Nationwide Temporal and Geographical Distribution of Tick Populations and Phylogenetic Analysis of Severe Fever with Thrombocytopenia Syndrome Virus in Ticks in Korea, 2020
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Tick Collection and Species Identification
2.3. Molecular Detection of SFTSV from Ticks
2.4. DNA Sequencing and Phylogenetic Analysis
2.5. Geographical Analysis
3. Results
3.1. Prevalence of Tick Populations
3.2. Prevalence of SFTSV in Ticks
3.3. Molecular and Phylogenetic Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abudurexiti, A.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Avšič-Županc, T.; Ballinger, M.J.; Bente, D.A.; Beer, M.; Bergeron, É.; Blair, C.D.; et al. Taxonomy of the order Bunyavirales: Update 2019. Arch. Virol. 2019, 164, 1949–1965. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; He, B.; Huang, S.Y.; Wei, F.; Zhu, X.Q. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet. Infect. Dis. 2014, 14, 763–772. [Google Scholar] [CrossRef]
- Fu, Y.; Li, S.; Zhang, Z.; Man, S.; Li, X.; Zhang, W.; Zhang, C.; Cheng, C. Phylogeographic analysis of severe fever with thrombocytopenia syndrome virus from Zhoushan Islands, China: Implication for transmission across the ocean. Sci. Rep. 2016, 6, 19563. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.M.; Park, S.J.; Kim, Y.I.; Park, S.W.; Yu, M.A.; Kwon, H.I.; Kim, E.H.; Yu, K.M.; Jeong, H.W.; Ryou, J.; et al. Genetic and pathogenic diversity of severe fever with thrombocytopenia syndrome virus (SFTSV) in South Korea. JCI Insight. 2020, 5, e129531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, S.M.; Lee, W.G.; Ryou, J.; Yang, S.C.; Park, S.W.; Roh, J.Y.; Lee, Y.J.; Park, C.; Han, M.G. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg. Infect. Dis. 2014, 20, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.S.; Kang, J.G.; Chae, J.B.; Cho, Y.K.; Shin, J.H.; Jheong, W.H.; Chae, J.S. Prevalence of severe fever with thrombocytopenia syndrome virus in ticks collected from national parks in Korea. Vector Borne. Zoonotic Dis. 2019, 19, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Korea Diseases Control and Prevention Agency. Infectious Disease Dortal. The Results of the National Infectious Disease Surveillance. 2020. Available online: http://kdca.go.kr/npt/biz/npp/ist/bass/bassDissStatsMain.do (accessed on 1 April 2021).
- Kim-Jeon, M.D.; Jegal, S.; Jun, H.; Jung, H.; Park, S.H.; Ahn, S.K.; Lee, J.; Gong, Y.W.; Joo, K.; Kwon, M.J.; et al. Four year surveillance of the vector hard ticks for SFTS, Ganghwa-do, Korea. Korean. J. Parasitol. 2019, 57, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.G.; Yang, S.C. Introduction of regional center for vector surveillance against climate change. Korea KDCA Public Health Weekly Rep. 2014, 7, 936–938. [Google Scholar]
- Yamaguti, N.; Tipton, V.; Keegan, H.; Toshioka, S. Ticks of Japan, Korea, and the Ryukyu Islands. Brigham Young Univ. Sci. Bull. Biol. Ser. 1971, 15, 1–256. [Google Scholar]
- Jung, M.; Kho, J.W.; Lee, W.G.; Roh, J.Y.; Lee, D.H. Seasonal occurrence of Haemaphysalis longicornis (Acari: Ixodidae) and Haemaphysalis flava, vectors of severe fever with thrombocytopenia syndrome (SFTS) in South Korea. J. Med. Entomol. 2019, 56, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.M.; Song, B.G.; Choi, W.; Roh, J.Y.; Lee, Y.J.; Park, W.I.; Han, M.G.; Ju, Y.R.; Lee, W.J. First isolation of severe fever with thrombocytopenia syndrome virus from Haemaphysalis longicornis ticks collected in severe fever with thrombocytopenia syndrome outbreak areas in the Republic of Korea. Vector. Borne. Zoonotic. Dis. 2016, 16, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Mays, S.E.; Houston, A.E.; Troutfryxell, R.T. Comparison of novel and conventional methods of trapping ixodid ticks in the southeastern U.S.A. Med. Vet. Entomol. 2016, 30, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Noh, B.E.; Lee, W.G.; Lee, H.I.; Cho, S.H. Surveillance of tick density in the Republic of Korea, 2019. Korea KDCA Public Health Weekly Rep. 2020, 13, 807–816. [Google Scholar]
- Casel, M.A.; Park, S.J.; Choi, Y.K. Severe fever with thrombocytopenia syndrome virus: Emerging novel phlebovirus and their control strategy. Exp. Mol. Med. 2021, 53, 713–722. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, M.; Kho, J.W.; Song, H.; Moon, K.; Kim, Y.H.; Lee, D.H. Characterization of overwintering sites of Haemaphysalis longicornis (Acari: Ixodidae) and tick infection rate with severe fever with thrombocytopenia syndrome virus from eight provinces in South Korea. Ticks. Tick. Borne. Dis. 2020, 11, 101490. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Ryou, J.; Choi, W.Y.; Han, M.G.; Lee, W.J. Epidemiological and clinical features of severe fever with thrombocytopenia syndrome during an outbreak in South Korea, 2013–2015. Am. J. Trop. Med. Hyg. 2016, 95, 1358–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.W.; Han, M.G.; Yun, S.M.; Park, C.; Lee, W.J.; Ryou, J. Severe fever with thrombocytopenia syndrome virus, South Korea, 2013. Emerg. Infect. Dis. 2014, 20, 1880–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, S.M.; Song, B.G.; Choi, W.Y.; Park, W.I.; Kim, S.Y.; Roh, J.Y.; Ryou, J.; Ju, Y.R.; Park, C.; Shin, E.H. Prevalence of tick-borne encephalitis virus in ixodid ticks collected from the Republic of Korea during 2011–2012. Osong Public Health Res. Perspect. 2012, 3, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Suh, J.H.; Kim, H.C.; Yun, S.M.; Lim, J.W.; Kim, J.H.; Chong, S.T.; Kim, D.H.; Kim, H.T.; Kim, H.; Klein, T.A.; et al. Detection of SFTS virus in Ixodes nipponensis and Amblyomma testudinarium (Ixodida: Ixodidae) collected from reptiles in the Republic of Korea. J. Med. Entomol. 2016, 53, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Song, B.G.; Shin, E.H.; Yun, S.M.; Han, M.G.; Park, M.Y.; Park, C.; Ryou, J. Prevalence of severe fever with thrombocytopenia syndrome virus in Haemaphysalis longicornis ticks in South Korea. Ticks. Tick. Borne. Dis. 2014, 5, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Lim, A.; Kang, H.J.; Han, M.G. Genotype analysis of severe fever with thrombocytopenia syndrome virus detected in patients. Korea KDCA. Public Health Weekly Rep. 2021, 14, 597–606. [Google Scholar]
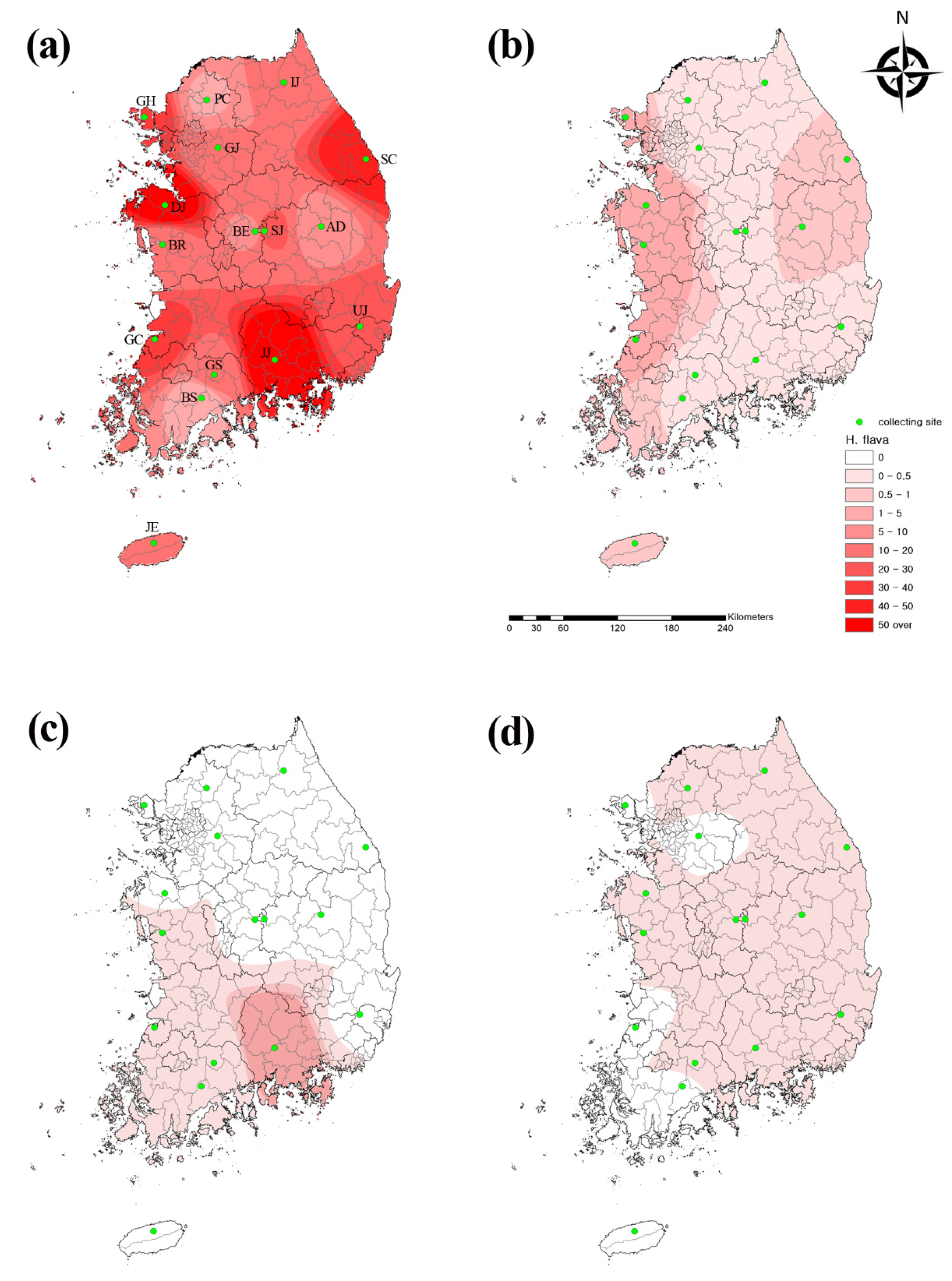

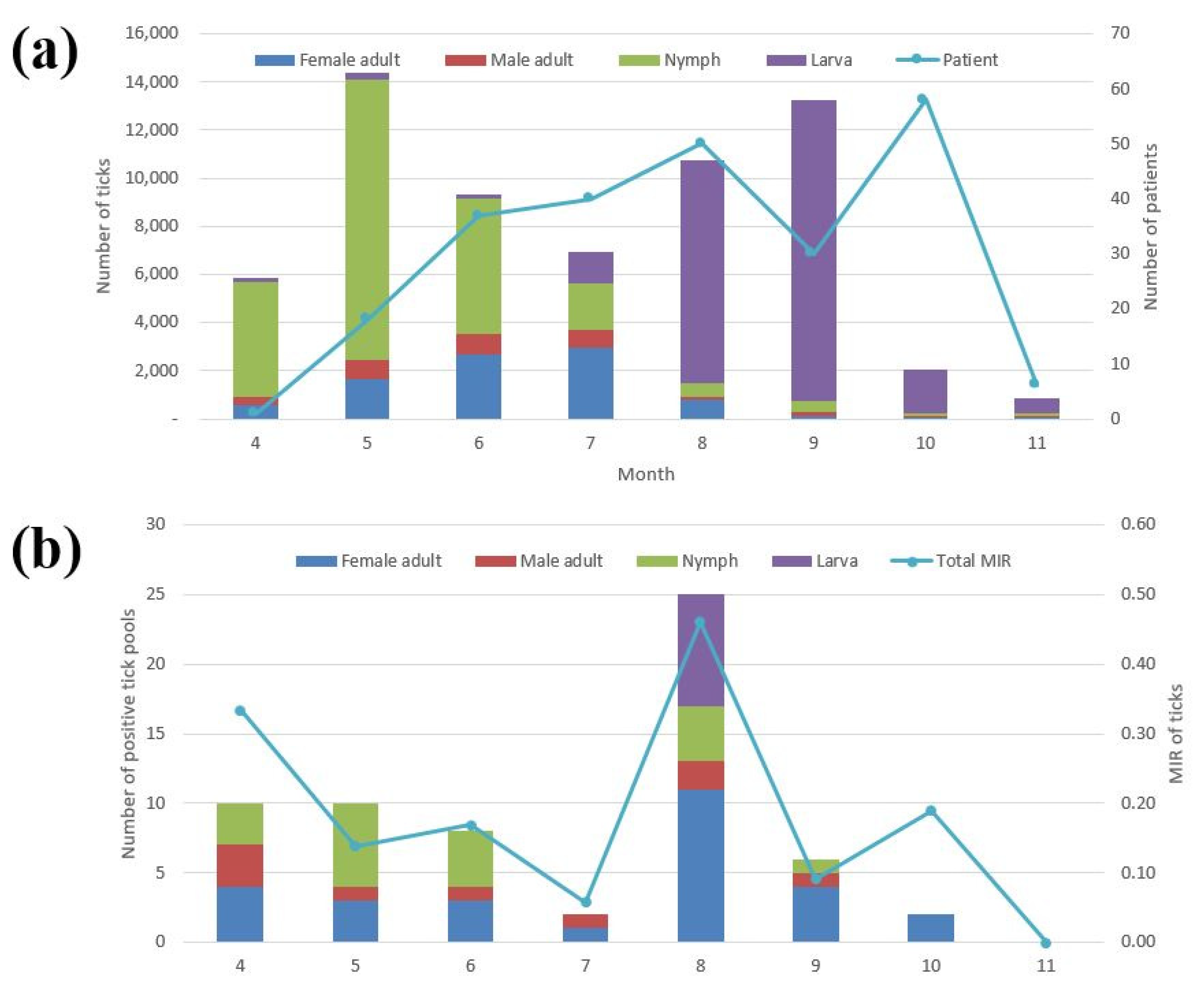
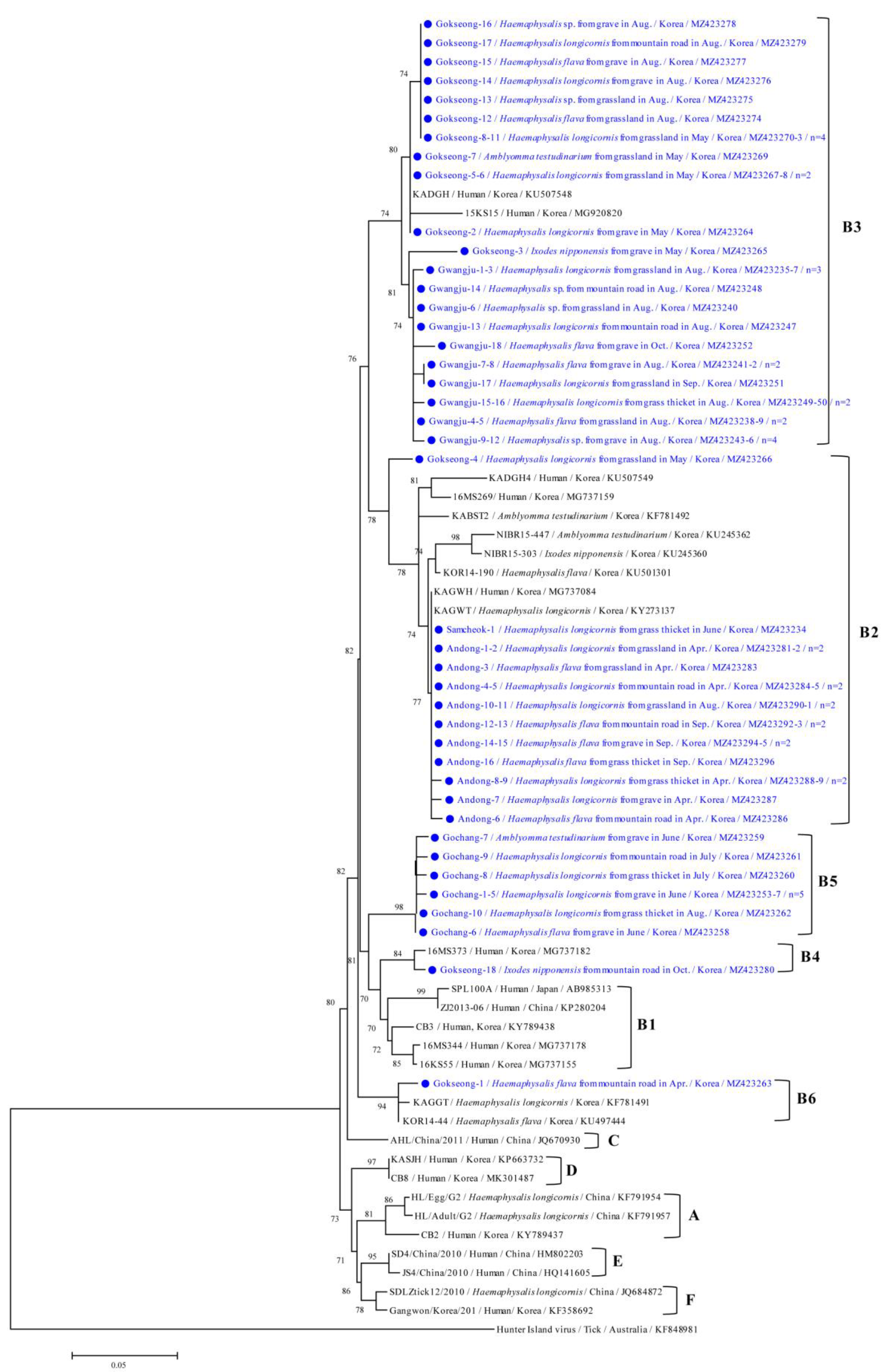
| Region | Number of Ticks Collected (Trap Index (TI)) | |||||||
|---|---|---|---|---|---|---|---|---|
| H. Longi | Haemaphysalis spp. | H. Flava | Ixodes spp. | A. Testu | I. Nippo | H. Japo | Total | |
| Pocheon | 447 (4.7) | 72 (0.8) | 14 (0.1) | 0 | 0 | 1 (0.01) | 0 | 534 (5.6) |
| Inje | 1140 (11.9) | 638 (6.6) | 20 (0.2) | 0 | 0 | 3 (0.03) | 2 (0.02) | 1803 (18.8) |
| Ganghwa | 3696 (38.5) | 39 (0.4) | 102 (1.1) | 0 | 0 | 0 | 0 | 3837 (40.0) |
| Samcheok | 4616 (48.1) | 7176 (74.8) | 59 (0.6) | 0 | 0 | 10 (0.1) | 0 | 11,861 (123.6) |
| Gwangju | 1005 (10.5) | 851 (8.9) | 23 (0.2) | 0 | 0 | 0 | 0 | 1879 (19.6) |
| Dangjin | 5748 (59.9) | 2669 (27.8) | 218 (2.3) | 445 (4.6) | 0 | 6 (0.06) | 0 | 9086 (94.6) |
| Boryeong | 969 (10.1) | 500 (5.2) | 156 (1.6) | 0 | 1 (0.01) | 1 (0.01) | 0 | 1627 (16.9) |
| Boeun | 527 (5.5) | 180 (1.9) | 50 (0.5) | 0 | 0 | 1 (0.01) | 0 | 758 (7.9) |
| Andong | 639 (6.7) | 28 (0.3) | 78 (0.8) | 0 | 0 | 7 (0.07) | 0 | 752 (7.8) |
| Sangju | 2400 (25.0) | 1359 (14.2) | 13 (0.1) | 1 (0.01) | 0 | 2 (0.02) | 0 | 3775 (39.3) |
| Gochang | 3301 (34.4) | 116 (1.2) | 122 (1.3) | 2 (0.02) | 42 (0.4) | 0 | 0 | 3583 (37.3) |
| Ulju | 2589 (27.0) | 2790 (29.1) | 23 (0.2) | 0 | 0 | 8 (0.08) | 0 | 5410 (56.4) |
| Gokseong | 1455 (15.2) | 625 (6.5) | 38 (0.4) | 0 | 9 (0.1) | 9 (0.09) | 0 | 2136 (22.3) |
| Boseong | 186 (1.9) | 1458 (15.2) | 52 (0.5) | 0 | 1 (0.01) | 0 | 0 | 1697 (17.7) |
| Jinju | 5978 (62.3) | 1990 (20.7) | 28 (0.3) | 0 | 130 (1.4) | 15 (0.16) | 0 | 8141 (84.8) |
| Jeju | 1247 (13.0) | 5197 (54.1) | 53 (0.6) | 0 | 0 | 0 | 0 | 6497 (67.7) |
| Total | 35,943 (23.4) | 25,688 (16.7) | 1049 (0.7) | 448 (0.3) | 183 (0.1) | 63 (0.04) | 2 (0.001) | 63,376 (41.3) |
| Ticks | Stage | Number of Collected Ticks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Apr. | May | Jun. | Jul. | Aug. | Sep. | Oct. | Nov. | Total | ||
| H. longi | Female | 556 | 1601 | 2683 | 2955 | 770 | 49 | 12 | 8 | 8634 |
| Male | 311 | 837 | 813 | 753 | 98 | 40 | 7 | 1 | 2860 | |
| Nymph | 4645 | 11,487 | 5412 | 1864 | 565 | 430 | 21 | 25 | 24,449 | |
| subtotal | 5512 | 13,925 | 8908 | 5572 | 1433 | 519 | 40 | 34 | 35,943 | |
| H. flava | Female | 29 | 15 | 5 | 1 | 8 | 81 | 52 | 56 | 247 |
| Male | 9 | 7 | 2 | 5 | 26 | 95 | 41 | 37 | 222 | |
| Nymph | 75 | 64 | 205 | 52 | 13 | 47 | 57 | 67 | 580 | |
| subtotal | 113 | 86 | 212 | 58 | 47 | 223 | 150 | 160 | 1049 | |
| I. nippo | Female | 4 | 5 | 0 | 1 | 0 | 1 | 5 | 10 | 26 |
| Male | 0 | 4 | 0 | 1 | 0 | 1 | 2 | 8 | 16 | |
| Nymph | 5 | 6 | 2 | 1 | 3 | 0 | 4 | 0 | 21 | |
| subtotal | 9 | 15 | 2 | 3 | 3 | 2 | 11 | 18 | 63 | |
| A. testu | Female | 1 | 3 | 0 | 2 | 1 | 1 | 0 | 0 | 8 |
| Male | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Nymph | 57 | 85 | 16 | 8 | 3 | 2 | 3 | 0 | 174 | |
| subtotal | 58 | 89 | 16 | 10 | 4 | 3 | 3 | 0 | 183 | |
| H. japo | Nymph | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| H. spp. | Larva | 146 | 255 | 212 | 887 | 9229 | 12,517 | 1819 | 623 | 25,688 |
| I. spp. | Larva | 0 | 0 | 0 | 433 | 14 | 1 | 0 | 0 | 448 |
| Total | Female | 590 | 1624 | 2688 | 2959 | 779 | 132 | 69 | 74 | 8915 |
| Male | 320 | 849 | 815 | 759 | 124 | 136 | 50 | 46 | 3099 | |
| Nymph | 4783 | 11,643 | 5635 | 1925 | 584 | 479 | 85 | 92 | 25,226 | |
| Larva | 146 | 255 | 212 | 1320 | 9243 | 12,518 | 1819 | 623 | 26,136 | |
| Total | 5839 | 14,371 | 9350 | 6963 | 10,730 | 13,265 | 2023 | 835 | 63,376 | |
| Regions. | Number of Collected Ticks | Trap Index | Number of Tested Ticks | Number of Pools | Number of SFTS-Positive Tick Pools | MIR (%) of Ticks |
|---|---|---|---|---|---|---|
| Pocheon | 534 | 5.6 | 288 | 92 | 0 | 0 |
| Inje | 1803 | 18.8 | 920 | 111 | 0 | 0 |
| Ganghwa | 3837 | 40.0 | 1945 | 271 | 0 | 0 |
| Samcheok | 11,861 | 123.6 | 5944 | 284 | 1 | 0.02 |
| Gwangju | 1879 | 19.6 | 961 | 119 | 18 | 1.9 |
| Dangjin | 9086 | 94.6 | 4582 | 438 | 0 | 0 |
| Boryeong | 1627 | 16.9 | 829 | 114 | 0 | 0 |
| Boeun | 758 | 7.9 | 411 | 122 | 0 | 0 |
| Andong | 752 | 7.8 | 399 | 99 | 16 | 4.0 |
| Sangju | 3775 | 39.3 | 1887 | 157 | 0 | 0 |
| Gochang | 3583 | 37.3 | 1825 | 232 | 10 | 0.5 |
| Ulju | 5410 | 56.4 | 2693 | 207 | 0 | 0 |
| Gokseong | 2136 | 22.3 | 1104 | 135 | 18 | 1.6 |
| Boseong | 1697 | 17.7 | 870 | 79 | 0 | 0 |
| Jinju | 8141 | 84.8 | 4106 | 335 | 0 | 0 |
| Jeju | 6497 | 67.7 | 3288 | 178 | 0 | 0 |
| Total | 63,376 | 41.3 | 32,052 | 2973 | 63 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.-G.; Noh, B.-E.; Lee, H.S.; Kim, T.-K.; Song, B.-G.; Lee, H.I. Nationwide Temporal and Geographical Distribution of Tick Populations and Phylogenetic Analysis of Severe Fever with Thrombocytopenia Syndrome Virus in Ticks in Korea, 2020. Microorganisms 2021, 9, 1630. https://doi.org/10.3390/microorganisms9081630
Seo M-G, Noh B-E, Lee HS, Kim T-K, Song B-G, Lee HI. Nationwide Temporal and Geographical Distribution of Tick Populations and Phylogenetic Analysis of Severe Fever with Thrombocytopenia Syndrome Virus in Ticks in Korea, 2020. Microorganisms. 2021; 9(8):1630. https://doi.org/10.3390/microorganisms9081630
Chicago/Turabian StyleSeo, Min-Goo, Byung-Eon Noh, Hak Seon Lee, Tae-Kyu Kim, Bong-Goo Song, and Hee Il Lee. 2021. "Nationwide Temporal and Geographical Distribution of Tick Populations and Phylogenetic Analysis of Severe Fever with Thrombocytopenia Syndrome Virus in Ticks in Korea, 2020" Microorganisms 9, no. 8: 1630. https://doi.org/10.3390/microorganisms9081630
APA StyleSeo, M.-G., Noh, B.-E., Lee, H. S., Kim, T.-K., Song, B.-G., & Lee, H. I. (2021). Nationwide Temporal and Geographical Distribution of Tick Populations and Phylogenetic Analysis of Severe Fever with Thrombocytopenia Syndrome Virus in Ticks in Korea, 2020. Microorganisms, 9(8), 1630. https://doi.org/10.3390/microorganisms9081630






