Remediation Strategies to Control Toxic Cyanobacterial Blooms: Effects of Macrophyte Aqueous Extracts on Microcystis aeruginosa (Growth, Toxin Production and Oxidative Stress Response) and on Bacterial Ectoenzymatic Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cyanobacterial Strains
2.2. Macrophyte Sampling and Extraction
2.3. Comparison of Anticyanobacterial Activity of Different Macrophytes Extracts
2.4. Allelopathic Effect of the Most Active Aqueous Extract on M. aeruginosa Growth, Physiology and Toxicity
2.4.1. Experimental Design
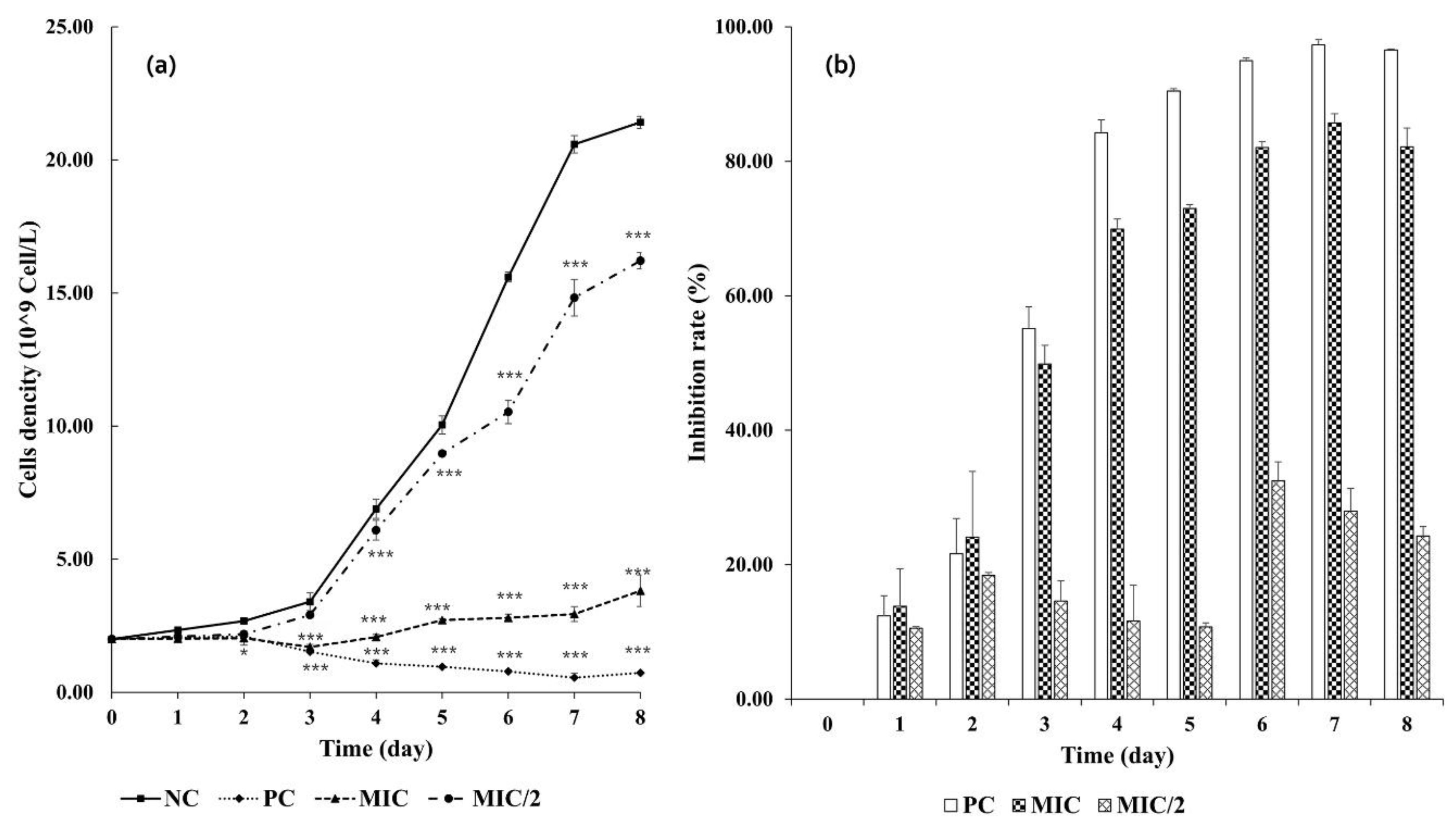
2.4.2. Cyanobacterial Density and Growth Rate Determination
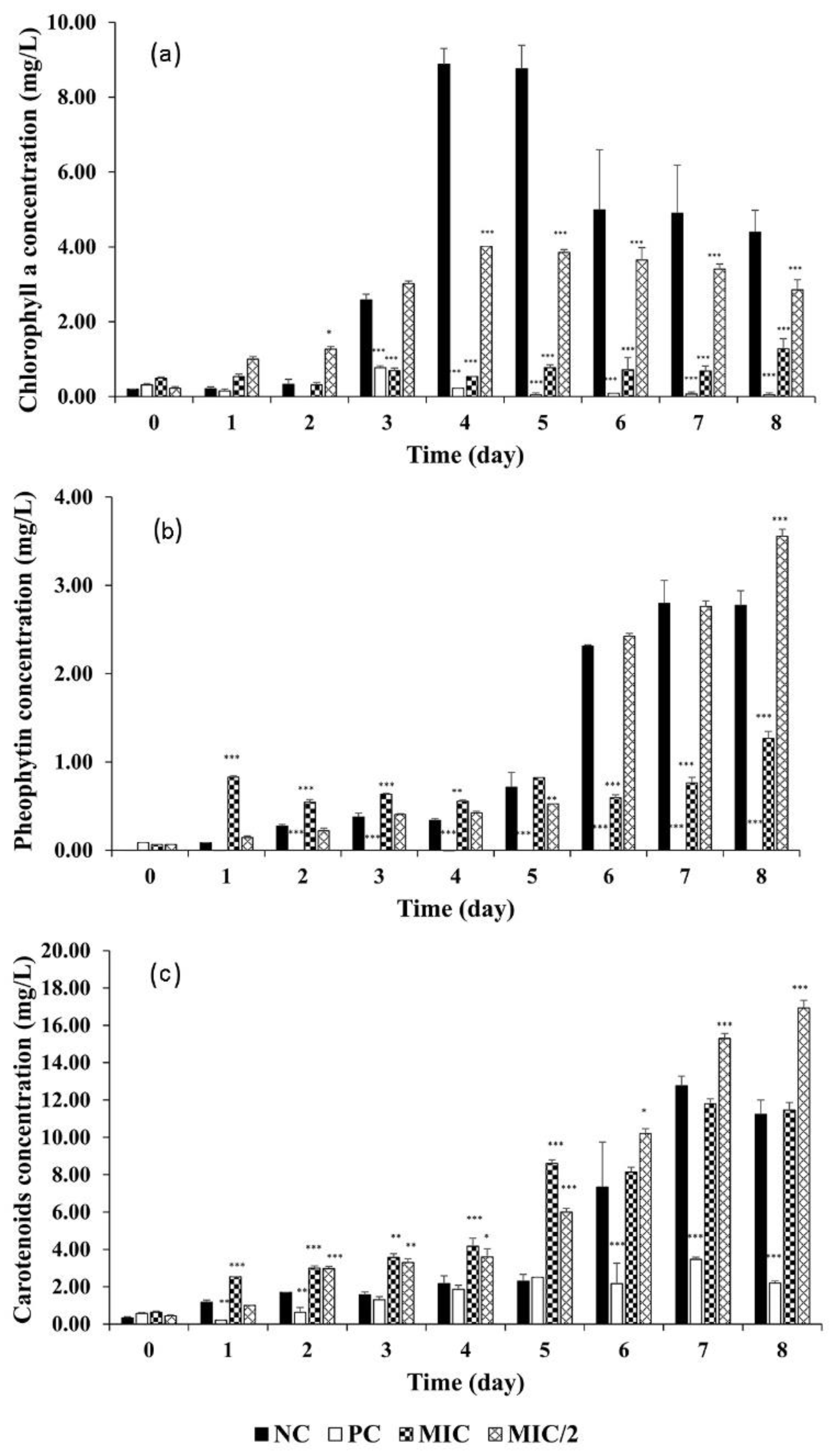
2.4.3. Pigment Contents and Pheophytin Concentrations
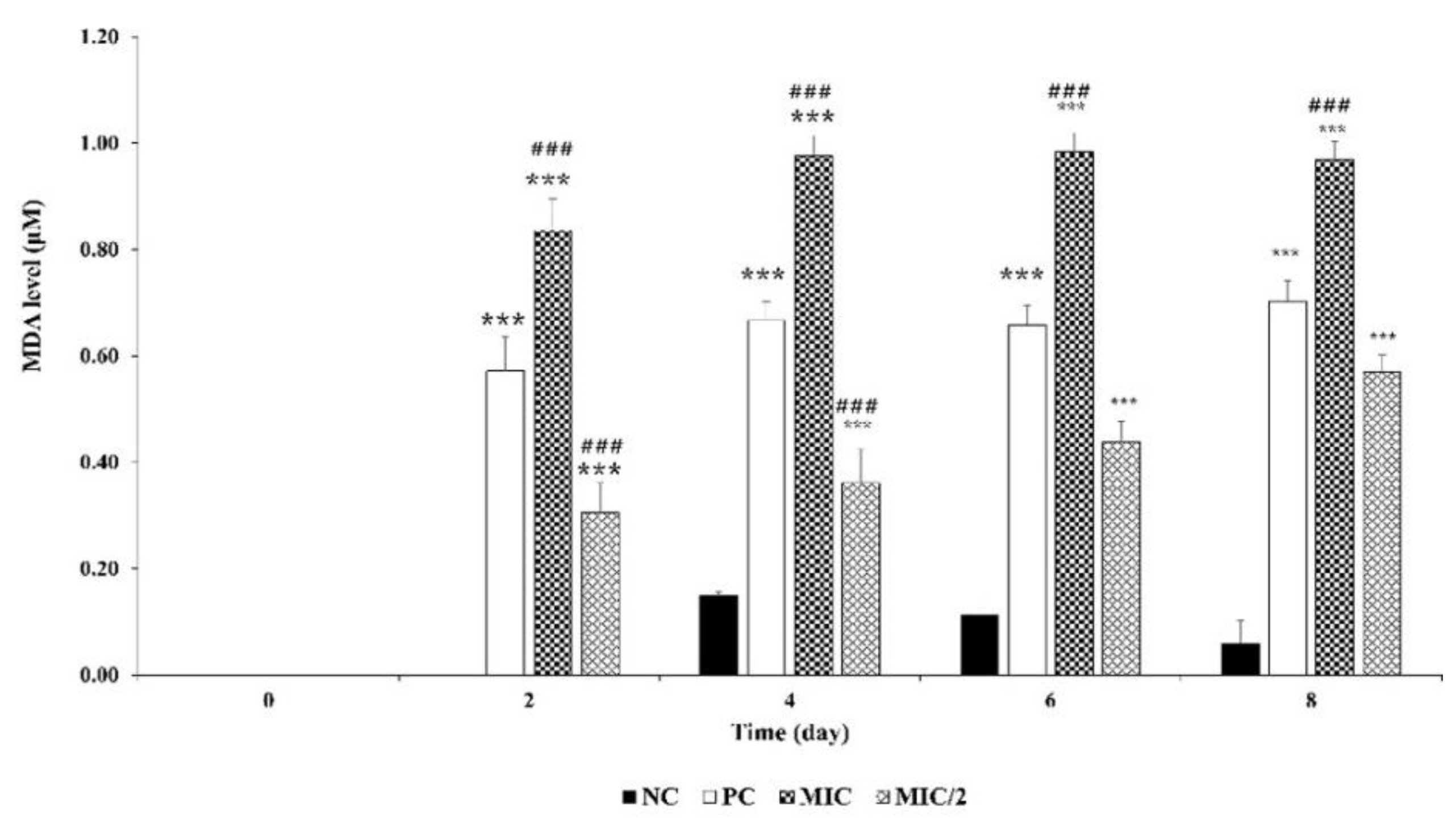
2.4.4. Antioxidant Response of M. aeruginosa
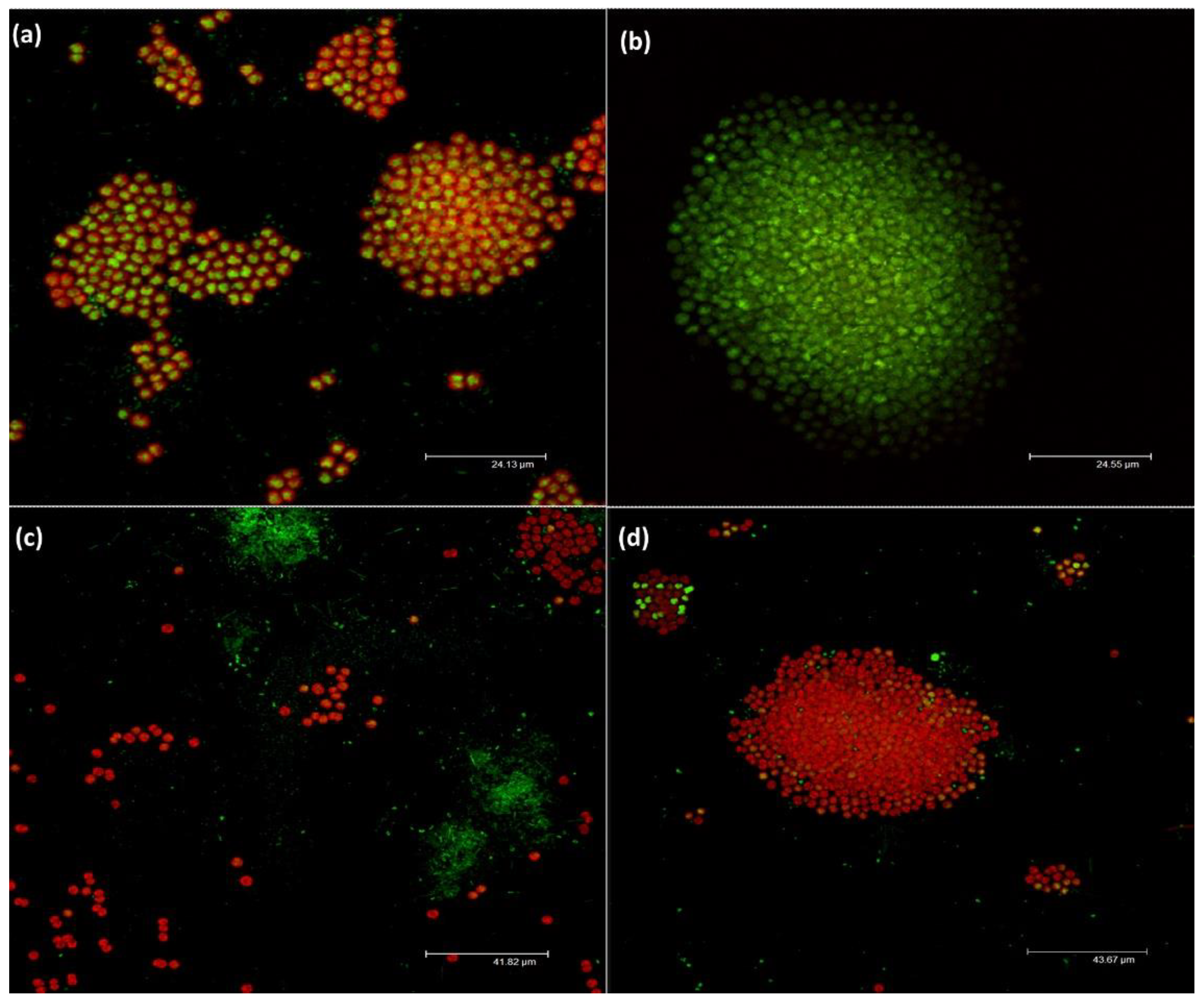
2.4.5. Cyanobacteria Morphology and Bacterial Abundance
2.4.6. Cyanotoxins Analysis
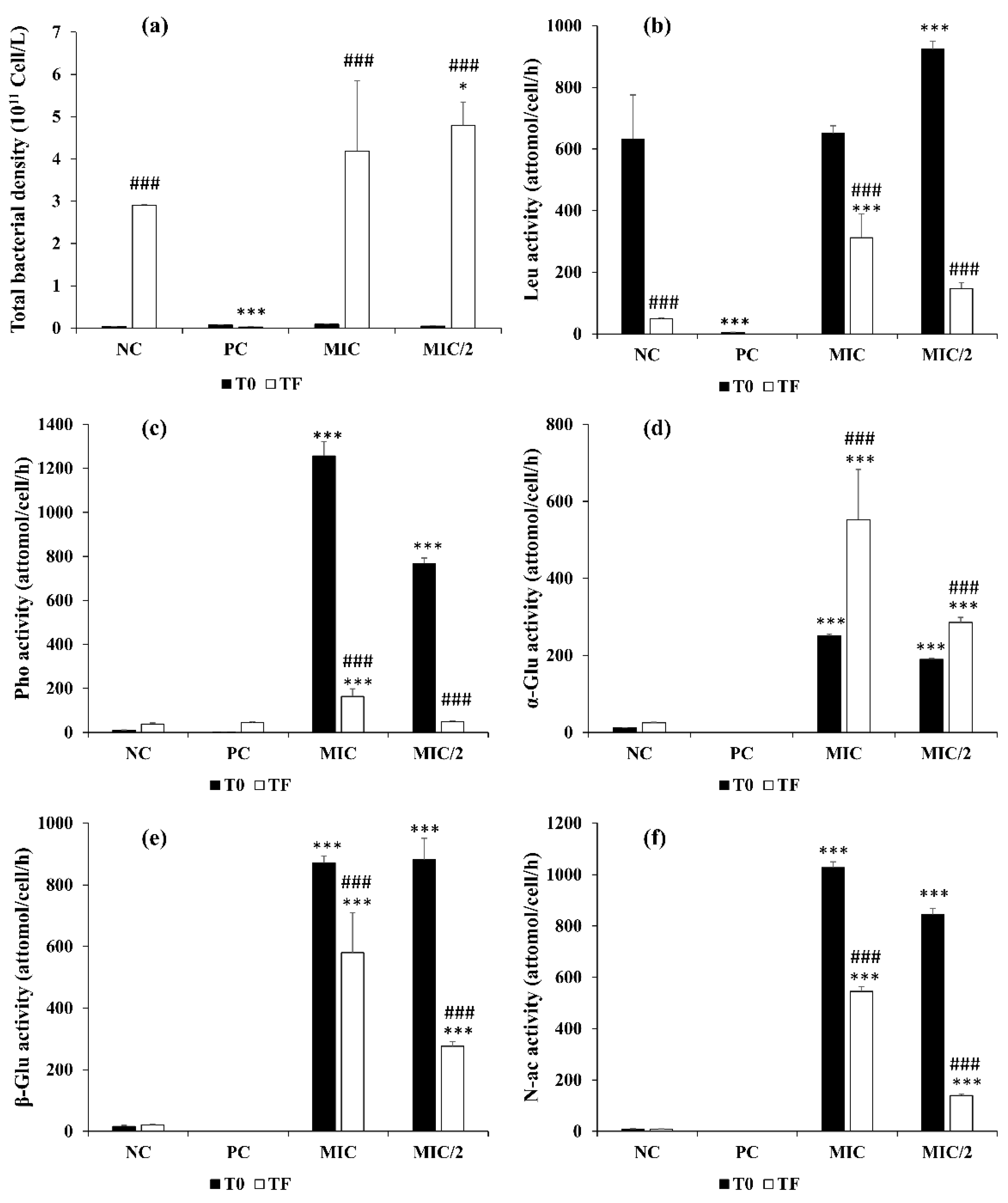
2.4.7. Ectoenzymatic Activities
2.5. Effects of the Most Active Aqueous Extract on Other Cyanobacterial Species
2.6. Data Analyses
3. Results
3.1. Comparison of Anticyanobacterial Activity Shown by Different Macrophyte Aqueous Extracts
3.2. Allelopathic Effect of R. aquatilis Aqueous Extract on M. aeruginosa and Their Associated Bacteria
3.2.1. Growth Inhibition
3.2.2. Effect on Pigments and Pheophytin Content of M. aeruginosa
3.2.3. Response of M. aeruginosa Cells to Oxidative Stress
3.2.4. Effect on Morphology of M. aeruginosa and Bacterial Community
3.2.5. Effects on Toxin Production
3.3. Allelopathic Effect of R. aquatilis Aqueous Extract on Growth and Toxicity of CS1101 R. raciborsky, CS1034 C. ovalisporum and P. rubescens F10
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action, and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef]
- D’Anglada, L.V. Public Health Surveillance, public communication and participation. In Toxic Cyanobacteria in Water, 2nd ed.; Chorus, I., Welker, M., Eds.; CRC Press, on behalf of the World Health Organization, Geneva, CH: Boca Raton, FL, USA, 2021; p. 839. [Google Scholar]
- Lee, J.; Kumar, P.; Jae, Y.; Kim, K.-h.; Kwon, E.E. The role of algae and cyanobacteria in the production and release of odorants in water. Environ. Pollut. 2017, 227, 252–262. [Google Scholar] [CrossRef]
- Kong, Y.; Peng, Y.; Zhang, Z.; Zhang, M.; Zhou, Y.; Duan, Z. Removal of Microcystis aeruginosa by ultrasound: Inactivation mechanism and release of algal organic matter. Ultrason. Sonochemistry. 2019, 56, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Y.; Xie, P.; Shang, R.; Ma, J. Removal of Microcystis aeruginosa by UV-activated persulfate: Performance and characteristics. Chem. Eng. J. 2016, 300, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Klapper, H. Technologies for lake restoration. J. Limnol. 2003, 62, 73–90. [Google Scholar] [CrossRef] [Green Version]
- Visser, P.M.; Ibelings, B.W.; Bormans, M.; Huisman, J. Artificial mixing to control cyanobacterial blooms: A review. Aquat. Ecol. 2016, 50, 423–441. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Xie, P.; Zhang, D.; Lei, H. In situ studies on the distribution patterns and dynamics of microcystins in a biomanipulation fish–bighead carp (Aristichthys nobilis). Environ. Pollut. 2007, 147, 150–157. [Google Scholar] [CrossRef]
- Burch, M.; Brookes, J.; Chorus, I. Assessing and controlling the risk of cyanobacterial blooms. Waterbody conditions. In Toxic Cyanobacteria in Water, 2nd ed.; Chorus, I., Welker, M., Eds.; CRC Press, on behalf of the World Health Organization, Geneva, CH: Boca Raton, FL, USA, 2021; pp. 505–562. [Google Scholar]
- Wang, X.; Zhao, Y.; Jiang, X.; Wang, Y.; Li, H.; Wang, L.; Liang, W. The growth and physiological activity of Microcystis aeruginosa after flocculation using modified tannin. Int. Biodeterior. Biodegrad. 2018, 133, 180–186. [Google Scholar] [CrossRef]
- Hilt, S.; Gross, E.M. Can allelopathically active submerged macrophytes stabilise clear-water states in shallow lakes? Basic. Appl. Ecol. 2008, 9, 422–432. [Google Scholar] [CrossRef] [Green Version]
- Mulderij, G.; Van Nes, E.H.; Van Donk, E. Macrophyte-phytoplankton interactions: The relative importance of allelopathy versus other factors. Ecol. Model. 2007, 204, 85–92. [Google Scholar] [CrossRef]
- Tazart, Z.; Douma, M.; Tebaa, L.; Loudiki, M. Use of macrophytes allelopathy in the biocontrol of harmful Microcystis aeruginosa blooms. Water. Sci. Technol. Water Supply. 2019, 19, 245–253. [Google Scholar] [CrossRef]
- Yuan, R.; Li, Y.; Li, J.; Ji, S.; Wang, S.; Kong, F. The allelopathic effects of aqueous extracts from Spartina alterniflora on controlling the Microcystis aeruginosa blooms. Sci. Total. Environ. 2020, 712, 136332. [Google Scholar] [CrossRef]
- Liu, B.Y.; Zhou, P.J.; Tian, J.R.; Jiang, S.Y. Effect of pyrogallol on the growth and pigment content of cyanobacteria-blooming toxic and nontoxic Microcystis aeruginosa. Bull. Environ. Contam. Toxicol. 2007, 78, 499–502. [Google Scholar] [CrossRef]
- Liu, R.; Ran, X.; Bai, F.; Xu, J.; Yang, S.; Shi, J.; Wu, Z. Use of chlorophyll a fluorescence to elucidate the toxicity target of N-phenyl-2-naphthylamine on photosynthetic system of Cylindrospermopsis raciborskii (Cyanobacteria). Phycologia 2015, 54, 12–19. [Google Scholar] [CrossRef]
- Shao, J.; Wu, Z.; Yu, G.; Peng, X.; Li, R. Allelopathic mechanism of pyrogallol to Microcystis aeruginosa PCC7806 (Cyanobacteria): From views of gene expression and antioxidant system. Chemosphere 2009, 75, 924–928. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Feng, J.; Lv, J.-p.p.; Xie, S.-l.l. Effect of high-doses pyrogallol on oxidative damage, transcriptional responses and microcystins synthesis in Microcystis aeruginosa TY001 (Cyanobacteria). Ecotoxicol. Environ. Saf. 2016, 134, 273–279. [Google Scholar] [CrossRef]
- Song, Y.; Mowe, M.A.D.; Mitrovic, S.M.; Tan, H.T.W.; Yeo, D.C.J. An ex-situ mesocosm study of emergent macrophyte effects on phytoplankton communities. Fundam. Appl. Limnol. Arch. Hydrobiol. 2019, 192, 225–235. [Google Scholar] [CrossRef]
- Vanderstukken, M.; Declerck, S.A.J.; Decaestecker, E.; Muylaert, K. Long-term allelopathic control of phytoplankton by the submerged macrophyte Elodea nuttallii. Freshw. Biol. 2014, 59, 930–941. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, B.; Huo, Y.; Liu, M.; Shi, J.; Jiang, T.; Zhang, Q.; Tang, C.; Bi, H.; He, P. Nutrient bioextraction and microalgae growth inhibition using submerged macrophyte Myriophyllum spicatum in a low salinity area of East China Sea. Mar. Pollut. Bull. 2018, 127, 67–72. [Google Scholar] [CrossRef]
- Wetzel, R.G. Extracellular Enzymatic Interactions: Storage, Redistribution, and Interspecific Communication. In Microbial Enzymes in Aquatic Environments; Chróst, R.J., Ed.; Brock/Springer Series in Contemporary Bioscience; Springer: New York, NY, USA, 1991; pp. 6–28. [Google Scholar] [CrossRef]
- Mecina, G.F.; Chia, M.A.; Cordeiro-Araújo, M.K.; Bittencourt-Oliveira, M.d.C.; Varela, R.M.; Torres, A.; González Molinillo, J.M.; Macías, F.A.; da Silva, R.M.G. Effect of flavonoids isolated from Tridax procumbens on the growth and toxin production of Microcystis aeruginos. Aquat. Toxicol. 2019, 211, 81–91. [Google Scholar] [CrossRef]
- Jang, M.-H.; Ha, K.; Takamura, N. Reciprocal allelopathic responses between toxic cyanobacteria (Microcystis aeruginosa) and duckweed (Lemna japonica). Toxicon 2007, 49, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Kucała, M.; Saładyga, M.; Kaminski, A. Phytoremediation of CYN, MC-LR and ANTX-a from Water by the Submerged Macrophyte Lemna trisulca. Cells 2021, 10, 699. [Google Scholar] [CrossRef] [PubMed]
- Douma, M.; Manaut, N.; Oudra, B.; Loudiki, M. First report of cyanobacterial diversity and microcystins in a Microcystis strain from Sidi Boughaba, a Moroccan coastal lagoon. Afr. J. Aquat. Sci. 2016, 41, 445–452. [Google Scholar] [CrossRef]
- Douma, M.; Ouahid, Y.; Campo, D.F.F.; Loudiki, M.; Mouhri, K.; Oudra, B. Identification and quantification of cyanobacterial toxins (microcystins) in two Moroccan drinking-water reservoirs (Mansour Eddahbi, Almassira). Environ. Monit. Assess. 2010, 160, 439–450. [Google Scholar] [CrossRef]
- Douma, M.; Ouahid, Y.; Loudiki, M.; del Campo, F.F.; Oudra, B. The first detection of potentially toxic Microcystis strains in two Middle Atlas Mountains natural lakes (Morocco). Environ. Monit. Assess. 2016, 189, 39. [Google Scholar] [CrossRef] [PubMed]
- Loudiki, M.; Oudra, B.; Sabour, B.; Sbiyyaa, B.; Vasconcelos, V. Taxonomy and geographic distribution of potential toxic cyanobacterial strains in Morocco. Ann. Limnol. 2002, 38, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Ndlela, L.L.; Oberholster, P.J.; Van Wyk, J.H.; Cheng, P.-H.H. An overview of cyanobacterial bloom occurrences and research in Africa over the last decade. Harmful Algae. 2016, 60, 11–26. [Google Scholar] [CrossRef]
- Oudra, B.; Loudiki, M.; Vasconcelos, V.; Sabour, B.; Sbiyyaa, B.; Oufdou, K.; Mezrioui, N. Detection and quantification of microcystins from cyanobacteria strains isolated from reservoirs and ponds in Morocco. Environ. Toxicol. 2002, 17, 32–39. [Google Scholar] [CrossRef]
- Sabour, B.; Loudiki, M.; Oudra, B.; Vasconcelos, V.; Martins, R.; Oubraim, S.; Fawzi, B. Toxicology of a Microcystis ichthyoblabe waterbloom from lake oued Mellah (Morocco). Environ. Toxicol. Int. J. 2002, 17, 24–31. [Google Scholar] [CrossRef]
- Samoudi, S.; Latour, D.; Robin, J.; Sabart, M.; Misson, B.; Ait Hammou, H.; Mouhri, K.; Loudiki, M. Horizontal distribution of the cell abundance and toxicity of Microcystis in a hypereutrophic Moroccan reservoir. Contemp. Probl. Ecol. 2016, 9, 554–562. [Google Scholar] [CrossRef]
- Ait Hammou, H.; Latour, D.; Samoudi, S.; Mouhri, K.; Douma, M.; Robin, J.; Loudiki, M. Occurrence of the First Toxic Microcystis Bloom in a Recent Moroccan Reservoir. Water. Resour. 2018, 45, 409–417. [Google Scholar] [CrossRef]
- Hammada, S. Études sur la végétation des zones humides du Maroc. Catalogue et analyse de la biodiversité et identification des principaux groupements végétaux. Ph.D. Thesis, Université Mohammed V de Rabat, Rabat, Morocco, February 2007. [Google Scholar]
- Tazart, Z.; Douma, M.; Caldeira, A.T.; Tebaa, L.; Mouhri, K.; Loudiki, M. Highlighting of the antialgal activity of organic extracts of Moroccan macrophytes: Potential use in cyanobacteria blooms control. Environ. Sci. Pollut. Res. 2020, 27, 19630–19637. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, M.; Scardala, S.; Cabras, P.; Orrù, A.; Vichi, S.; Testai, E.; Funari, E.; Manganelli, M. Cyanobacterial dynamics and toxins concentrations in Lake Alto Flumendosa, Sardinia, Italy. Adv. Oceanogr. Limnol. 2017, 8, 71–86. [Google Scholar] [CrossRef] [Green Version]
- WHO/HEP/ECH/WSH/2020.4. In WHO/HEP/ECH/WSH/2020.4 Cyanobacterial Toxins: Cylindrospermopsins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020.
- Luo, J.; Wang, Y.; Tang, S.; Liang, J.; Lin, W.; Luo, L. Isolation and Identification of Algicidal Compound from Streptomyces and Algicidal Mechanism to Microcystis aeruginosa. PLoS ONE 2013, 8, 1–14. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Supply and Services Canada: Ottawa, ON, Canada, 1972; pp. 175–206.
- Siedlewicz, G.; Żak, A.; Sharma, L.; Kosakowska, A.; Pazdro, K. Effects of oxytetracycline on growth and chlorophyll a fluorescence in green algae (Chlorella vulgaris), diatom (Phaeodactylum tricornutum) and cyanobacteria (Microcystis aeruginosa and Nodularia spumigena). Oceanologia 2020, 62, 214–225. [Google Scholar] [CrossRef]
- Lichtenthaler, H.; Wellburn, A. Determinations of total carotenoids and chlorophylls b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Lorenzen, C.J. Determination of chlorophyll and pheo-pigments: Spectrophotometric equations 1. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Rao, V.M.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and Ozone-Induced Biochemical Changes in Antioxidant Enzymes of Arabidopsis thaliana. Plant. Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; Volume 186, pp. 407–421. [Google Scholar]
- Tazart, Z.; Caldeira, A.T.; Douma, M.; Salvador, C.; Loudiki, M. Inhibitory effect and mechanism of three macrophytes extract on Microcystis aeruginosa growth and physiology. Water. Environ. J. 2021, 35, 580–592. [Google Scholar] [CrossRef]
- Patel, A.; Noble, R.T.; Steele, J.A.; Schwalbach, M.S.; Hewson, I.; Fuhrman, J.A. Virus and prokaryote enumeration from planktonic aquatic environments by epifluorescence microscopy with SYBR Green, I. Nat. Protoc. 2007, 2, 269–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO/HEP/ECH/WSH/2020.6. In WHO/HEP/ECH/WSH/2020.6 Cyanobacterial Toxins: Microcystins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020.
- Buratti, F.M.; Scardala, S.; Funari, E.; Testai, E. The conjugation of microcystin-RR by human recombinant GSTs and hepatic cytosol. Toxicol. Lett. 2013, 219, 231–238. [Google Scholar] [CrossRef]
- Nigro Di Gregorio, F.; Bogialli, S.; Ferretti, E.; Lucentini, L. First evidence of MC-HtyR associated to a Plankthothrix rubescens blooming in an Italian lake based on a LC-MS method for routinely analysis of twelve microcystins in freshwaters. Microchem. J. 2017, 130, 329–335. [Google Scholar] [CrossRef]
- Manganelli, M.; Scardala, S.; Stefanelli, M.; Vichi, S.; Mattei, D.; Bogialli, S.; Ceccarelli, P.; Corradetti, E.; Petrucci, I.; Gemma, S.; et al. Health risk evaluation associated to Planktothrix rubescens: An integrated approach to design tailored monitoring programs for human exposure to cyanotoxins. Water. Res. 2010, 44, 1297–1306. [Google Scholar] [CrossRef]
- Hoppe, H.-G. Significance of exoenzymatic activities in the ecology of brackish water: Measurements by means of methylumbelliferyl-substrates. Mar. Ecol. Prog. Ser. 1983, 11, 299–308. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, P.; Zeng, G.; Cai, X.; Liu, S.; Yin, Y.; Hu, X.; Hu, X.; Tan, X. Growth inhibition and oxidative damage of Microcystis aeruginosa induced by crude extract of Sagittaria trifolia tubers. J. Environ. Sci. (China) 2016, 43, 40–47. [Google Scholar] [CrossRef]
- Hua, Q.; Liu, Y.-g.; Yan, Z.-l.; Zeng, G.-m.; Liu, S.-b.; Wang, W.-j.; Tan, X.-f.; Deng, J.-q.; Tang, X.; Wang, Q.-p. Allelopathic effect of the rice straw aqueous extract on the growth of Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2018, 148, 953–959. [Google Scholar] [CrossRef]
- Ni, L.; Rong, S.; Gu, G.; Hu, L.; Wang, P.; Li, D.; Yue, F.; Wang, N.; Wu, H.; Li, S. Inhibitory effect and mechanism of linoleic acid sustained-release microspheres on Microcystis aeruginosa at different growth phases. Chemosphere. 2018, 212, 654–661. [Google Scholar] [CrossRef]
- Gallardo-Rodríguez, J.J.; Astuya-Villalón, A.; Llanos-Rivera, A.; Avello-Fontalba, V.; Ulloa-Jofré, V. A critical review on control methods for harmful algal blooms. Rev. Aquac. 2019, 11, 661–684. [Google Scholar] [CrossRef]
- Wu, Y.; Wan, L.; Zhang, W.; Ding, H.; Yang, W. Resistance of cyanobacteria Microcystis aeruginosa to erythromycin with multiple exposure. Chemosphere 2020, 249, 126147. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mao, F.; Kirumba, G.C.; Jiang, C.; Manefield, M.; He, Y. Changes in metabolites, antioxidant system, and gene expression in Microcystis aeruginosa under sodium chloride stress. Ecotoxicol. Environ. Saf. 2015, 122, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Hu, H.Y.; Li, F.M. Physiological and biochemical effects of allelochemical ethyl 2-methyl acetoacetate (EMA) on cyanobacterium Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2008, 71, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, Y.; Gao, Y.; Liu, B.; Sun, X.; He, F.; Zhou, Q.; Wu, Z. Effects of pyrogallic acid on Microcystis aeruginosa: Oxidative stress related toxicity. Ecotoxicol. Environ. Saf. 2016, 132, 413–419. [Google Scholar] [CrossRef]
- Hou, X.; Huang, J.; Tang, J.; Wang, N.; Zhang, L.; Gu, L.; Sun, Y.; Yang, Z.; Huang, Y. Allelopathic inhibition of juglone (5-hydroxy-1,4-naphthoquinone) on the growth and physiological performance in Microcystis aeruginosa. J. Environ. Manag. 2019, 232, 382–386. [Google Scholar] [CrossRef]
- Zhang, S.; Benoit, G. Comparative physiological tolerance of unicellular and colonial Microcystis aeruginosa to extract from Acorus calamus rhizome. Aquat. Toxicol. 2019, 215, 105271. [Google Scholar] [CrossRef]
- Schuurmans, J.M.; Brinkmann, B.W.; Makower, A.K.; Dittmann, E.; Huisman, J.; Matthijs, H.C.P. Microcystin interferes with defense against high oxidative stress in harmful cyanobacteria. Harmful. Algae. 2018, 78, 47–55. [Google Scholar] [CrossRef]
- Neilan, B.A.; Pearson, L.A.; Muenchhoff, J.; Moffitt, M.C.; Dittmann, E. Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ. Microbiol. 2013, 15, 1239–1253. [Google Scholar] [CrossRef]
- Kaplan, A.; Weiss, G.; Sukenik, A. Cyanobacterial secondary metabolites mediate interspecies–intraspecies communication in the water body. Environ. Microbiol. 2016, 18, 305–306. [Google Scholar] [CrossRef]
- Zilliges, Y.; Kehr, J.-C.; Meissner, S.; Ishida, K.; Mikkat, S.; Hagemann, M.; Kaplan, A.; Boerner, T.; Dittmann, E. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. PLoS ONE 2011, 6, e17615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mecina, G.F.; Dokkedal, A.L.; Saldanha, L.L.; Chia, M.A.; Cordeiro-Araújo, M.K.; do Carmo Bittencourt-Oliveira, M.; da Silva, R.M.G. Response of Microcystis aeruginosa BCCUSP 232 to barley (Hordeum vulgare L.) straw degradation extract and fractions. Sci. Total. Environ. 2017, 599–600, 1837–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Chen, Y.; Cai, F.; Liu, X.; Wang, Y.; Li, R. Toxicity-associated changes in the invasive cyanobacterium Cylindrospermopsis raciborskii in response to nitrogen fluctuations. Environ. Pollut. 2018, 237, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Akcaalan, R.; Köker, L.; Oğuz, A.; Spoof, L.; Meriluoto, J.; Albay, M. First Report of Cylindrospermopsin Production by Two Cyanobacteria (Dolichospermum mendotae and Chrysosporum ovalisporum) in Lake Iznik, Turkey. Toxins 2014, 6, 3173–3186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, E.M.; Meyer, H.; Schilling, G. Release and ecological impact of algicidal hydrolysable polyphenols in Myriophyllum spicatum. Phytochem 1996, 41, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Ling, F.; Yi, Y.L.; Zhang, H.Y.; Wang, G.X. Algicidal activity and potential mechanisms of ginkgolic acids isolated from Ginkgo biloba exocarp on Microcystis aeruginosa. J. Appl. Phycol. 2014, 26, 323–332. [Google Scholar] [CrossRef]

| Treatments | ELISA (µg/L) | LC-MS/MS (µg/L) | HPLC-DAD (µg/L) | Cell Quota (fg/cell) |
|---|---|---|---|---|
| NC | 10.20 (±6.68) | 15.75 (±4.37) | 14.40 | 0.74 (±0.43) |
| PC | 0.22 (±0.01) *** | 0.08 (±0.06) *** | 5.40 | 0.11 (±0.09) * |
| MIC | 1.58 (±0.74) *** | 2.27 (±0.24) ** | 22.06 | 0.66 (±0.13) |
| MIC/2 | 3.79 (±1.25) * | 4.01 (±0.44) * | 22.04 | 0.25 (±0.04) * |
| Cell Density (cell/L∙109) | Growth Rate (d−1) | CTX (µg/L) | Cell Quota fg MC-LReq/cell | |
|---|---|---|---|---|
| CS1101 C | 1.95 (±0.28) | 0.17 | 82.4 (±10.7) 1 | 39.66 (±1.57) |
| CS1101 MIC | 0.11 (±0.02) | −0.19 | 35.9 (±11.7) 1 | 220.13 (±71.41) |
| CS1034 C | 0.29 (±0.03) | 0.08 | 97.1 (±37.8) 1 | 335.66 (±112.34) |
| CS1034 MIC | 0.06 (±0.00) | −0.30 | 80.5 (±33.7) 1 | 1304.50 (±580.85) |
| F10 C | 2.20 (±0.33) | 0.14 | 109.2 (±10.1) 2 | 48.41 (±1.75) |
| F10 MIC | 4.92 (±0.93) | 0.07 | 275.9 (±41.4) 2 | 57.86 (±22.81) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tazart, Z.; Manganelli, M.; Scardala, S.; Buratti, F.M.; Nigro Di Gregorio, F.; Douma, M.; Mouhri, K.; Testai, E.; Loudiki, M. Remediation Strategies to Control Toxic Cyanobacterial Blooms: Effects of Macrophyte Aqueous Extracts on Microcystis aeruginosa (Growth, Toxin Production and Oxidative Stress Response) and on Bacterial Ectoenzymatic Activities. Microorganisms 2021, 9, 1782. https://doi.org/10.3390/microorganisms9081782
Tazart Z, Manganelli M, Scardala S, Buratti FM, Nigro Di Gregorio F, Douma M, Mouhri K, Testai E, Loudiki M. Remediation Strategies to Control Toxic Cyanobacterial Blooms: Effects of Macrophyte Aqueous Extracts on Microcystis aeruginosa (Growth, Toxin Production and Oxidative Stress Response) and on Bacterial Ectoenzymatic Activities. Microorganisms. 2021; 9(8):1782. https://doi.org/10.3390/microorganisms9081782
Chicago/Turabian StyleTazart, Zakaria, Maura Manganelli, Simona Scardala, Franca Maria Buratti, Federica Nigro Di Gregorio, Mountasser Douma, Khadija Mouhri, Emanuela Testai, and Mohammed Loudiki. 2021. "Remediation Strategies to Control Toxic Cyanobacterial Blooms: Effects of Macrophyte Aqueous Extracts on Microcystis aeruginosa (Growth, Toxin Production and Oxidative Stress Response) and on Bacterial Ectoenzymatic Activities" Microorganisms 9, no. 8: 1782. https://doi.org/10.3390/microorganisms9081782
APA StyleTazart, Z., Manganelli, M., Scardala, S., Buratti, F. M., Nigro Di Gregorio, F., Douma, M., Mouhri, K., Testai, E., & Loudiki, M. (2021). Remediation Strategies to Control Toxic Cyanobacterial Blooms: Effects of Macrophyte Aqueous Extracts on Microcystis aeruginosa (Growth, Toxin Production and Oxidative Stress Response) and on Bacterial Ectoenzymatic Activities. Microorganisms, 9(8), 1782. https://doi.org/10.3390/microorganisms9081782







