The Discovery of Vitreoscilla Hemoglobin and Early Studies on Its Biochemical Functions, the Control of Its Expression, and Its Use in Practical Applications
Abstract
:1. The Discovery, Purification, and Biochemical Characterization of Vitreoscilla Hemoglobin
2. Early Studies on the Effects of VHb Expression on Cell Metabolism
3. Molecular Biology of the VHb Gene (vgb)
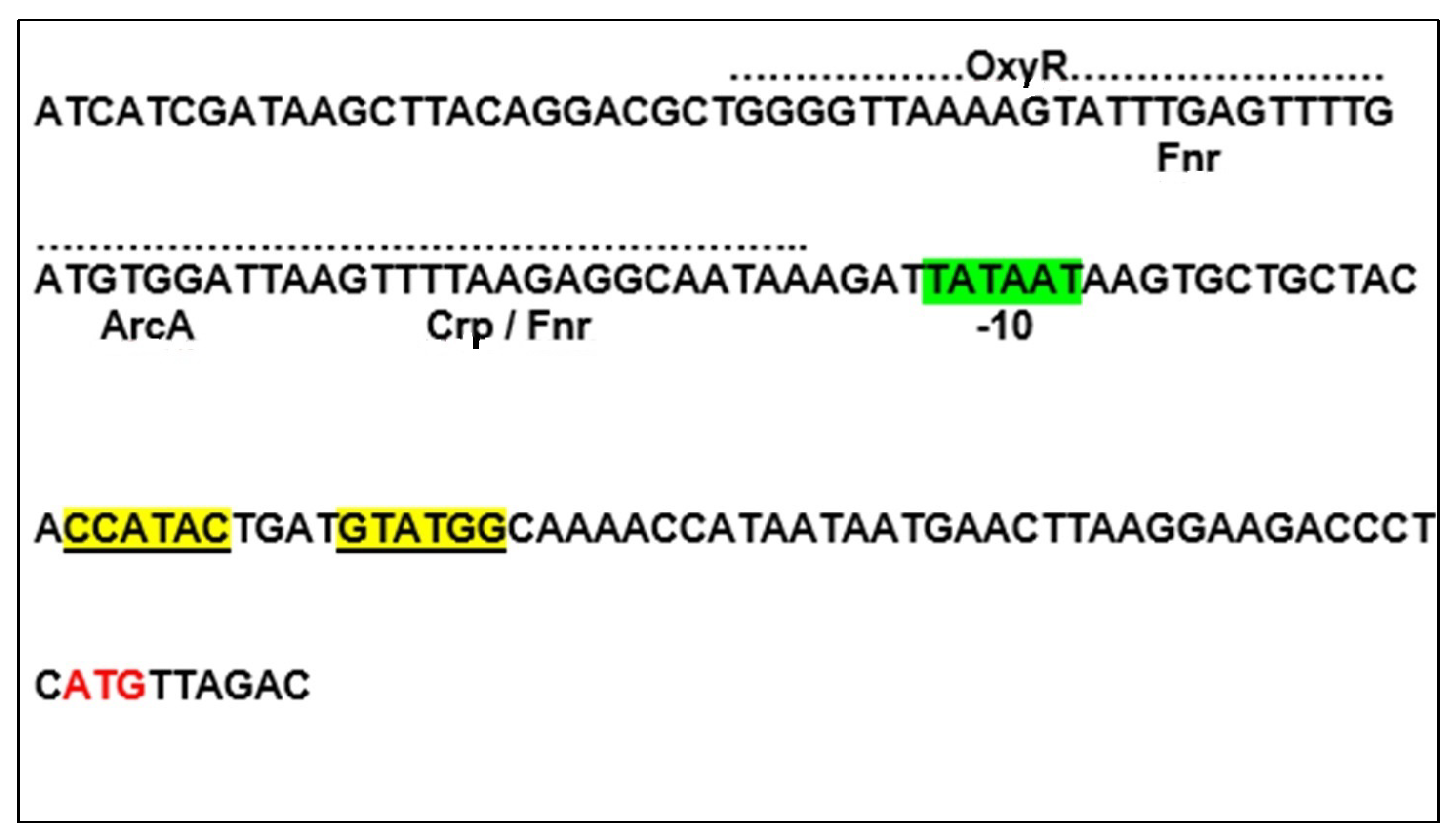
3.1. The Isolation and Sequencing of vgb
3.2. The Control of vgb Gene Expression by Oxygen
3.3. VHb Mediated Transcription Regulation of Oxidative Stress
4. Early Work on Genetic Engineering with vgb
4.1. Enhancement of Protein and Metabolite Production
4.2. Enhancement of Bioremediation
5. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Appleby, C.A. Electron transport systems of Rhizobium japonicum. II. Rhizobium haemoglobin, cytochromes and oxidases in free-living (cultured) cells. Biochim. Biophys. Acta 1969, 172, 88–105. [Google Scholar] [CrossRef]
- Webster, D.A.; Hackett, D.P. The purification and properties of cytochrome o from Vitreoscilla. J. Biol. Chem. 1966, 241, 3308–3315. [Google Scholar] [CrossRef]
- Webster, D.A.; Liu, C.Y. Reduced nicotinamide adenine dinucleotide cytochrome o reductase associated with cytochrome o purified from Vitreoscilla: Evidence for an intermediate oxygenated form of cytochrome o. J. Biol. Chem. 1974, 249, 4257–4260. [Google Scholar] [CrossRef]
- Webster, D.A. Structure and function of bacterial hemoglobin and related proteins. In Advances in Inorganic Biochemistry, Heme Proteins; Eichhorn, G.L., Marzilli, L., Eds.; Elsevier Science Publishing Co.: New York, NY, USA, 1987; Volume 7, pp. 245–265. [Google Scholar]
- Webster, D.A.; Orii, Y. Oxygenated cytochrome o an active intermediate observed in whole cells of Vitreoscilla. J. Biol. Chem. 1977, 252, 1834–1836. [Google Scholar] [CrossRef]
- Wakabayashi, S.; Matsubara, H.; Webster, D.A. Primary sequence of a dimeric bacterial hemoglobin (Vitreoscilla). Nature 1986, 322, 481–483. [Google Scholar] [CrossRef]
- Tarricone, C.; Galizzi, A.; Coda, A.; Ascenzi, P.; Bolognesi, M. Unusual structure of the oxygen-binding site in the dimeric bacterial hemoglobin from Vitreoscilla sp. Structure 1997, 5, 497–507. [Google Scholar] [CrossRef] [Green Version]
- Ratakonda, S.; Anand, A.; Dikshit, K.L.; Stark, B.C.; Howard, A.J. Crystallographic structure determination of B10 mutants of Vitreoscilla hemoglobin: Role of Tyr29 (B10) in the structure of the ligand binding site. Acta Cryst. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 215–222. [Google Scholar] [CrossRef] [Green Version]
- DeMaio, R.A.; Webster, D.A.; Chance, B. Spectral evidence for the existence of a second cytochrome bo in whole cells of Vitreoscilla. J. Biol. Chem. 1983, 258, 13768–13771. [Google Scholar] [CrossRef]
- Georgiou, C.; Webster, D.A. Purification and partial characterization of the membrane-bound cytochrome o (561,564) from Vitreoscilla. Biochemistry 1987, 26, 6521–6526. [Google Scholar] [CrossRef]
- Boerman, S.J.; Webster, D.A. Control of heme content in Vitreoscilla by oxygen. J. Gen. Appl. Microbiol. 1982, 28, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Pringsheim, E.G. The Vitreoscillaceae: A family of colourless, gliding, filamentous organisms. J. Gen. Microbiol. 1951, 5, 124–149. [Google Scholar] [CrossRef] [Green Version]
- Hwang, K.W.; Raje, M.; Kim, K.J.; Stark, B.C.; Dikshit, K.L.; Webster, D.A. Vitreoscilla hemoglobin: Intracellular localization and binding to membranes. J. Biol. Chem. 2001, 276, 24781–24789. [Google Scholar]
- Park, K.-W.; Kim, K.-J.; Howard, A.J.; Stark, B.C.; Webster, D.A. Vitreoscilla hemoglobin binds to subunit I of cytochrome bo ubiquinol oxidases. J. Biol. Chem. 2002, 277, 33334–33337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikshit, R.J.; Dikshit, K.L.; Liu, Y.; Webster, D.A. The bacterial hemoglobin from Vitreoscilla can support the aerobic growth of Escherichia coli lacking terminal oxidases. Arch. Biochem. Biophys. 1992, 293, 241–245. [Google Scholar] [CrossRef]
- Stark, B.C.; Dikshit, K.L.; Pagilla, K.R. The biochemistry of Vitreoscilla hemoglobin. Comp. Struct. Biotechnol. J. 2012, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sanny, T.; Arnaldos, M.; Kunkel, S.A.; Pagilla, K.R.; Stark, B.C. Engineering of ethanolic E. coli with the Vitreoscilla hemoglobin gene enhances ethanol production from both glucose and xylose. Appl. Microbiol. Biotechnol. 2010, 88, 1103–1112. [Google Scholar] [CrossRef]
- Roos, V.; Andersson, C.I.; Bülow, L. Gene expression profiling of Escherichia coli expressing double Vitreoscilla haemoglobin. J. Biotechnol. 2004, 114, 107–120. [Google Scholar] [CrossRef]
- Dikshit, K.L.; Webster, D.A. Cloning, characterization and expression of the bacterial globin gene from Vitreoscilla in Escherichia coli. Gene 1988, 70, 377–386. [Google Scholar] [CrossRef]
- Khosla, C.; Bailey, J.E. The Vitreoscilla hemoglobin gene: Molecular cloning, nucleotide sequence and genetic expression in Escherichia coli. Mol. Gen. Genet. 1988, 214, 158–161. [Google Scholar] [CrossRef]
- Dikshit, K.L.; Spaulding, D.; Braun, A.; Webster, D.A. Oxygen inhibition of globin gene transcription and bacterial haemoglobin synthesis in Vitreoscilla. J. Gen. Microbiol. 1989, 135, 2601–2609. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.C.; Liu, Y.X.; Webster, D.A.; Stark, B.C. Sequence of the region downstream of the Vitreoscilla hemoglobin gene: Vgb is not part of a multigene operon. Appl. Microbiol. Biotechnol. 1994, 42, 304–308. [Google Scholar] [CrossRef]
- Dikshit, K.L.; Dikshit, R.P.; Webster, D.A. Study of Vitreoscilla globin (vgb) gene expression and promoter activity in E. coli through transcription fusion. Nucleic Acids Res. 1990, 18, 4149–4155. [Google Scholar] [CrossRef] [Green Version]
- Joshi, M.; Dikshit, K.L. Oxygen dependent regulation of Vitreoscilla globin gene: Evidence for positive regulation by FNR. Biochem. Biophys. Res. Commun. 1994, 202, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Yang., J.; Webster, D.A.; Stark, B.C. ArcA works with Fnr as a positive regulator of Vitreoscilla (bacterial) hemoglobin gene expression in Escherichia coli. Microbiol. Res. 2005, 160, 405–415. [Google Scholar] [CrossRef]
- Abrams, J.J.; Webster, D.A. Purification, partial characterization, and possible role of catalase in the bacterium Vitreoscilla. Arch. Biochem. Biophys. 1990, 279, 54–59. [Google Scholar] [CrossRef]
- Geckil, H.; Gencer, S.; Kahraman, H.; Erenler, S.O. Genetic engineering of Enterobacter aerogenes with the Vitreoscilla hemoglobin gene: Cell growth, survival, and antioxidant enzyme status under oxidative stress. Res. Microbiol. 2003, 154, 425–431. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, Y.; Chen, W.; Tang, K.; Zhang, L. Functional expression of Vitreoscilla hemoglobin (VHb) in Arabidopsis relieves submergence, nitrosative, photo-oxidative stress and enhances antioxidants metabolism. Plant Sci. 2009, 176, 66–77. [Google Scholar] [CrossRef]
- Akbas, M.Y.; Daruk, T.; Ozdemir, T.; Stark, B.C. Further investigation on the mechanism of Vitreoscilla hemoglobin (VHb) protection from oxidative stress in Escherichia coli. Biologia 2011, 66, 735–740. [Google Scholar] [CrossRef]
- Anand, A.; Duk, B.T.; Singh, S.; Akbas, M.Y.; Webster, D.A.; Stark, B.C.; Dikshit, K.L. Redox-mediated interactions of VHb (Vitreoscilla haemoglobin) with OxyR: Novel regulation of VHb biosynthesis under oxidative stress. Biochem. J. 2010, 426, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Aslund, F.; Storz, G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 1998, 279, 1718–1721. [Google Scholar] [CrossRef]
- Khosla, C.; Bailey, J.E. Heterologous expression of a bacterial hemoglobin improves the growth properties of recombinant Escherichia coli. Nature 1988, 331, 633–635. [Google Scholar] [CrossRef]
- Khosla, C.; Curtis, J.E.; DeModena, J.; Rinas, U.; Bailey, J.E. Expression of intracellular hemoglobin improves protein synthesis in oxygen-limited Escherichia coli. Biotechnology 1990, 8, 849–853. [Google Scholar] [CrossRef]
- Khosravi, M.; Webster, D.A.; Stark, B.C. Presence of the bacterial hemoglobin gene improves alpha-amylase production of a recombinant E. coli strain. Plasmid 1990, 24, 190–194. [Google Scholar] [CrossRef]
- Stark, B.C.; Dikshit, K.L.; Pagilla, K.R. Recent advances in understanding the structure, function, and biotechnological usefulness of the hemoglobin from the bacterium Vitreoscilla. Biotechnol. Lett. 2011, 33, 1705–1714. [Google Scholar] [CrossRef]
- Stark, B.C.; Pagilla, K.R.; Dikshit, K.L. Recent applications of Vitreoscilla hemoglobin technology in bioproduct synthesis and bioremediation. Appl. Microbiol. Biotechnol. 2015, 99, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.D.; Kallio, P.T. Bacterial hemoglobins and flavohemoglobins: Versatile proteins and their impact on microbiology and biotechnology. FEMS Microbiol. Rev. 2003, 27, 525–545. [Google Scholar] [CrossRef] [Green Version]
- Urgun-Demirtas, M.; Stark, B.; Pagilla, K. Use of genetically engineered microorganisms (GEMs) for the bioremediation of contaminants. Critical Rev. Biotechnol. 2006, 26, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Stark, B.C.; Urgun-Demirtas, M.; Pagilla, K.R. Role of hemoglobin in improving biodegradation of aromatic contaminants under hypoxic conditions. J. Mol. Microbiol. Biotechnol. 2008, 15, 181–189. [Google Scholar] [CrossRef]
- So, J.; Pagilla, K.R.; Kim, H.; Stark, B.C. Kinetics of growth, oxygen uptake, and substrate utilization of wild type and genetically engineered Burkholderia sp. Biotechnol. Bioprocess Eng. 1999, 4, 176–180. [Google Scholar] [CrossRef]
- So, J.; Webster, D.A.; Stark, B.C.; Pagilla, K.R. Enhancement of 2,4, -dinitrotoluene biodegradation by Burkholderia sp. in sand bioreactors using bacterial hemoglobin technology. Biodegradation 2004, 15, 161–171. [Google Scholar] [CrossRef]
- Urgun-Demirtas, M.; Pagilla, K.R.; Stark, B.C.; Webster, D.A. Biodegradation of 2-chlorobenzoate by recombinant Burkholderia cepacia expressing Vitreoscilla hemoglobin under variable levels of oxygen availability. Biodegradation 2003, 14, 357–365. [Google Scholar] [CrossRef]
- Urgun-Demirtas, M.; Stark, B.; Pagilla, K. Comparison of 2-chlorobenzoic acid biodegradation in a membrane bioreactor by B. cepacia and B. cepacia bearing the bacterial hemoglobin gene. Water Res. 2006, 40, 3123–3130. [Google Scholar] [CrossRef]
- Krooneman, J.; Wieringa, E.B.A.; Moore, E.R.B.; Gerritse, J.; Prins, R.A.; Gottschal, J.C. Isolation of Alcaligenes sp. strain L6 at low oxygen concentrations and degradation of 3-chlorobenzoate via a pathway not involving (chloro) catechols. Appl. Environ. Microbiol. 1996, 62, 2427–2433. [Google Scholar] [CrossRef] [Green Version]
- Arnaldos, M.; Kunkel, S.A.; Stark, B.C.; Pagilla, K.R. Characterization of heme protein expressed by ammonia-oxidizing bacteria under low dissolved oxygen conditions. Appl. Microbiol. Biotechnol. 2014, 98, 3231–3239. [Google Scholar] [CrossRef]
- Kunkel, S.A.; Pagilla, K.R.; Stark, B.C. Engineering of Nitrosomonas europaea to express Vitreoscilla hemoglobin enhances oxygen uptake and conversion of ammonia to nitrite. Appl. Microbiol. Biotechnol. Express 2015, 5, 43. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, W.; Li, H.; Zhang, J.; Zhang, Y.; Cao, Y.; Ma, J.; Li, Z. Construction and Characterization of Vitreoscilla hemoglobin (VHb) with enhanced peroxidase activity for efficient degradation of textile dye. J. Microbiol. Biotechnol. 2015, 25, 1433–1441. [Google Scholar] [CrossRef]
- Vinogradov, S.N.; Hoogewijs, D.; Bailey, X.; Arrendondo-Peter, R.; Gough, J.; Dewilde, S.; Moens, L.; Vanfleteren, J.R. A phylogenetic profile of globins. BMC Evolut. Biol. 2006, 6, 31. [Google Scholar] [CrossRef] [Green Version]
| Metabolite(s) | Effect(s) Reported |
|---|---|
| NAD(P)H | both increases and decreases in net generation; increase in flux |
| ATP | increases, decreases, no change |
| membrane DpH | no change |
| acids | increases, decreases, no change |
| alcohols, acetoin | increases, decreases |
| Organism Type | Product or Property Enhanced by VHb |
|---|---|
| Bacteria | antibiotics, polymers, enzymes, various metabolites, ethanol, biomass |
| Fungi | antibiotics, enzymes, various metabolites |
| Plants | growth, germination, productivity, submergence and stress tolerance |
| Organism Type | Bioremediation Application |
|---|---|
| bacteria | metabolism of aromatics, chlorinated aromatics, dinitrotoluene |
| bacteria | sulfur removal from diesel oil and benzothiophene |
| bacteria | uptake of heavy metals (Cd, Pb, Co, Cu); oxidation of Mn in contaminated water |
| bacteria | solubilization of phosphate in soil |
| bacteria | production of biological surfactants |
| bacteria | nitrification in artificial waste water |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Webster, D.A.; Dikshit, K.L.; Pagilla, K.R.; Stark, B.C. The Discovery of Vitreoscilla Hemoglobin and Early Studies on Its Biochemical Functions, the Control of Its Expression, and Its Use in Practical Applications. Microorganisms 2021, 9, 1637. https://doi.org/10.3390/microorganisms9081637
Webster DA, Dikshit KL, Pagilla KR, Stark BC. The Discovery of Vitreoscilla Hemoglobin and Early Studies on Its Biochemical Functions, the Control of Its Expression, and Its Use in Practical Applications. Microorganisms. 2021; 9(8):1637. https://doi.org/10.3390/microorganisms9081637
Chicago/Turabian StyleWebster, Dale A., Kanak L. Dikshit, Krishna R. Pagilla, and Benjamin C. Stark. 2021. "The Discovery of Vitreoscilla Hemoglobin and Early Studies on Its Biochemical Functions, the Control of Its Expression, and Its Use in Practical Applications" Microorganisms 9, no. 8: 1637. https://doi.org/10.3390/microorganisms9081637
APA StyleWebster, D. A., Dikshit, K. L., Pagilla, K. R., & Stark, B. C. (2021). The Discovery of Vitreoscilla Hemoglobin and Early Studies on Its Biochemical Functions, the Control of Its Expression, and Its Use in Practical Applications. Microorganisms, 9(8), 1637. https://doi.org/10.3390/microorganisms9081637





