Intestinal Colonization with Tropheryma whipplei—Clinical and Immunological Implications for HIV Positive Adults in Ghana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Data Collection and Laboratory Methods
2.3. Statistical Analyses
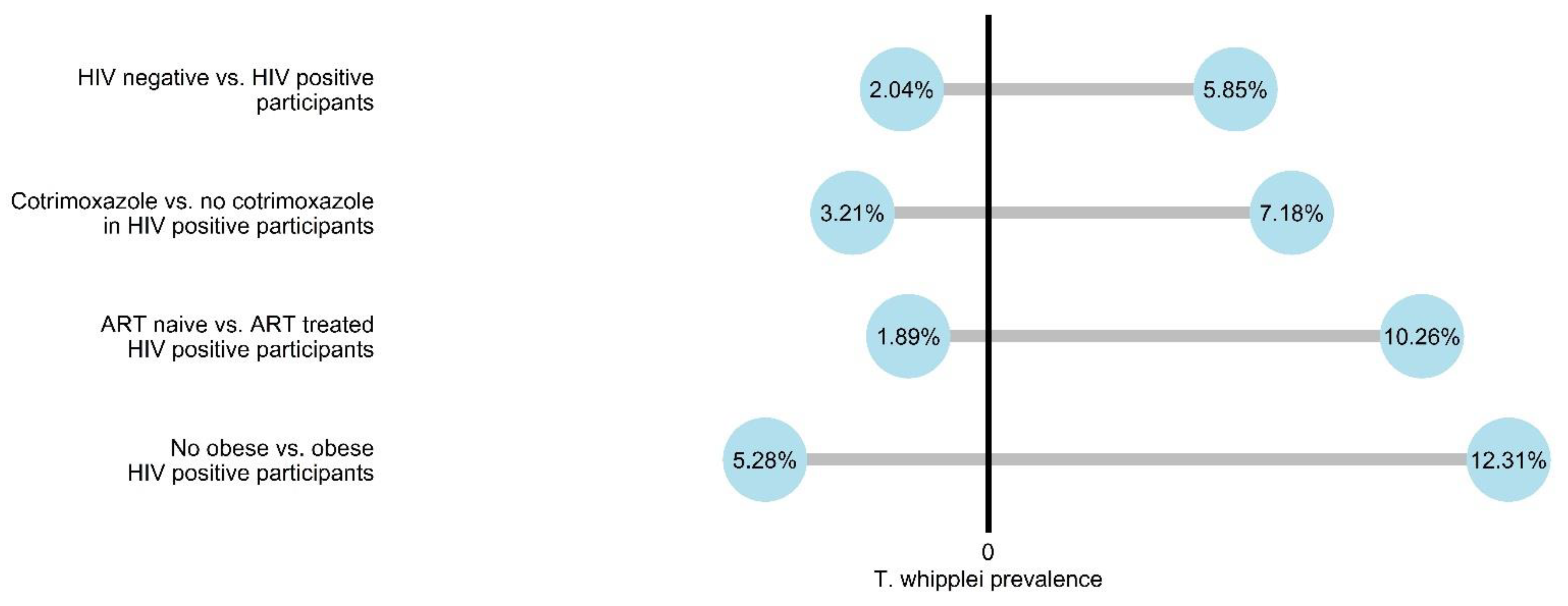
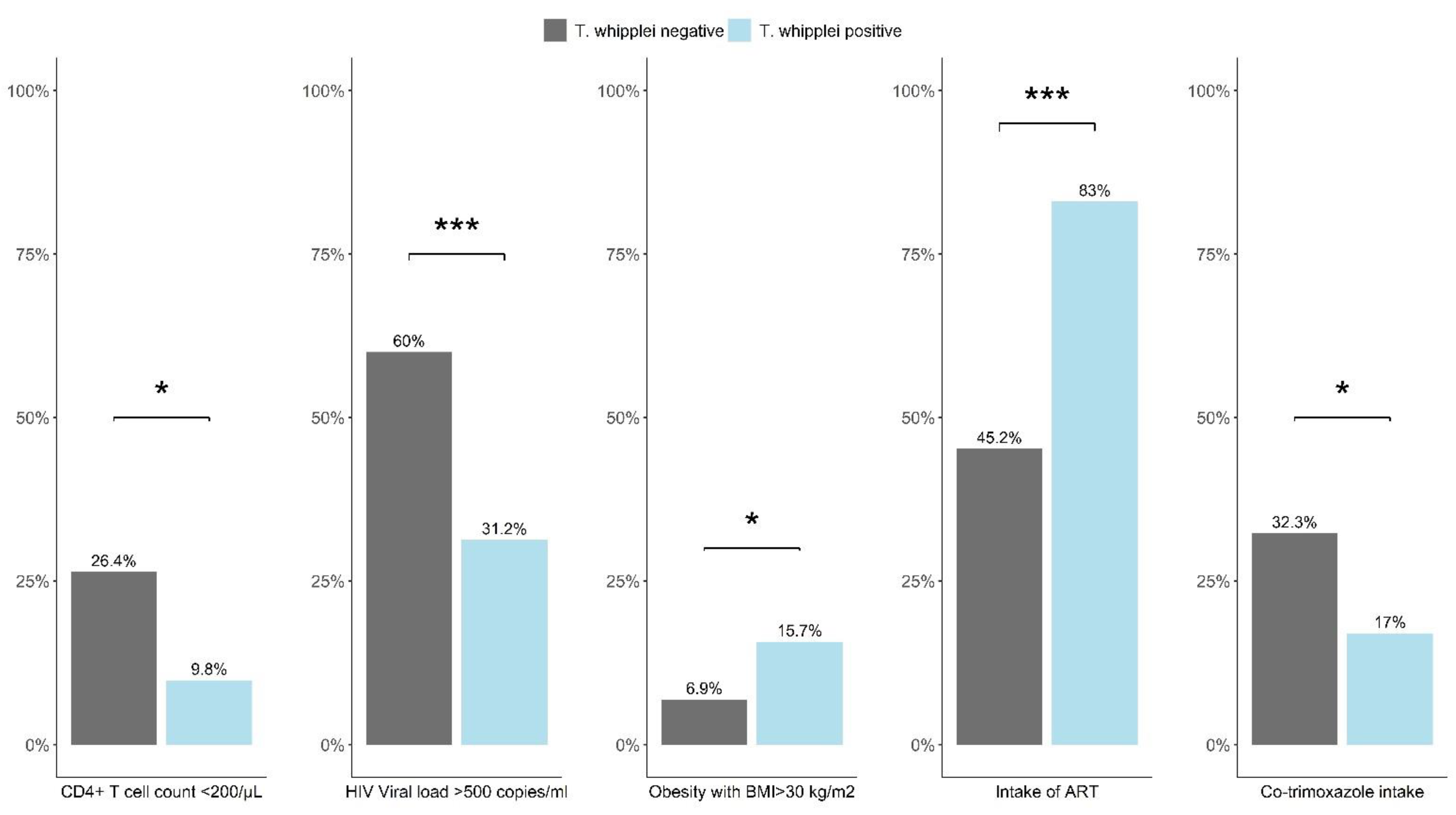
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, T.; Moos, V.; Loddenkemper, C.; Marth, T.; Fenollar, F.; Raoult, D. Whipple’s disease: New aspects of pathogenesis and treatment. Lancet Infect. Dis. 2008, 8, 179–190. [Google Scholar] [CrossRef]
- Fenollar, F.; Célard, M.; Lagier, J.-C.; Lepidi, H.; Fournier, P.-E.; Raoult, D. Tropheryma whipplei Endocarditis. Emerg. Infect. Dis. 2013, 19, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Bousbia, S.; Papazian, L.; Auffray, J.P.; Fenollar, F.; Martin, C.; Li, W.; Chiche, L.; La Scola, B.; Raoult, D. Tropheryma whipplei in patients with pneumonia. Emerg. Infect. Dis. 2010, 16, 258–263. [Google Scholar] [CrossRef]
- Coria, F.; Cuadrado, N.; Velasco, C.; JJ, J.C.; Jiménez, M.I.; Mena, F.J.; Acebes, J.M. Whipple’s disease with isolated central nervous system symptomatology diagnosed by molecular identification of Tropheryma whippelii in peripheral blood. Neurologia 2000, 15, 173–176. [Google Scholar]
- Keita, A.K.; Raoult, D.; Fenollar, F. Tropheryma whipplei as a commensal bacterium. Future Microbiol. 2013, 8, 57–71. [Google Scholar] [CrossRef]
- Ramharter, M.; Harrison, N.; Bühler, T.; Herold, B.; Lagler, H.; Lötsch, F.; Mombo-Ngoma, G.; Müller, C.; Adegnika, A.A.; Kremsner, P.G.; et al. Prevalence and risk factor assessment of Tropheryma whipplei in a rural community in Gabon: A community-based cross-sectional study. Clin. Microbiol. Infect. 2014, 20, 1189–1194. [Google Scholar] [CrossRef] [Green Version]
- Keïta, A.K.; Bassene, H.; Tall, A.; Sokhna, C.; Ratmanov, P.; Trape, J.-F.; Raoult, D.; Fenollar, F. Tropheryma whipplei: A Common Bacterium in Rural Senegal. PLoS Negl. Trop. Dis. 2011, 5, e1403. [Google Scholar] [CrossRef]
- Beltrame, A.; Ragusa, A.; Perandin, F.; Formenti, F.; Fenollar, F.; Edouard, S.; Laroche, M.; Zavarise, G.; Doro, F.; Giorli, G.; et al. Tropheryma whipplei intestinal colonization in Italian and migrant population: A retrospective observational study. Future Microbiol. 2019, 14, 283–292. [Google Scholar] [CrossRef]
- Keita, A.K.; Dubot-Pérès, A.; Phommasone, K.; Sibounheuang, B.; Vongsouvath, M.; Mayxay, M.; Raoult, D.; Newton, P.; Fenollar, F. High Prevalence of Tropheryma whipplei in Lao Kindergarten Children. PLoS Neglected Trop. Dis. 2015, 9, e0003538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shams, S.; Rezaei, N.; Beltrame, A.; Moro, L.; Piubelli, C.; Amiri, F.B.; Esmaeili, S. Tropheryma whipplei in Immunocompromised Children in Iran: A Preliminary Study. Preprints 2021, 2021020261. [Google Scholar] [CrossRef]
- Fenollar, F.; Trape, J.F.; Bassene, H.; Sokhna, C.; Raoult, D. Tropheryma whipplei in fecal samples from children, Senegal. Emerg. Infect. Dis. 2009, 15, 922–924. [Google Scholar] [CrossRef] [PubMed]
- Frickmann, H.; Hanke, M.; Hahn, A.; Schwarz, N.G.; Landt, O.; Moter, A.; Kikhney, J.; Hinz, R.; Rojak, S.; Dekker, D.; et al. Detection of Tropheryma whipplei in stool samples by one commercial and two in-house real-time PCR assays. Trop. Med. Int. Health 2019, 24, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurens, M.B.; Mungwira, R.G.; Nampota, N.; Nyirenda, O.M.; Divala, T.H.; Kanjala, M.; A Mkandawire, F.; Galileya, L.T.; Nyangulu, W.; Mwinjiwa, E.; et al. Revisiting Co-Trimoxazole Prophylaxis for African Adults in The Era of Antiretroviral Therapy: A Randomized Controlled Clinical Trial. Clin. Infect. Dis. 2021, ciab252. [Google Scholar] [CrossRef]
- Qin, S.; Clausen, E.; Nouraie, S.M.; Kingsley, L.; McMahon, D.; Kleerup, E.; Huang, L.; Ghedin, E.; Greenblatt, R.M.; Morris, A. Tropheryma whipplei colonization in HIV-infected individuals is not associated with lung function or inflammation. PLoS ONE 2018, 13, e0205065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Álvarez, L.; Pérez-Matute, P.; Blanco, J.-R.; Ibarra, V.; Oteo, J.A. High prevalence of asymptomatic carriers of Tropheryma whipplei in different populations from the North of Spain. Enferm. Infecc. Microbiol. Clin. 2016, 34, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Cota-Gomez, A.; Palmer, B.E.; Linderman, D.J.; Charlson, E.S.; Sodergren, E.; Mitreva, M.; Abubucker, S.; Martin, J.; Yao, G.; et al. Widespread colonization of the lung by Tropheryma whipplei in HIV infection. Am. J. Respir. Crit. Care Med. 2013, 187, 1110–1117. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Clausen, E.; Lucht, L.; Michael, H.; Beck, J.M.; Curtis, J.L.; Freeman, C.M.; Morris, A. Presence of Tropheryma whipplei in Different Body Sites in a Cohort of Healthy Subjects. Am. J. Respir. Crit. Care Med. 2016, 194, 243–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duro, M.; Manso, M.D.C.; Barreira, S.; Rebelo, I.; Medeiros, R.; Almeida, C. Metabolic syndrome in human immunodeficiency virus-infected patients. Int. J. STD AIDS 2018, 29, 1089–1097. [Google Scholar] [CrossRef]
- Sarfo, F.S.; Eberhardt, K.; Dompreh, A.; Kuffour, E.O.; Soltau, M.; Schachscheider, M.; Drexler, J.F.; Eis-Hübinger, A.M.; Häussinger, D.; Oteng-Seifah, E.E.; et al. Helicobacter pylori Infection Is Associated with Higher CD4 T Cell Counts and Lower HIV-1 Viral Loads in ART-Naïve HIV-Positive Patients in Ghana. PLoS ONE 2015, 10, e0143388. [Google Scholar] [CrossRef] [Green Version]
- Di Cristanziano, V.; D´Alfonso, R.; Berrilli, F.; Sarfo, F.S.; Santoro, M.; Fabeni, L.; Knops, E.; Heger, E.; Kaiser, R.; Dompreh, A.; et al. Lower prevalence of Blastocystis sp. infections in HIV positive compared to HIV negative adults in Ghana. PLoS ONE 2019, 14, e0221968. [Google Scholar]
- Di Cristanziano, V.; Weimer, K.; Böttcher, S.; Sarfo, F.S.; Dompreh, A.; Cesar, L.-G.; Knops, E.; Heger, E.; Wirtz, M.; Kaiser, R.; et al. Molecular Characterization and Clinical Description of Non-Polio Enteroviruses Detected in Stool Samples from HIV-Positive and HIV-Negative Adults in Ghana. Viruses 2020, 12, 221. [Google Scholar] [CrossRef] [Green Version]
- Eberhardt, K.; Sarfo, F.S.; Dompreh, A.; Kuffour, E.O.; Geldmacher, C.; Soltau, M.; Schachscheider, M.; Drexler, J.F.; Eis-Hübinger, A.M.; Häussinger, D.; et al. Helicobacter pylori Coinfection Is Associated with Decreased Markers of Immune Activation in ART-Naive HIV-Positive and in HIV-Negative Individuals in Ghana. Clin. Infect. Dis. 2015, 61, 1615–1623. [Google Scholar] [CrossRef] [Green Version]
- Fenollar, F.; Laouira, S.; Lepidi, H.; Rolain, J.-M.; Raoult, D. Value of Tropheryma whipplei Quantitative Polymerase Chain Reaction Assay for the Diagnosis of Whipple Disease: Usefulness of Saliva and Stool Specimens for First-Line Screening. Clin. Infect. Dis. 2008, 47, 659–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinnemeier, C.; Klupp, E.; Krumkamp, R.; Rolling, T.; Fischer, N.; Owusu-Dabo, E.; Addo, M.; Adu-Sarkodie, Y.; Käsmaier, J.; Aepfelbacher, M.; et al. Tropheryma whipplei in children with diarrhoea in rural Ghana. Clin. Microbiol. Infect. 2016, 22, 65.e1–65.e3. [Google Scholar] [CrossRef]
- Heinemann, M.; Strauchs, C.; Lütgehetmann, M.; Aepfelbacher, M.; Klupp, E.-M.; Owusu-Dabo, E.; Rolling, T.; Cramer, J.P.; Vinnemeier, C.D. Polymicrobial enteric infections in African infants with diarrhoea—Results from a longitudinal prospective case–control study. Clin. Microbiol. Infect. 2021. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Fenollar, F.; Raoult, D. Acute infections caused by Tropheryma whipplei. Future Microbiol. 2017, 12, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Fenollar, F.; Minodier, P.; Boutin, A.; Laporte, R.; Brémond, V.; Noël, G.; Miramont, S.; Richet, H.; Benkouiten, S.; Lagier, J.-C.; et al. Tropheryma whipplei associated with diarrhoea in young children. Clin. Microbiol. Infect. 2016, 22, 869–874. [Google Scholar] [CrossRef] [Green Version]
- Feurle, G.E.; Moos, V.; Landt, O.; Corcoran, C.; Reischl, U.; Maiwald, M. Tropheryma whipplei in Feces of Patients with Diarrhea in 3 Locations on Different Continents. Emerg. Infect. Dis. 2021, 27, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Moos, V.; Schneider, T. The role of T cells in the pathogenesis of classical Whipple’s disease. Expert Rev. Anti-Infect. Ther. 2012, 10, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Hasse, B.; Iff, M.; Ledergerber, B.; Calmy, A.; Schmid, P.; Hauser, C.; Cavassini, M.; Bernasconi, E.; Marzolini, C.; Tarr, P.E.; et al. Obesity Trends and Body Mass Index Changes After Starting Antiretroviral Treatment: The Swiss HIV Cohort Study. Open Forum Infect. Dis. 2014, 1, ofu040. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | HIV Positive T. whipplei Negative, n = 853 (94.15%) | HIV Positive T. whipplei Positive, n = 53 (5.85%) | p-Value | |
|---|---|---|---|---|
| Demographics | Age in years ± SD | 40.5 ± 9.5 | 38.5 ± 7.9 | 0.127 |
| Female, n (%) | 643 (75.38) | 42 (79.25) | 0.638 | |
| Socioeconomic parameters | Access to tap water, n (%) | 449 (52.64) | 29 (54.72) | 0.879 |
| Electricity in household, n (%) | 792 (92.85) | 49 (92.45) | 0.787 | |
| Television in household, n (%) | 689 (80.77) | 42 (79.25) | 0.925 | |
| Refrigerator in household, n (%) | 603 (70.69) | 34 (64.15) | 0.392 | |
| Owning a car, n (%) | 80 (9.38) | 4 (7.55) | 0.810 | |
| Clinical symptoms during the last six months | Any acute or chronic cough, n (%) | 100 (11.74) | 3 (5.66) | 0.262 |
| Any acute or chronic gastrointestinal symptoms, n (%) | 105 (12.32) | 4 (7.55) | 0.387 | |
| Any acute or chronic fever, n (%) | 84 (9.86) | 2 (3.77) | 0.222 | |
| Weigh loss during last six months, n (%) | 201 (23.59) | 6 (11.32) | 0.058 | |
| Any acute or chronic symptoms of the above, n (%) | 265 (31.10) | 10 (18.87) | 0.084 |
| Variable | HIV Positive T. whipplei Negative, Median (IQR) | HIV Positive T. whipplei Positive, Median (IQR) | p-Value |
|---|---|---|---|
| Viral load, log10 copies/ml | 4.0 (1.6–5.3) | 1.6 (0.0–3.3) | <0.001 |
| CD4+ T-cell count/µL | 396.0 (184.0–632.5) | 525.0 (371.0–654.5) | 0.007 |
| CD8+ T-cell count/µL | 960.0 (637.0–1381.0) | 1034.0 (588.5–1220.0) | 0.763 |
| CD4+/CD8+ T-cell ratio | 0.4 (0.2–0.7) | 0.5 (0.4–0.8) | 0.020 |
| HLA-DR+CD38+CD4+ (%) | 18.4 (10.4–32.8) | 11.2 (7.3–19.7) | 0.009 |
| HLA-DR+CD38+CD8+ (%) | 42.3 (27.5–55.7) | 29.1 (22.7–40.5) | 0.006 |
| CD57+CD4+ (%) | 16.4 (9.4–27.6) | 13.9 (9.8–26.2) | 0.788 |
| CD57+CD8+ (%) | 49.0 (38.9–60.3) | 54.6 (44.8–68.5) | 0.046 |
| PD-1+CD4+ (%) | 34.5 (23.8–49.5) | 32.8 (22.3–46.3) | 0.602 |
| PD-1+CD8+ (%) | 31.6 (19.9–43.6) | 20.7 (14.9–40.3) | 0.074 |
| Ki67+CD4+ (%) | 13.0 (6.9–27.2) | 9.3 (7.9–11.6) | 0.076 |
| Ki67+CD8+ (%) | 10.2 (6.0–17.5) | 8.4 (6.2–16.8) | 0.834 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eberhardt, K.A.; Sarfo, F.S.; Klupp, E.-M.; Dompreh, A.; Di Cristanziano, V.; Osei Kuffour, E.; Boateng, R.; Norman, B.; Phillips, R.O.; Aepfelbacher, M.; et al. Intestinal Colonization with Tropheryma whipplei—Clinical and Immunological Implications for HIV Positive Adults in Ghana. Microorganisms 2021, 9, 1781. https://doi.org/10.3390/microorganisms9081781
Eberhardt KA, Sarfo FS, Klupp E-M, Dompreh A, Di Cristanziano V, Osei Kuffour E, Boateng R, Norman B, Phillips RO, Aepfelbacher M, et al. Intestinal Colonization with Tropheryma whipplei—Clinical and Immunological Implications for HIV Positive Adults in Ghana. Microorganisms. 2021; 9(8):1781. https://doi.org/10.3390/microorganisms9081781
Chicago/Turabian StyleEberhardt, Kirsten Alexandra, Fred Stephen Sarfo, Eva-Maria Klupp, Albert Dompreh, Veronica Di Cristanziano, Edmund Osei Kuffour, Richard Boateng, Betty Norman, Richard Odame Phillips, Martin Aepfelbacher, and et al. 2021. "Intestinal Colonization with Tropheryma whipplei—Clinical and Immunological Implications for HIV Positive Adults in Ghana" Microorganisms 9, no. 8: 1781. https://doi.org/10.3390/microorganisms9081781
APA StyleEberhardt, K. A., Sarfo, F. S., Klupp, E.-M., Dompreh, A., Di Cristanziano, V., Osei Kuffour, E., Boateng, R., Norman, B., Phillips, R. O., Aepfelbacher, M., & Feldt, T. (2021). Intestinal Colonization with Tropheryma whipplei—Clinical and Immunological Implications for HIV Positive Adults in Ghana. Microorganisms, 9(8), 1781. https://doi.org/10.3390/microorganisms9081781






