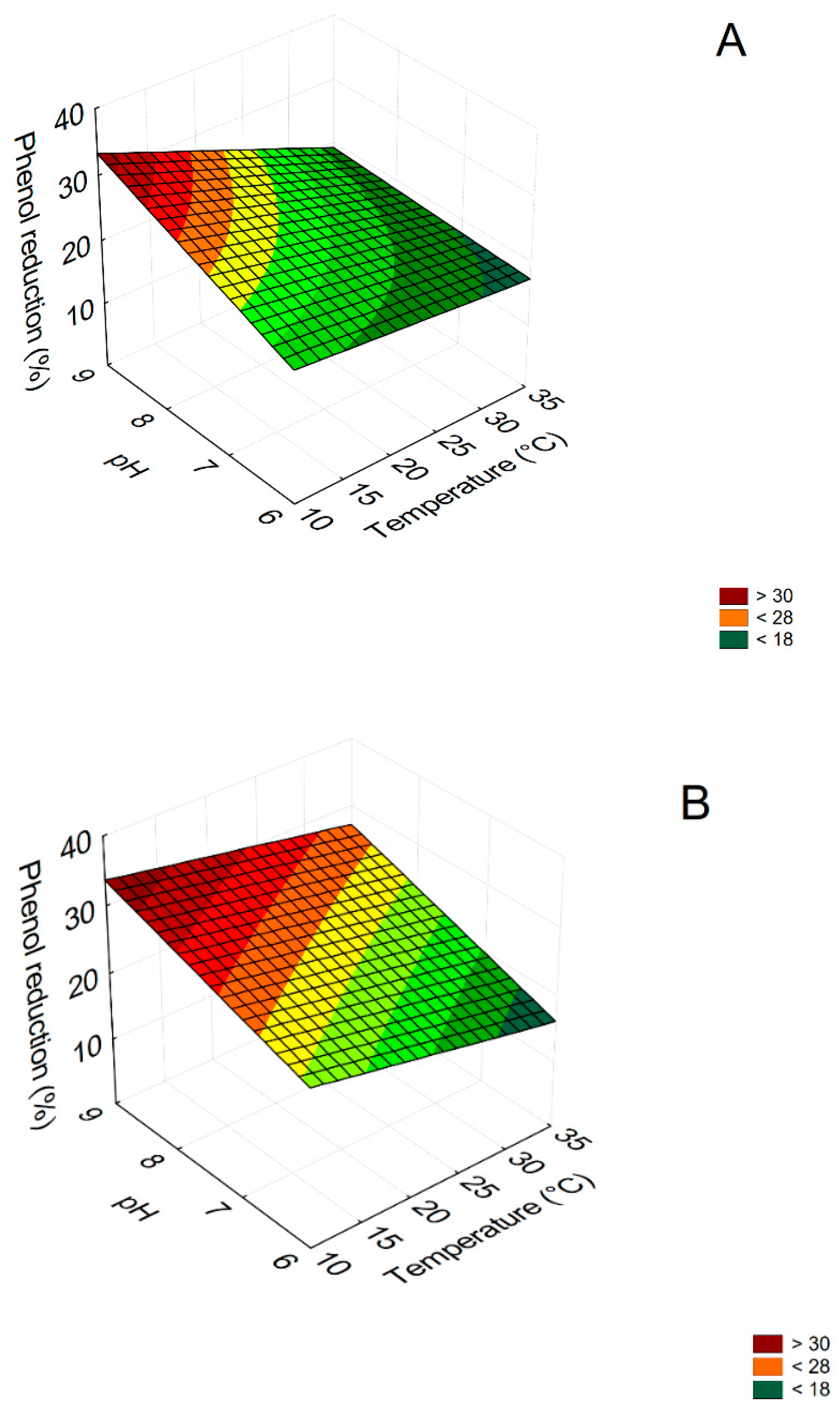
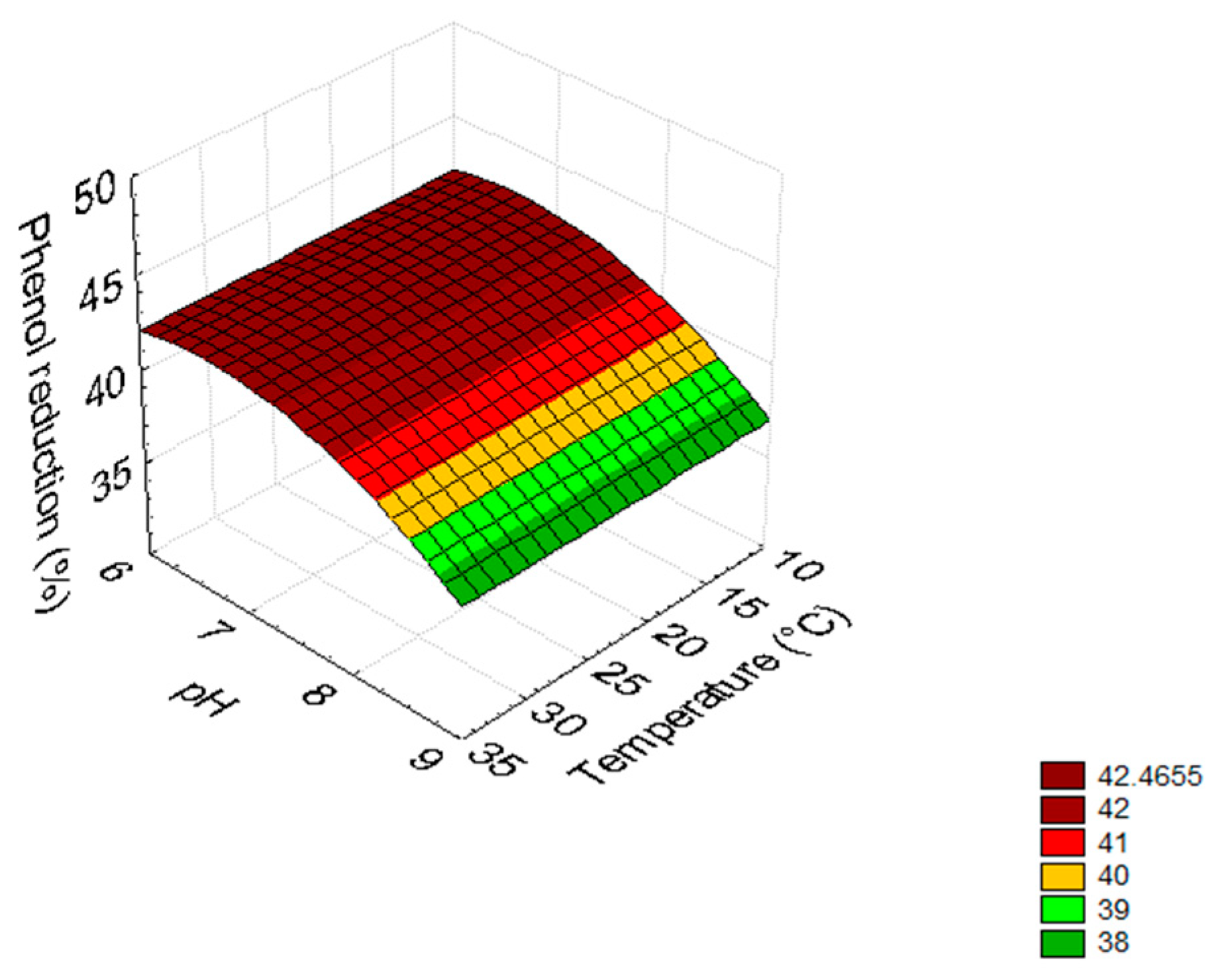
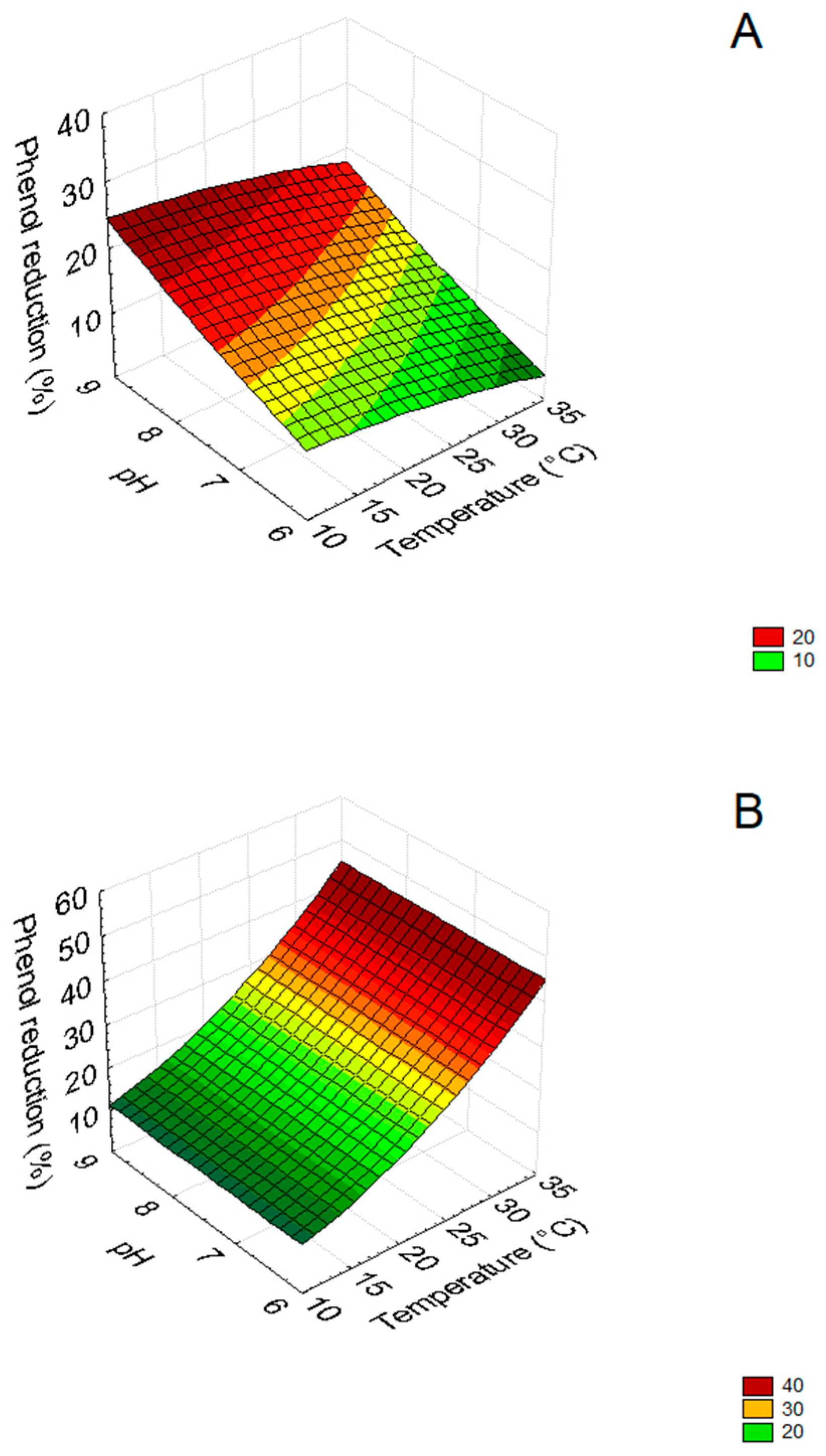
3.2. Mathematical Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IOC International Olive Council. Madrid, Spain, 2020. Available online: https://www.internationaloliveoil.org (accessed on 18 February 2020).
- Patsios, S.L.; Kontogiannopoulos, K.N.; Pouliou, N.; Karabelas, A.J. Performance of a membrane bioreactor and a moving bed biofilm reactor–membrane bioreactor treating table olive processing wastewater: A comparative study. J. Chem. Technol. Biotechnol. 2021, 96, 1030–1039. [Google Scholar] [CrossRef]
- Rincón-Llorente, B.; De la Lama-Calvente, D.; Fernández-Rodríguez, M.J.; Borja-Padilla, R. Table olive wastewater: Problem, treatments and future strategy. Front. Microbiol. 2018, 9, 1641. [Google Scholar] [CrossRef] [Green Version]
- Ayed, L.; Chammen, N.; Asses, N.; Hamdi, M. Optimization of biological pretreatment of green table olive processing wastewaters using Aspergillus niger. J. Bioremediat. Biodegrad. 2013, 4, 212. [Google Scholar] [CrossRef] [Green Version]
- Ayed, L.; Asses, N.; Chammem, N.; Ben Othman, N.; Hamdi, M. Advanced oxidation process and biological treatments for table olive processing wastewaters: Constraints and a novel approach to integrated recycling process: A review. Biodegradation 2017, 28, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, A.; Lasaridi, L.; Kotsou, M.; Balis, C.; Pilidis, G. Combined bioremediation and advanced oxidation of green table olive processing wastewater. Process Biochem. 2005, 40, 1401–1408. [Google Scholar] [CrossRef]
- Ayed, L.; Asses, N.; Chammem, N.; Hamdi, M. Improvement of green table olive processing wastewater decolorization by Geotrichum candidum. Desalin. Water Treat. 2015, 57, 17322–17332. [Google Scholar] [CrossRef]
- Campus, M.; Değirmencioğlu, N.; Comunian, R. Technologies and trends to improve table olive quality and safety. Front. Microbiol. 2018, 9, 617. [Google Scholar] [CrossRef] [Green Version]
- Dutournié, P.; Jeguirim, M.; Khiari, B.; Goddard, M.L.; Jellali, S. Olive mill wastewater: From a pollutant to green fuels, agricultural water source, and bio-fertilizer. Part 2: Water recovery. Water 2019, 11, 768. [Google Scholar] [CrossRef] [Green Version]
- Campaniello, D.; Carlucci, A.; Speranza, B.; Raimondo, M.L.; Cibelli, F.; Corbo, M.R.; Bevilacqua, A. A comparative study on Trichoderma harzianum and a combination of Candida/Bacillus as tools for the bioremediation of table olive processing water. Microorganisms 2020, 8, 878. [Google Scholar] [CrossRef]
- Lasaridi, K.E.; Kyriacou, A.; Chroni, C.; Abeliotis, K.; Chatzipavlidis, I.; Ayed, L.; Chammam, N.; Hamdi, M. Estimating the bioremediation of green table olive processing wastewater using a selected strain of Aspergillus niger. Desalin. Water Treat. 2010, 23, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Cormenzana, A.; Juarez-Jimenez, B.; Garcia-Pareja, M.P. Antimicrobial activity of olive mill wastewaters (Alpechin) and biotransformed olive oil mill wastewater. Int. Biodeterior. Biodegrad. 1996, 38, 283–290. [Google Scholar] [CrossRef]
- Ben Othman, N.; Ayed, L.; Assas, N.; Kachouri, F.; Hammami, M.; Hamdi, M. Ecological removal of recalcitrant phenolic compounds of treated olive mill wastewater by Pediococcus pentosaceus. Bioresour. Technol. 2008, 99, 2996–3001. [Google Scholar] [CrossRef] [PubMed]
- Ayed, L.; Hamdi, M. Fermentative decolorization of olive mill wastewater by Lactobacillus plantarum. Process Biochem. 2003, 39, 59–65. [Google Scholar]
- Knupp, G.; Rucker, G.; Ramos-Cormenzana, A.; Hoyos, S.E.G.; Neugebauer, M.; Ossenkop, T. Problems of identifying phenolic compounds during the microbial degradation of olive mill wastewater. Int. Biodeterior. Biodegrad. 1996, 38, 277–282. [Google Scholar] [CrossRef]
- Constantinos, E.; Papadopoulou, K.; Kotsou, M.; Mari, I.; Constantinos, B. Adaptation and population dynamics of Azotobacter vinelandii during aerobic biological treatment of olive mill wastewater. FEMS Microbiol. Ecol. 1999, 30, 301–311. [Google Scholar]
- Balis, C.; Chatzipavlidis, J.; Flouri, F. Olive mill waste as a substitute for nitrogen fixation. Int. Biodeterior. Biodegrad. 1996, 38, 169–178. [Google Scholar] [CrossRef]
- Piperidou, C.I.; Chaidou, C.I.; Stalikas, D.; Soulti, K.; Pilidis, G.A.; Balis, C. Bioremediation of olive mill wastewater: Chemical alterations induced by Azotobacter vinelandii. J. Agric. Food Chem. 2000, 48, 1941–1948. [Google Scholar] [CrossRef]
- Borja, R.; Martin, A.; Alonso, V.; Garcia, I.; Banks, C.J. Influence of different aerobic pre- treatments on the kinetics of anaerobic digestion of olive mill wastewater. Water Res. 1995, 29, 489–495. [Google Scholar] [CrossRef]
- Di Gioia, D.; Bertin, L.; Fava, F.; Marchetti, L. Biodegradation of hydroxylated and methoxylated benzoic, phenylacetic and phenylpropenoic acids present in olive mill wastewaters by two bacterial strains. Res. Microbiol. 2001, 152, 83–93. [Google Scholar] [CrossRef]
- Di Gioia, D.; Fava, F.; Bertin, L.; Marchetti, L. Biodegradation of synthetic and naturally occurring mixtures of mono-cyclic aromatic compounds present in olive mill wastewaters by two aerobic bacteria. Appl. Microbiol. Biotechnol. 2001, 55, 619–626. [Google Scholar] [CrossRef] [PubMed]
- D’Annibale, A.; Brozzoli, V.; Crognale, S.; Gallo, A.N.; Federici, F.; Petruccioli, M. Optimisation by response surface methodology of fungal lipase production on olive mill wastewater. J. Chem. Technol. Biotechnol. 2006, 81, 1586–1593. [Google Scholar] [CrossRef]
- D’Annibale, A.; Sermanni, G.G.; Federici, F.; Petruccioli, M. Olive-mill wastewaters: A promising substrate for microbial lipase production. Bioresour. Technol. 2006, 97, 1828–1833. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, G.; Johnson, A.C.; Bachman, R.T.; Williams, C.J.; Burgoyne, A.; Edyvean, R.G.J. Two-stage biological treatment of olive mill wastewater with whey as cosubstrate. Int. Biodeterior. Biodegrad. 2007, 59, 273–282. [Google Scholar] [CrossRef]
- Aloui, F.; Abid, N.; Roussos, S.; Sayadi, S. Decolorization of semisolid olive residues of “Alperujo” during the solid state fermentation by Phanerochaete chrysosporium, Trametes versicolor, Pycnoporus cinnabarinus and Aspergillus niger. Biochem. Eng. J. 2007, 35, 120–125. [Google Scholar] [CrossRef]
- Garcìa Garcìa, I.; Jiménez Pena, P.R.; Venceslada, B.; Martìn Martìn, A.; Martin Santos, M.A.; Ramos Gomez, E. Removal of phenol compounds from olive mill wastewater using Phanerochaete chrysosporium, Aspergillus niger, Aspergillus terreus and Geotrichum candidum. Process Biochem. 2000, 35, 751–758. [Google Scholar] [CrossRef]
- Jaouani, A.; Sayadi, S.; Vanthournhout, M.; Penninckx, M.J. Potent fungi for decolourisation of olive mill wastewaters. Enzym. Microb. Technol. 2003, 33, 802–809. [Google Scholar] [CrossRef]
- Kissi, M.; Mountadar, M.; Assobhei, O.; Gargiulo, E.; Palmieri, G.; Giardina, P.; Sannita, G. Roles of two white-rot Basidiomyces fungi in decolorisation and detoxification of olive mill wastewater. Appl. Microbiol. Biotechnol. 2001, 57, 221–226. [Google Scholar] [PubMed]
- Tsioulpas, A.; Dimou, D.; Iconomou, D.; Aggelis, G. Phenolic removal in olive oil mill wastewater by strains of Pleurotus spp. in respect to their phenol oxidase (Laccase) activity. Bioresour. Technol. 2002, 84, 251–257. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Petruzzi, L.; Corbo, M.R.; Sinigaglia, M. Bioremediation of olive mill wastewater by yeasts—A review of the criteria for the selection of promising strains. In Applied Bioremediation—Active and Passive Approaches; Patil, Y.B., Rao, P., Eds.; Intech: Rijeka, Croatia, 2013; pp. 53–68. [Google Scholar]
- Lanciotti, R.; Gianotti, A.; Baldi, D.; Angrisani, R.; Suzzi, G.; Mastrocola, D.; Guerzoni, M.E. Use of Yarrowia lipolytica strains for the treatment of olive mill wastewaters. Bioresour. Technol. 2005, 96, 317–322. [Google Scholar] [CrossRef]
- Martinez-Garcia, G.; Johnson, A.C.; Bachmann, R.T.; Williams, C.J.; Burgoyne, A.; Edyvean, R.G.J. Anaerobic treatment of olive mill wastewater and piggery effluents fermented with Candida tropicalis. J. Hazard. Mater. 2009, 164, 1398–1405. [Google Scholar] [CrossRef]
- Bray, H.G.; Thorpe, W.V. Analysis of phenolic compounds of interest in metabolism. In Methods of Biochemical Analysis; Blick, D., Ed.; Interscience: New York, NY, USA, 1954; pp. 27–52. [Google Scholar]
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. Modeling, geometry, and experimental designs. In Statistics for Experimenters. Design, Innovation, and Discovery, 2nd ed.; Box, G.E.P., Hunter, J.S., Hunter, W.G., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 437–488. [Google Scholar]
- Bevilacqua, A.; Campaniello, D.; Sinigaglia, M.; Ciccarone, C.; Corbo, M.R. Sodium benzoate and citrus extract increase the effect of homogenization towards spores of Fusarium oxysporum in pineapple juice. Food Control 2012, 28, 199–204. [Google Scholar] [CrossRef]
- Papadaki, E.; Mantzouridou, F.T. Current status and future challenges of table olive processing wastewater valorization. Biochem. Eng. J. 2016, 112, 103–113. [Google Scholar] [CrossRef]
- Patil, S.S.; Jena, H.M. Statistical optimization of phenol degradation by Bacillus pumilus OS1 using plackett–burman design and response surface methodology. Arab. J. Sci. Eng. 2015, 40, 2141–2151. [Google Scholar] [CrossRef]
- Pradeep, N.V.; Anupama, S.; Navya, K.; Shalini, H.N.; Idris, M.; Hampannavar, U.S. Biological removal of phenol from wastewaters: A mini review. Appl. Water Sci. 2015, 5, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Sinigaglia, M.; Di Benedetto, N.; Bevilacqua, A.; Corbo, M.R.; Capece, A.; Romano, P. Yeasts isolated from olive mill wastewaters from Southern Italy: Technological characterization and potential use for phenol removal. Appl. Microbiol. Biotechnol. 2010, 87, 2345–2354. [Google Scholar] [CrossRef]
- Campaniello, D.; Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. Selection of promising bacterial strains as potential tools for the bioremediation of olive mill wastewater. In Wastewater Treatment Engineering; Samer, M., Ed.; InTech: Rijeka, Croatia, 2015; pp. 85–100. [Google Scholar]
- Aissam, H. Etude des Effluents des Huileries de la Ville de Fés (Margines): Caractérisation, Biotraitement et Valorisation. Ph.D. Thesis, University of Fés, Fes, Morocco, 2003. [Google Scholar]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments. Desalination 2010, 261, 3–18. [Google Scholar] [CrossRef] [Green Version]
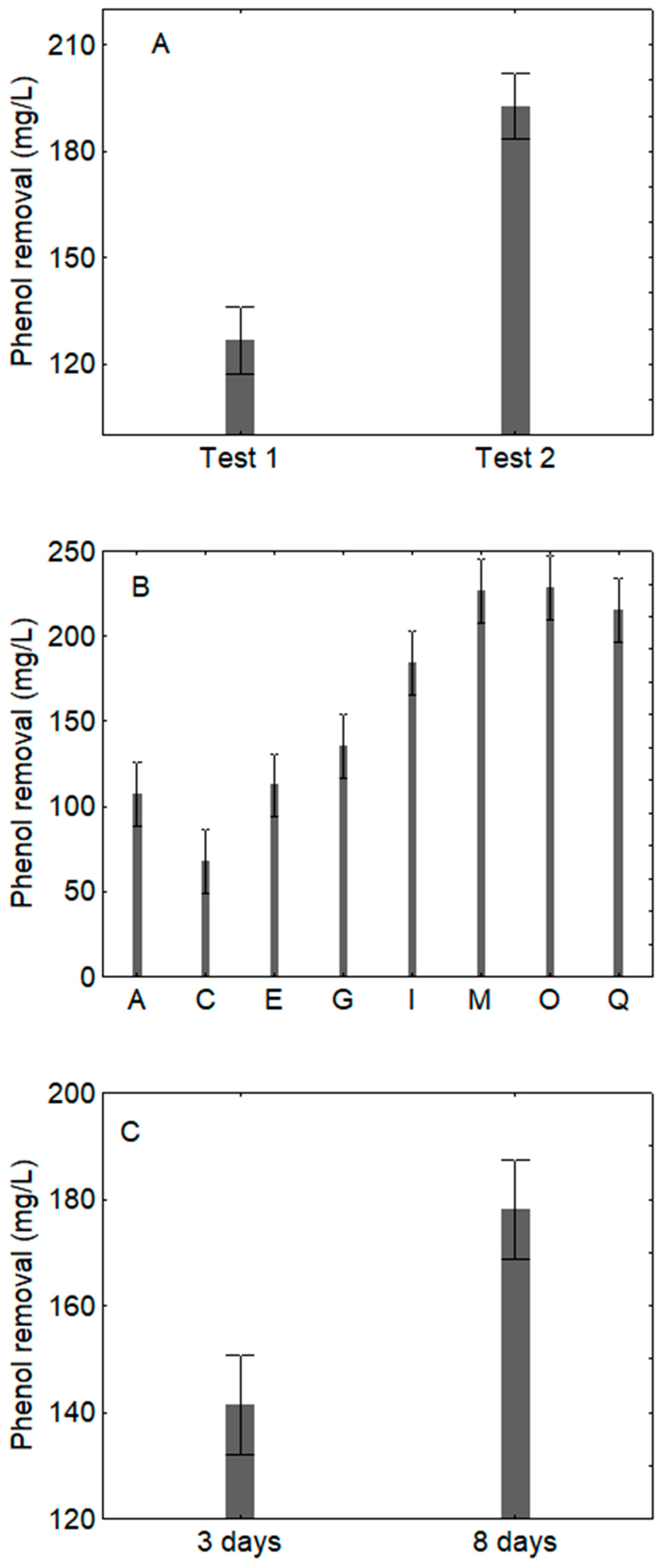

| Coded Values | Actual Values | |||||
|---|---|---|---|---|---|---|
| Run | pH | Dilution | Temperature | pH | Dilution | Temperature |
| A | −1 | −1 | −1 | 6 | 0 | 10 °C |
| C | +1 | −1 | −1 | 9 | 0 | 10 °C |
| E | −1 | +1 | −1 | 6 | 1:1 | 10 °C |
| G | +1 | +1 | −1 | 9 | 1:1 | 10 °C |
| I | −1 | −1 | +1 | 6 | 0 | 35 °C |
| M | +1 | −1 | +1 | 9 | 0 | 35 °C |
| O | −1 | +1 | +1 | 6 | 1:1 | 35 °C |
| Q | +1 | +1 | +1 | 9 | 1:1 | 35 °C |
| FTOPW | ATOPW | |
|---|---|---|
| Inoculum (test 1 or test 2) | 98.778 | 279.971 |
| Combination of the design | 43.787 | 53.440 |
| Time of sampling | 30.514 | 45.614 |
| Inoculum vs. combination | 69.665 | 93.794 |
| Inoculum vs. time | 4.656 | 9.749 |
| Combination vs. time | 3.170 | 3.732 |
| Inoculum vs. combination vs. time | 11.416 | 4.599 |
| Test 1 | Test 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Inoculum of C. boidinii Followed by B. pumilus | Inoculum of B. pumilus Followed by C. boidinii | |||||||
| A | B | C | D | E | F | G | H | |
| pH | 11.845 | 14.0612 | 7.198 | 12.269 | - | - | - | - |
| Temperature | - | - | - | - | - | - | - | - |
| Dilution | - | - | - | - | - | - | - | - |
| pH2 | - | - | −4.448 | −6.699 | - | 8.331 | - | - |
| Temperature2 | - | −2.488 | - | - | - | −2.40 | 3.013 | 6.209 |
| Dilution2 | - | - | - | - | - | - | - | - |
| pH × temperature | −5.601 | - | - | - | - | - | - | - |
| pH × dilution | - | - | - | - | - | - | - | - |
| T × dilution | 3.123 | - | - | - | - | - | - | - |
| R2 | 0.993 | 0.983 | 0.980 | 0.997 | - | 0.941 | 0.982 | 0.865 |
| R2adjusted | 0.985 | 0.977 | 0.973 | 0.994 | - | 0.921 | 0.971 | 0.843 |
| F-test | 133.52 | 171.79 | 148.55 | 517.29 | - | 47.515 | 91.522 | 38.556 |
| p-level | 0.0016 | 0.00001 | 0.0001 | 0.0000 | - | 0.0021 | 0.00009 | 0.00081 |
| SE | 2.906 | 3.954 | 6.502 | 3.152 | - | 4.650 | 5.933 | 7.811 |
| Equations | ||||||||
| A | R = 4.404 × pH − 0.08 × pH × temperature + 0.452 × temperature × dilution | |||||||
| B | R = 3.870 × pH − 0.006 × temperature2 | |||||||
| C | R = 12.803 × pH − 0.965 × pH2 | |||||||
| D | R = 11.241 × pH − 0.718 × pH2 | |||||||
| E | - | |||||||
| F | R = 0.311 × pH2 − 0.006 × temperature2 | |||||||
| G | R = 0.06 × temperature2 | |||||||
| H | R = 8.274 + 0.03 × temperature2 | |||||||
| FTOPW | ATOPW | |||||||
|---|---|---|---|---|---|---|---|---|
| Test 1 | Test 2 | Test 1 | Test 2 | |||||
| 13M | 682 | 13M | 682 | 13M | 682 | 13M | 682 | |
| A | 5.91 | 5.76 | 7.17 | 5.34 | 5.75 | 6.59 | 7.26 | 5.26 |
| C | 6.26 | 5.49 | 7.78 | 5.28 | 5.91 | 5.07 | 7.32 | 5.71 |
| E | 6.31 | 6.83 | 7.13 | 5.32 | 5.68 | 5.76 | 7.53 | 5.71 |
| G | 6.02 | 5.71 | 7.81 | 5.60 | 6.14 | 5.63 | 7.54 | 5.57 |
| I | 5.71 | 5.49 | 7.72 | 4.48 | 5.86 | 4.20 | 7.15 | 5.15 |
| M | 6.17 | 5.34 | 7.40 | 4.15 | 5.85 | 4.32 | 7.41 | 3.91 |
| O | 6.03 | 4.84 | 7.73 | 4.32 | 5.94 | 4.40 | 7.66 | 5.08 |
| Q | 6.05 | 6.86 | 7.58 | 4.00 | 6.04 | 6.03 | 7.54 | 5.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campaniello, D.; Speranza, B.; Altieri, C.; Sinigaglia, M.; Bevilacqua, A.; Corbo, M.R. Removal of Phenols in Table Olive Processing Wastewater by Using a Mixed Inoculum of Candida boidinii and Bacillus pumilus: Effects of Inoculation Dynamics, Temperature, pH, and Effluent Age on the Abatement Efficiency. Microorganisms 2021, 9, 1783. https://doi.org/10.3390/microorganisms9081783
Campaniello D, Speranza B, Altieri C, Sinigaglia M, Bevilacqua A, Corbo MR. Removal of Phenols in Table Olive Processing Wastewater by Using a Mixed Inoculum of Candida boidinii and Bacillus pumilus: Effects of Inoculation Dynamics, Temperature, pH, and Effluent Age on the Abatement Efficiency. Microorganisms. 2021; 9(8):1783. https://doi.org/10.3390/microorganisms9081783
Chicago/Turabian StyleCampaniello, Daniela, Barbara Speranza, Clelia Altieri, Milena Sinigaglia, Antonio Bevilacqua, and Maria Rosaria Corbo. 2021. "Removal of Phenols in Table Olive Processing Wastewater by Using a Mixed Inoculum of Candida boidinii and Bacillus pumilus: Effects of Inoculation Dynamics, Temperature, pH, and Effluent Age on the Abatement Efficiency" Microorganisms 9, no. 8: 1783. https://doi.org/10.3390/microorganisms9081783
APA StyleCampaniello, D., Speranza, B., Altieri, C., Sinigaglia, M., Bevilacqua, A., & Corbo, M. R. (2021). Removal of Phenols in Table Olive Processing Wastewater by Using a Mixed Inoculum of Candida boidinii and Bacillus pumilus: Effects of Inoculation Dynamics, Temperature, pH, and Effluent Age on the Abatement Efficiency. Microorganisms, 9(8), 1783. https://doi.org/10.3390/microorganisms9081783








