1. Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that affects both upper and lower motor neurons, determining progressive paralysis and death, within 3–5 years post diagnosis. About 5–10% of ALS cases are familial, the remaining are apparently sporadic of unknown etiology [
1]. A poor knowledge of ALS pathophysiology is the major reason for the absence of specific diagnostic tests in clinical practice and the lack of an effective treatment. Thus, it is important to find reliable diagnostic and prognostic markers that can guide the clinical diagnostic process at an early stage of the disease. There is no cure for ALS, and the FDA-approved drugs are only marginally effective in slowing the progression of the disease by a few months [
1]. Advances in understanding pathogenic mechanisms are essential to identify new possible therapeutic interventions. Several studies have demonstrated the presence of reverse transcriptase activity in serum samples of both ALS patients and their unaffected relatives [
2,
3], indicating that a common inherited endogenous retrovirus may be the trigger of the disease [
4]. Endogenous retroviral sequences constitute 8% of human genomes, in which they have been integrated through repeated infections during evolution [
5]. In particular, RNA sequences of the human endogenous retrovirus of the K family (HERV-K) have been detected in motor neurons of ALS patients [
6,
7], and it has been observed that increased expression of HERV-K envelope protein in upper and lower motor neurons was neurotoxic and able to cause cellular degeneration [
7], thus indicating the reactivation of HERV-K in the affected tissues. Moreover, in a transgenic mouse model, increased HERV-K envelope protein expression in motor neurons induces a clinical and pathological phenotype that resembles ALS, strongly suggesting that HERV-K may play a contributing role in the pathophysiology of this disorder [
7].
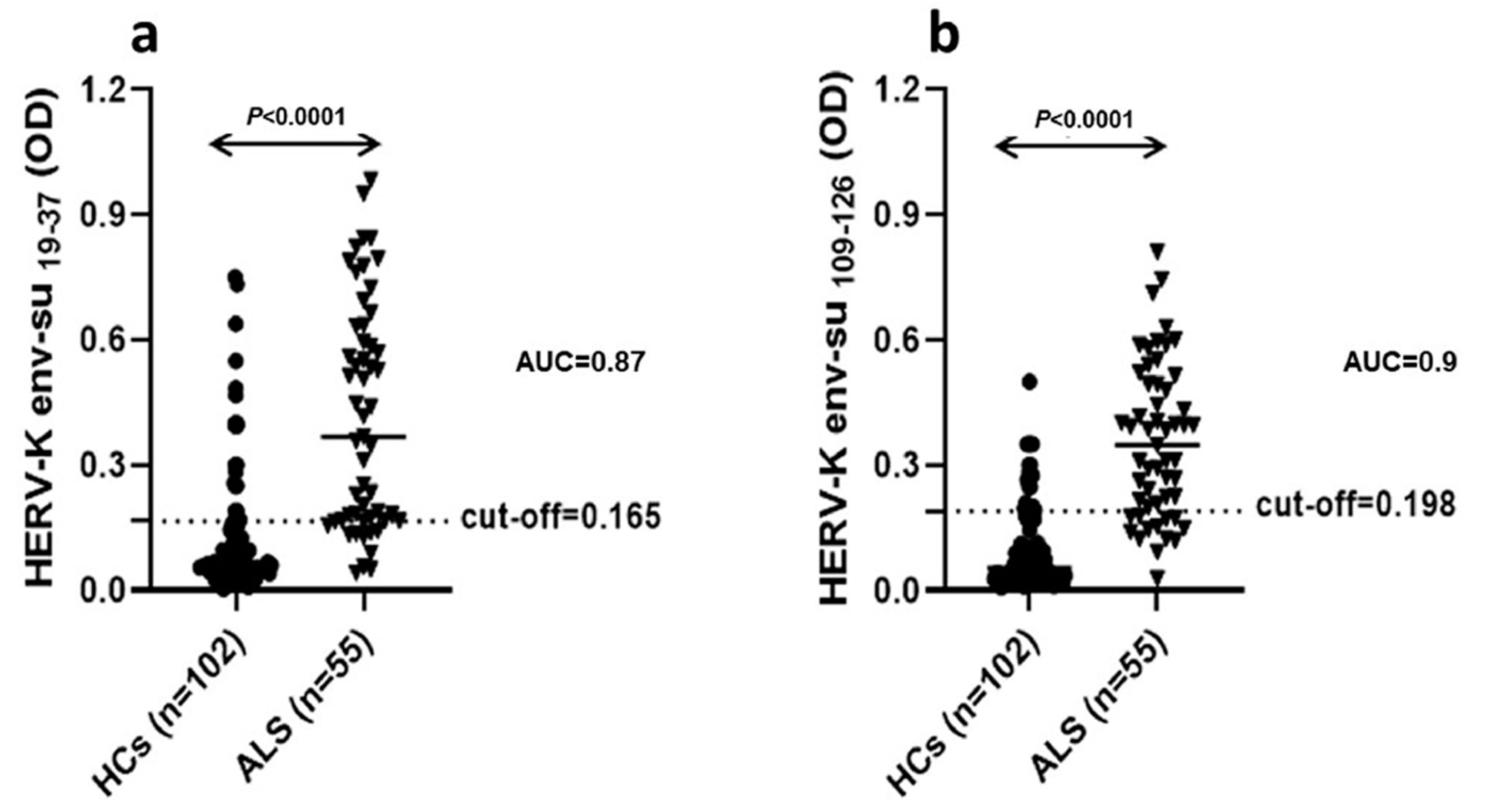
Recently, we investigated the specific humoral immune response against four antigenic peptides derived from the HERV-K env-su glycoprotein in serum and cerebrospinal fluid (CSF) of ALS patients, reporting a significant immune response directed toward the HERV-K env-su
19–37 peptide both in serum and CSF of ALS patients, but not in healthy controls (HCs). In addition, we observed a specific intrathecal IgG synthesis against HERV-K peptides and a functionally intact blood–brain barrier in most of the patients analyzed, indicating that there is active antibody production within the CNS. Moreover, in ALS patients, the HERV-K env-su
19–37 antibody levels were significantly correlated with clinical measures of disease severity, both in serum and CSF [
8]. In this paper, we shed light on the role of HERV-K in the pathophysiology of ALS by evaluating the humoral and cell-medi ated immune response to HERV-K in ALS patients compared to HCs, using different methodological approaches: (1) cytometric analysis to quantify HERV-K env-su glycoprotein expression on peripheral blood mononuclear cells (PBMCs) membrane and test the T and B mediated immune response after antigenic stimulation with HERV-K env
19–37 and HERV-K env
109–126, by quantification of Interleukin-6 (IL-6), Interferon- γ (IFN-γ), Tumor Necrosis Factor-α (TNF-α), Macrophage Inflammatory Protein-1α (MIP-1α), and Monocyte Chemoattractant Protein-1 (MCP-1); (2) investigation of HERV-K transcripts in PBMCs by RT qPCR method; (3) analysis by ELISA of specific antibodies against env-surface peptides; and (4) quantification of IgG1 and IgG4 subclasses by nephelometry. We detected IgG1 plasma levels significantly higher in ALS patients compared to HCs; conversely, no difference between the two groups was observed for IgG4 plasma levels.
The results obtained highlighted the ability of HERV-K to modulate the immune system at different levels, generating mediators mainly involved in proinflammatory response. Of note, despite depletion, B cells of ALS patients express higher levels of HERV-K env protein compared to HCs feeding the inflammatory loop, by induction of a continuous proinflammatory stimulus of T lymphocytes sustained by exposition of viral protein on B lymphocytes membrane.
2. Material and Methods
The study protocol was approved by the institutional review board of the Sassari University Hospital (Azienda Ospedaliero-Universitaria, Sassari, Italy: IRB number 2395/2016). All participants provided written informed consent.
2.1. Patients
For this study, we consecutively included, from January 2016 to December 2019, (1) sporadic, newly diagnosed ALS (sALS) patients, seen at the Neurology Unit of the University Hospital of Sassari, (2) long-survivor ALS patients reported by primary doctors and doctors in the Sassari local district, and (3) age and sex matched HC subjects from the Blood Transfusion Centre of Sassari. The diagnosis of ALS was made by the treating neurologists, according to the revised El Escorial criteria [
9]. Clinical measures of ALS severity were evaluated through the ALS Functional Rating Scale—Revised [
10].
Table 1 shows the demographic data of investigated groups and the different methodological approaches used. Patients with known infections at the time of study enrollment were excluded.
2.2. Blood Samples
All the subjects recruited for the study provided 20 mL of blood in EDTA tubes. PBMCs were isolated by centrifugation on a discontinuous density gradient (Ficoll); the obtained cells were washed twice in phosphate-buffered saline (PBS) and counted. They were then divided into two aliquots, one of which was frozen in dimethylsulfoxide solution and stored in liquid nitrogen to maintain cell viability in view of the flow cytometry analysis. The second aliquot was used for RNA extraction and quantification of HERV-K env transcripts by RT-qPCR (see below).
2.3. Antigens
Synthetic peptides derived from HERV-K env protein were used in this study for the antigenic stimulation. Peptides, described in detail in the previous paper [
8], were synthesized commercially at 90% purity (LifeTein, South Plainfield, NJ 07080, USA).
2.4. Antibody Reactivity against HERV-K Immunogenic Peptides
Antibody response against two antigenic peptides derived from HERV-K envelope proteins, namely HERV-K env
19–37 [VWVPGPTDDRCPAKPEEEG] and HERV-K env
109–126 [RPKGKTCPKEIPKGSKNT], belonging to the surface region of the protein, was assessed with a previously described methodology [
8]. Briefly, 96-well plates (NuncImmuno™ Plates, Maxi Sorp, Nalgen Nunc International, Sigma Aldrich, St. Louis, USA) were coated overnight at 4 °C with 10 µg/mL of each peptide in a solution 0.05 M of carbonate-bicarbonate, pH 9.5 (Sigma Aldrich, St. Louis, MO, USA). Plates were washed twice with 0.1% Tween-20 (in TBS) and blocked with 5% skimmed dried milk in PBS for 1 h at room temperature (25 °C). Plasma samples (1:1000 dilution) were added and incubated for 2 h at room temperature. Secondary antibody was alkaline phosphatase-conjugated goat anti-human immunoglobulin G polyclonal Ab (1:1000; Sigma Aldrich, St. Louis, MO, USA). Plates were washed between each incubation. Alkaline phosphatase was detected with para nitrophenyl phosphate (Sigma). Absorbance was read at 405 nm on a plate reader (SpectraMax Plus 384, Molecular Devices, Sunnyvale, CA, USA). All incubation volumes were 100 µL/well. Each sample was run in duplicate, and normalization was performed with a positive control (absorbance reactivity set at 1.0 arbitrary units) included in each assay. Background activity was calculated as the mean signal of an immobilized peptide with secondary Ab alone.
In our previous ELISA study, these two peptides only triggered a significantly increased antibody response in patients with ALS, the HERV-K env
109–126, only in serum, the HERV-Kenv
19–37 in serum, and CSF [
8].
2.5. Cell Preparation and Antigenic Stimulation
Cells of ALS patients and HCs were quickly thawed using RPMI, added to 10% of autologous human serum, and counted. They were then divided into 4 tubes, each containing 1,000,000 cells. The conditions were: positive and negative controls and the two HERV-K env stimulation peptides.
Positive control included: 500 µL of RPMI, 500 µL of cells suspension, the PMA (phorbol 12-myristate 13-acetate) 50 µg/mL, and calcium ionophore A23187 0.5 µg/mL (Sigma Al drich). Negative control included: 500 µL of RPMI and 500 µL of cells suspension. Antigenic stimulation sample were performed with: 500 µL of RPMI, 500 µL of cells suspension, the costimulatory mAB CD28/49d d 5 µL/mL, and two different peptides (HERV-K env 19–37 and HERV-K env 109–126, 20 µM). The culture tubes were incubated in a diagonal position at 37 °C of 5% CO2 for 8 h; the first 2 h of incubation were without Brefeldin A (BFA) to enable antigen processing by antigen presenting cells (APC). The BFA (10 µg/mL) was included for the final 6 h of activation in order to inhibit the secretion and paralysis of the Golgi apparatus. Afterwards, the samples were centrifuged for 10 min at 1200 rpm, then the supernatant was aspired and discerned. The pellet was vortexed and the samples were incubated for 15 min in the dark with 500 µL of 1 × Fixation Buffer (R&D) at room temperature (RT), than supplemented with 500 µL of Stain Buffer (BD Pharmigen, San Diego, CA USA). Cells were centrifuged for 10 min at 1200 rpm, and then the supernatant was discarded by inversion. The samples were gently resuspended in 2 mL of Stain Buffer (BD Pharmigen) and incubated at 4 °C overnight (o/n).
2.6. Intracellular Cytokine Staining and Phenotyping Analysis
The following day, cells were centrifuged for 10 min at 1200 rpm, the supernatant was discarded by inversion, then 200 µL of permeabilizing solution (0.05% saponin in PBS solution) were added to each sample and incubated for 10 min. Subsequently, the solution containing 100 µL of cells suspension and 100 µL of permeabilizing solution was divided into two tubes. Mix of antibodies was added for marking and samples were further incubated for 30 min in the dark. After washing with 2 mL of permeabilizing solution, centrifuging for 10 min at 1200 rpm, and discarding the supernatant by inversion, cells were resuspended in 300 µL of FACS Flow solution. The labeled cells were analyzed by flow cytometry on FACSCanto™ using FACSDiva 2.2 (Becton and Dickinson, Milano, Italia).
2.7. Cytokines and Phenotyping Antibodies
By using the intracellular cytokine (ICC) method, we evaluated in PBMCs, the immune response T and B mediated after stimulation with specific peptides. The ICC method permits to quantify the intracellular production of cytokines in specific subpopulations of CD4, CD8, CD19, CD14, and CD56/16 following specific peptide stimulus. In particular, monoclonal antibodies (mAbs) CD3 FITC, CD 19 PE, CD8 ACP-Cy7, INF-γ PE-Cy7, and IL-6 APC were used for the analysis of T and B lymphocytes, while monocytes, natural killer (NK) cells and T lymphocytes were detected using: CD3 FITC, CD14 APC-Cy7, MIP1-α PE, TNF -α PE Cy 7, CD56/CD16 PerCP-Cy5.5, MCP-1 APC, and CD8 APC-Cy7. The antibodies were used at the manufacturer’s recommended concentrations (BD Biosciences, San Diego, CA USA).
2.8. HERV-K env Protein Detection
The performance characteristics for the flow cytometry to detect HERV-K env protein on the plasma membrane were as follows: anti-HERV-K env mouse monoclonal antibody(clone 215/H4) (Space Import) and secondary allophycocyanin crosslinked goat antimouse IgG (Invitrogen, Thermo Fisher Scientific, CA USA) were used. The isotype control was a preimmune rabbit serum (Santa Cruz Biotechnology, Inc, Heidelberg, Germany). To determine the phenotype of cell subpopulations, PBMCs were stained with monoclonal antibodies (mAbs) CD3 FITC, CD19 PE, CD8 ACP, CD56/CD16 PerCP-Cy5.5, and CD14 APC-Cy7 (BD Biosciences, San Diego CA). For each sample, 1 × 106 cells in a 100 µL final volume were incubated in the dark for 30 minutes on ice. Stained cells were analyzed by flow cytometry on FACSCanto™ using FACSDiva 2.2 (Becton and Dickinson), a total of at least 5 × 104 events for each sample were acquired.
2.9. RNA Extraction and Real-Time RT-PCR
RNAs were extracted from 3 × 106 PBMCs isolated from peripheral blood by RNeasy
® Plus Mini Kit (Qiagen, Hilden, Germany) and treated with RNase free DNase (Qiagen). The concentration and the quality of RNA were evaluated with the Thermo Scientific ™ NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). Reverse transcription of 1 µg of total RNA into cDNA was performed using SuperScript™ III First-Strand Synthesis SuperMix (Invitrogen) according to manufacturer’s instructions. Gene expression levels for HERV-K env and glyceraldehyde 3-phosphotate dehydrogenase (GAPDH) were determined by quantitative PCR performed on C1000 Touch Thermal Cycler (Bio Rad CFX96 Real-Time System, Hercules CA, USA). PCR primers and probes for quantitative PCR were designed by the Beacon Designer software (PREMIER Biosoft International Corina Way Palo Alto, CA, USA), which designs the most favorable primers and probes for a specific target region, and are listed in
Table 2.
Absence of DNA contamination in the RNA samples was confirmed when target gene was not detected by PCR when RT was omitted during reverse transcription. The amount of RNA in PBMCs was expressed as relative levels to controls samples after normalization with the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) invariant housekeeping gene. Data were expressed according to the 2−ΔCt method.
2.10. IgG 1 and IgG 4 Subclasses Analysis
Forty-eight ALS serum samples, newly diagnosed patients (n = 27), long-survivor patients (n = 21), were analyzed for the quantitative determination of IgG1 (N AS IgG1 (Siemens, PA, USA) ref: OQXI09) and IgG4 (N Latex IgG4 (Siemens) ref: OPAU03) subclasses using immuno-nephelometry on the BN II System. Fifty-eight, HCs serum samples, matched by age and sex were also analyzed. The assay protocols for IgG in serum and CSF, respectively, were given in the BN II System Instruction Manual and software of the instrument. Screening at the starting dilution of 1:2000 in serum to arrive to a concentrate solution of the sample until 1:1 and titration of the positive cases were performed.
2.11. Statistical Analysis
All data obtained were analyzed using GraphPad Prism 8.4.1 software (GraphPad Software, San Diego, CA, USA). Continuous variables were presented as the mean ± standard deviation (SD), and categorical variables were presented as numbers and percentages. The analysis between two groups was executed using the non-parametric Mann–Whitney test and a value of p < 0.05 was considered statistically significant. The diagnostic value of the indirect ELISA assays was evaluated by the receiver operating characteristic (ROC) curve. The optimal cut off values were chosen according to ROC analysis, setting the most appropriate specificity and sensitivity for all serum and CSF samples measured. In ALS patients, linear regression for the different serum HERV-K antibodies levels against HERV-K env expression on the B cells membrane, was calculated using Spearman test for non-parametric data.
4. Discussion
ALS is characterized by heterogeneous clinical signs and symptoms, and a definite diagnosis may require 13 to 18 months, once of other disorders that can affect upper and lower motor neurons have been excluded [
11,
12].
The identification of specific and sensitive circulating biomarkers for ALS could permit an early diagnosis and avoid unnecessary and potentially harmful therapeutic trials. In particular, an increase in HERVs expression has been observed in some neurological diseases, including ALS, although there is no evidence that this may be the primary causative factor for the pathology.
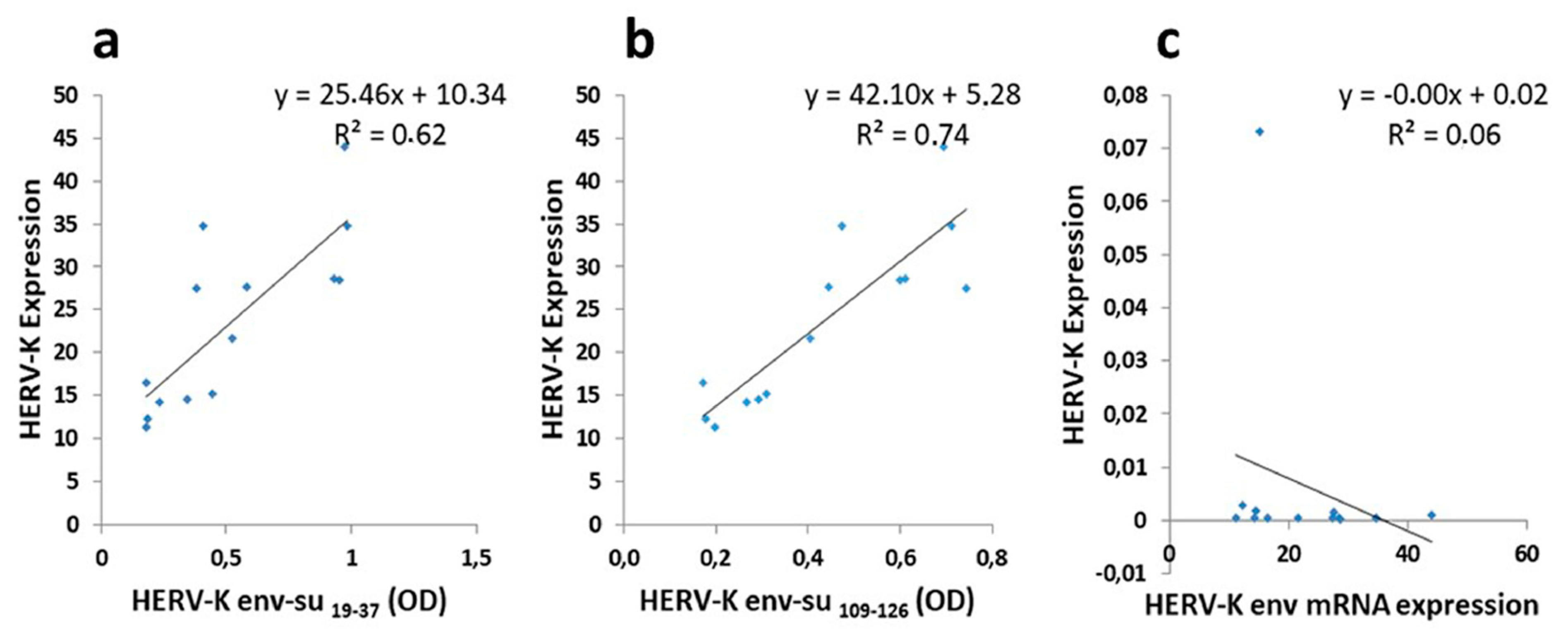
In a previous study, we documented an HERV-K env antigen humoral immunity response by detecting specific IgG antibodies in serum and CSF of Sardinian patients affected by ALS, with respect to other neurological diseases [
8]. In this study, to better secure the role of retroviruses in ALS, and validate the use of HERV-K as a possible diagnostic biomarker, we expanded the size of our previously studied cohort to 55, and respective healthy controls to 102, confirming the data obtained in the previous paper [
8]. We also used different methodological approaches to better understand the role of HERV-K on humoral and cell-mediated immune responses both by using cytometric analysis to quantify the HERV-K env peptides expression on the PBMCs membrane of ALS patients and controls and by investigating HERV-K transcripts in PBMCs by RT-qPCR method. Notably, PBMCs have been proposed as a good non-invasive option for studying ALS [
13].
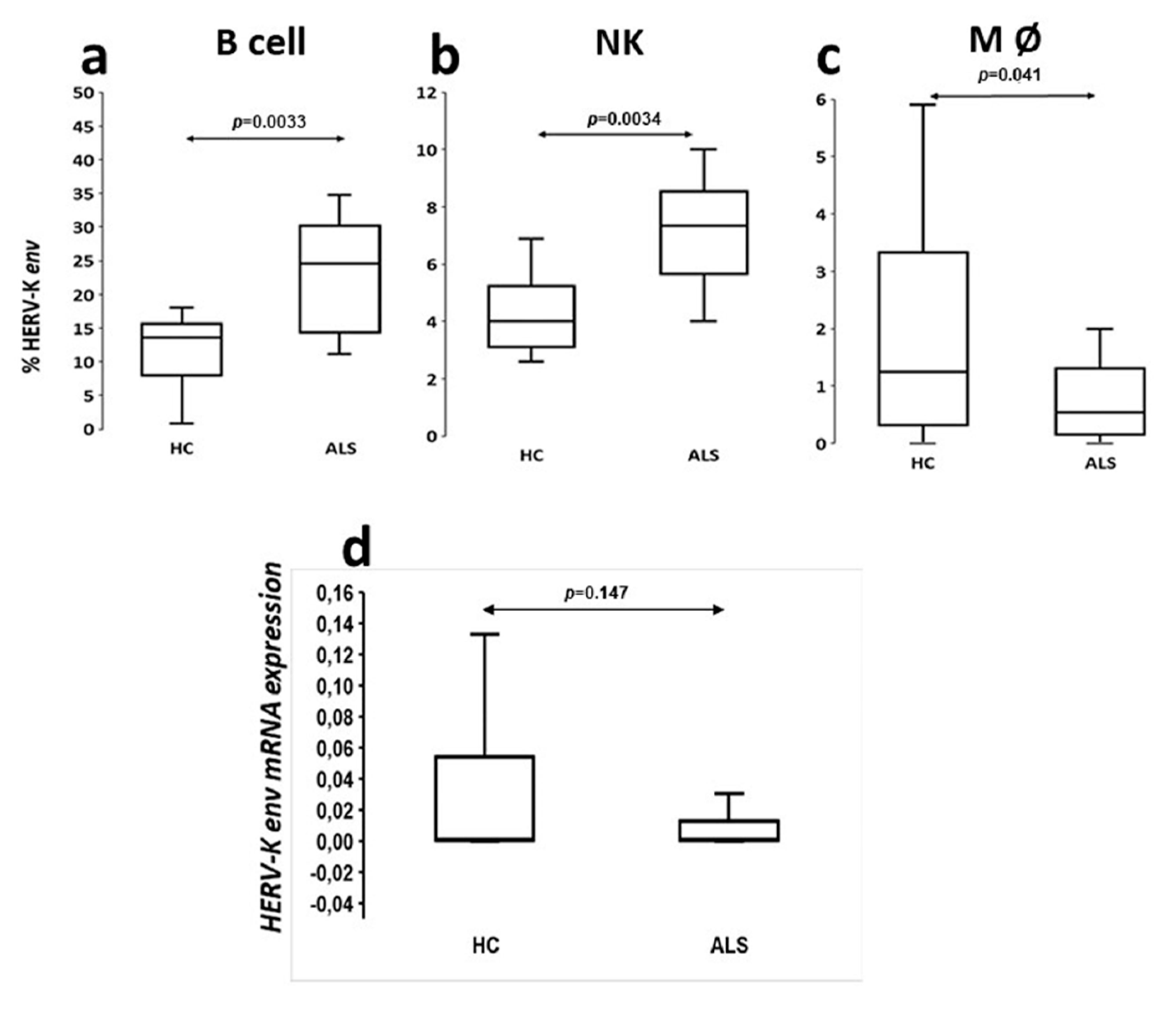
The analysis of viral protein expression on lymphomonocytes showed that HERV-K env protein was expressed with greater percentage frequency in B cells of ALS patients. This finding supports the results of ELISA tests in serum and CSF showing that the production of specific immunoglobulins towards selective retroviral peptides was significantly higher in patients with ALS with respect to HCs [
8]. Instead, the analysis of the HERV-K env transcripts has not proven any significant difference between patients and controls as previously observed by Garson [
14]. Interestingly, other HERVs families have been associated to different neurological diseases, such as HERV-W to multiple sclerosis (MS) from different authors [
15,
16], and HERV-W env expression on the membrane of PBMCs in MS patients seems modulated by natalizumab [
17]. Recently, we have also shown that circulating antibody levels directed against the HERV-W env-su
93–108 and HERV-W env-su
248–262 peptide fragments are different in different CNS demyelinating disorders [
18,
19].
Based on our findings, HERV-K env protein appeared to be poorly expressed on the CD4+ and CD8+ lymphocyte membranes of both ALS patients and controls (data not shown), despite retroviral antigens also determining a humoral response T helps mediate.
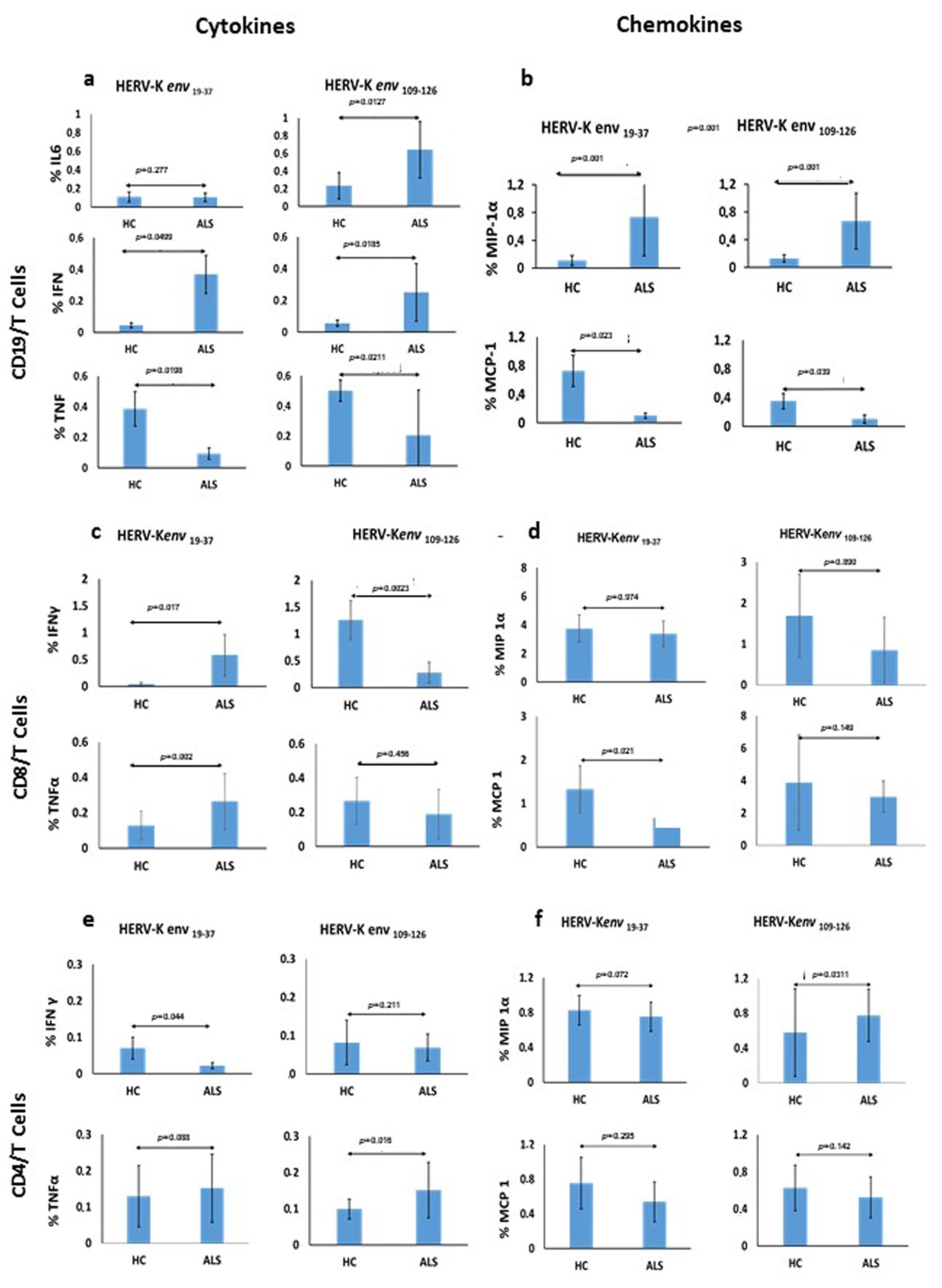
The specific immune response which T and B mediated after stimulation of PBMCs with two antigenic peptides derived from the HERV-K env-su glycoprotein, namely HERV-K env 19–37 and HERV-K env 109–126, in patients with ALS and HCs, showed significant differences among the cytokines produced, indicating a good and broad reactivity of the immune system. In particular, the expression of IL-6 by CD19/B cells was significantly different after stimulation with HERV-K env 109–126 peptide in ALS patients in comparison to HCs, suggesting that this peptide is likely responsible for the B-cell activation, considered the autocrine activity of this cytokine. Instead, the HERV-K env 19–37 peptide seems able to foster a pro-inflammatory response by stimulation of CD19/B cells and a statistically significant greater production of IFN-γ. In CD19/B cells, both peptides were not able to stimulate the TNF-α production, while the HERV-K env 19–37 peptide induced a greater production of TNF-α in CD8/T cells.
The mechanism for this latter effect is unknown. We believe it might be due to an insufficient immune innate response in ALS patients after a 6-hour stimulus of PBMCs. This is consistent with an increased retroviral expression observed on monocytes of HCs compared to patients, as TNF-α is a pro-inflammatory cytokine that appears at the early stages of the innate immune response. Previous studies have reported higher levels of IL-6 in ALS patients in comparison to HCs [
20,
21,
22,
23,
24] and rising IL-6 levels in plasma were also associated with risk for disease progression [
20]. Interestingly, although IL-6 may have an established bivalent role in inflammation, namely both a pro and anti-inflammatory action, a peripheral IL-6 upregulation usually is related to an inflammatory cell response also able to induce an endothelial cells damage at blood–brain barrier. [
24]. Thus, our data support the possibility that an increased expression of HERV-K can stimulate IL-6 and IFN-γ determining a chronic inflammatory state through a T lymphocytes/Th1-type cytokines response which, in patients genetically predisposed, may play a role in worsening the disease course.
Moreover, we studied the expression of MIP-1α and MCP-1 by CD19/B-cells stimulated with both peptides, and found a significant increase in the expression of MIP-1α in ALS patients. This finding fits previous data in the literature showing either a negative correlation between expression of MIP-1α and disease progression rate or a positive correlation with disease duration, thus suggesting a possible protective role of this chemokine on ALS outcome [
25]. The antigenic specific stimulation generated by HERV-K env
19–37 and HERV-K env
109–126 contribute to the specific increase in MIP-1 α levels as observed in serum and CSF by other authors [
21,
25]. Regarding MCP-1, we document a lower expression by CD19/B cells in ALS patients compared to HCs, following stimulation with both peptides. As higher MCP-1 levels were associated with worse disease severity and faster progression by several authors [
21], and due to the HERV-K related lower expression of this chemokine documented in our study, it is likely to work together with the increased expression of MIP-1α to produce a better ALS outcome.
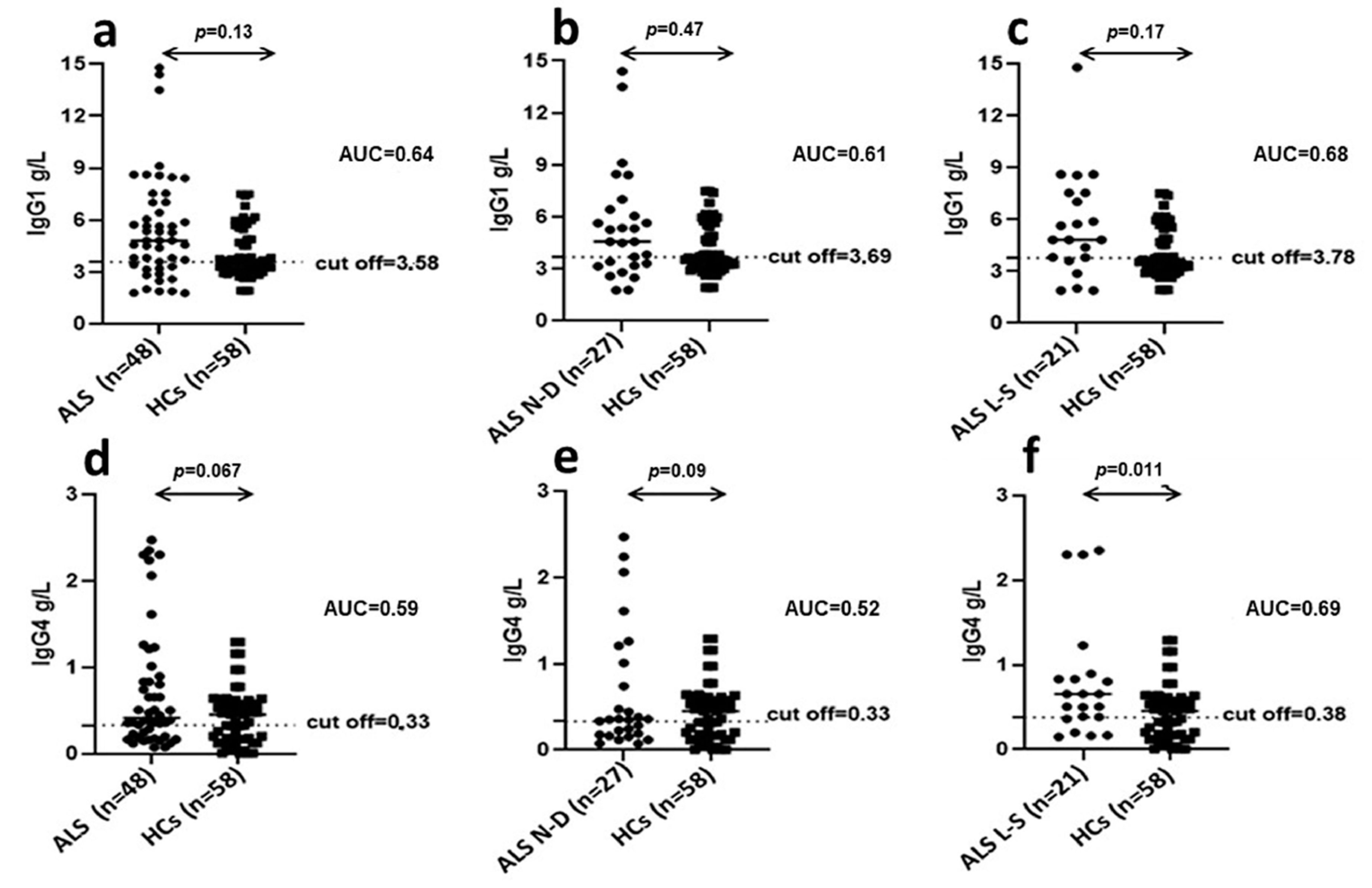
In this context, in order to investigate which IgG subclass of antibodies anti-HERV-K were predominant in patients with ALS, we determined plasma levels of IgG1 and IgG4 immunoglobulins. This is an important issue in order to clarify if the high levels of IgG found in ALS are protective (IgG4) or harmful (IgG1) [
26]. Indeed, recent data have indicated that IgG4 antibodies may fulfil a protective role dampening the more harmful effects of IgG1 when directed against the same epitopes [
26]. In addition, we evaluated if the observed IgG1 and IgG4 production could be correlated with disease progression and prognosis. We detected IgG1 plasma levels significantly higher in ALS patients compared to HCs; conversely, no difference between the two groups was observed for IgG4 plasma levels. Of note, IgG1 levels were not significantly different in newly diagnosed ALS patients with respect to long-survivor patients, thus this humoral finding does not have prognostic significance.
Interestingly, in long-survivor ALS patients, IgG1 and IgG4 plasma levels were both significantly increased with respect to HCs. This data indicates that, in long-survivors, the detrimental effect of increased IgG1 levels in plasma may be progressively dampened by the parallel increment in plasma of IgG4 levels. The mechanisms behind the pathological contribution of HERV-K activation in ALS remain yet to be completely clarified. Here, we document divergent mechanisms, related to humoral and cell-mediated immune response to antigenic peptides derived from HERV-K in ALS patients. In this context, our study provides useful information to better understand the possible role of endogenous retroviruses in neurodegenerative diseases. In particular, we highlighted the ability of HERV-K to modulate the immune system at different levels, generating mediators mainly involved in proinflammatory response. Of note, despite depletion, B cells of ALS patients express higher levels of HERV-K env protein compared to HCs feeding the inflammatory loop, by induction of a continuous proinflammatory stimulus of T lymphocytes sustained by exposition of viral protein on B lymphocytes membrane. Furthermore, we better understand the role of the cytokine IL 6 in the early stage of ALS as responsible for the injury of the BBB, such that to support neuronal damage. Certainly, an early knowledge is sorely needed to diagnose ALS, as recently described by Keon et al. [
27]. Adequate translation of our results into clinical practice may speed up the use of HERV-K as biomarker of disease progression due to its ability to modulate cytokines and chemokines as mediators of inflammation partly responsible for motor neurons damage.