Microorganisms Associated with Mosquito Oviposition Sites: Implications for Habitat Selection and Insect Life Histories
Abstract
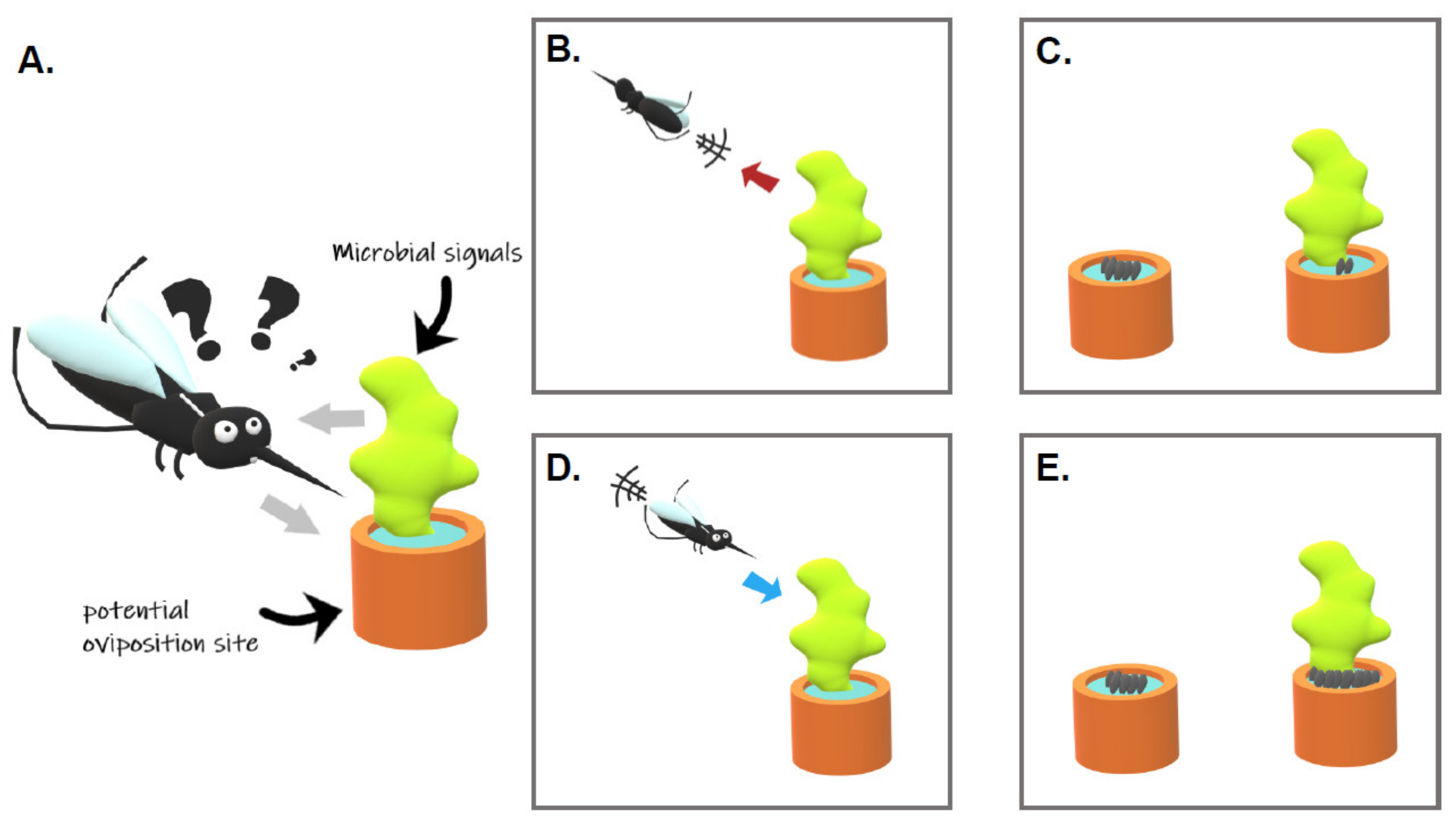
1. Introduction
2. Influence of Microorganisms on the Mosquito Oviposition Site Selection
2.1. Do Microorganisms from Water Habitats Attract/Stimulate Gravid Females?
2.2. Do Microorganisms Repel/Deter Gravid Females?
2.3. How Dose-Response Effects Influence the Female Oviposition?
3. Influence of Microorganisms Colonizing Water Habitats on Mosquitoes’ Premature Life History Traits
3.1. Can Microorganisms Be Used as a Food Source by Mosquito Larvae?
3.2. What Is the Influence of Microorganisms on Mosquitoes’ Development?
3.2.1. Egg Hatching
3.2.2. Post-Embryonic Development
3.3. Do microbes Impact the Post-Embryonic Survival?
4. Did Mosquitoes and Microorganisms Evolve toward Traits Influencing the Behavior?
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eisthen, H.L.; Theis, K.R. Animal–Microbe Interactions and the Evolution of Nervous Systems. Philos. Trans. R Soc. Lond. B Biol. Sci. 2016, 371, 20150052. [Google Scholar] [CrossRef]
- Dillon, R.J.; Vennard, C.T.; Charnley, A.K. Exploitation of Gut Bacteria in the Locust. Nature 2000, 403, 851. [Google Scholar] [CrossRef]
- Dillon, R.J.; Vennard, C.T.; Charnley, A.K. A Note: Gut Bacteria Produce Components of a Locust Cohesion Pheromone. J. Appl. Microbiol. 2002, 92, 759–763. [Google Scholar] [CrossRef]
- Dillon, R.; Charnley, K. Mutualism between the Desert Locust Schistocerca Gregaria and Its Gut Microbiota. Res. Microbiol. 2002, 153, 503–509. [Google Scholar] [CrossRef]
- Arakawa, H.; Cruz, S.; Deak, T. From Models to Mechanisms: Odorant Communication as a Key Determinant of Social Behavior in Rodents during Illness-Associated States. Neurosci. Biobehav. Rev. 2011, 35, 1916–1928. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, T.E.; Broederdorf, L.J.; Burkert, B.A.; Hirwa, I.H.; Mark, D.B.; Waldrip, Z.J.; Kopper, R.A.; Sutherland, M.V.; Freeman, E.W.; Hollister-Smith, J.A.; et al. Chemical Signals of Elephant Musth: Temporal Aspects of Microbially-Mediated Modifications. J. Chem. Ecol. 2012, 38, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, T.E.; Harelimana, I.H.; MacDonald, L.J.; Mark, D.B.; Juru, A.U.; Yin, Q.; Engman, J.A.; Kopper, R.A.; Lichti, C.F.; Mackintosh, S.G.; et al. The Role of Bacteria in Chemical Signals of Elephant Musth: Proximate Causes and Biochemical Pathways. In Chemical Signals in Vertebrates 13; Schulte, B.A., Goodwin, T.E., Ferkin, M.H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 63–85. [Google Scholar]
- Stensmyr, M.C.; Dweck, H.K.M.; Farhan, A.; Ibba, I.; Strutz, A.; Mukunda, L.; Linz, J.; Grabe, V.; Steck, K.; Lavista-Llanos, S.; et al. A Conserved Dedicated Olfactory Circuit for Detecting Harmful Microbes in Drosophila. Cell 2012, 151, 1345–1357. [Google Scholar] [CrossRef]
- Wilkerson, R.C.; Linton, Y.-M.; Fonseca, D.M.; Schultz, T.R.; Price, D.C.; Strickman, D.A. Making Mosquito Taxonomy Useful: A Stable Classification of Tribe Aedini That Balances Utility with Current Knowledge of Evolutionary Relationships. PLoS ONE 2015, 10, e0133602. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2016. Available online: http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/ (accessed on 28 December 2020).
- Shaw, W.R.; Catteruccia, F. Vector Biology Meets Disease Control: Using Basic Research to Fight Vector-Borne Diseases. Nat. Microbiol. 2019, 4, 20–34. [Google Scholar] [CrossRef]
- Ranson, H.; Abdallah, H.; Badolo, A.; Guelbeogo, W.M.; Kerah-Hinzoumbé, C.; Yangalbé-Kalnoné, E.; Sagnon, N.; Simard, F.; Coetzee, M. Insecticide Resistance in Anopheles Gambiae: Data from the First Year of a Multi-Country Study Highlight the Extent of the Problem. Malar. J. 2009, 8, 299. [Google Scholar] [CrossRef]
- Benelli, G.; Mehlhorn, H. Declining Malaria, Rising of Dengue and Zika Virus: Insights for Mosquito Vector Control. Parasitol. Res. 2016, 115, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Demok, S.; Endersby-Harshman, N.; Vinit, R.; Timinao, L.; Robinson, L.J.; Susapu, M.; Makita, L.; Laman, M.; Hoffmann, A.; Karl, S. Insecticide Resistance Status of Aedes Aegypti and Aedes Albopictus Mosquitoes in Papua New Guinea. Parasites Vectors 2019, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.G.; Birkett, M.A. Semiochemicals for Biting Fly Control: Their Identification and Exploitation. Pest Manag. Sci. 2007, 63, 647–657. [Google Scholar] [CrossRef]
- Bhattacharya, P.R. Microbial Control of Mosquitoes with Special Emphasis on Bacterial Control. Indian J. Malariol. 1998, 35, 206–224. [Google Scholar] [PubMed]
- Minard, G.; Mavingui, P.; Moro, C.V. Diversity and Function of Bacterial Microbiota in the Mosquito Holobiont. Parasites Vectors 2013, 6, 1. [Google Scholar] [CrossRef]
- Dickens, J.C.; Bohbot, J.D. Mini Review: Mode of Action of Mosquito Repellents. Pestic. Biochem. Physiol. 2013, 106, 149–155. [Google Scholar] [CrossRef]
- Guégan, M.; Zouache, K.; Démichel, C.; Minard, G.; Tran Van, V.; Potier, P.; Mavingui, P.; Valiente Moro, C. The Mosquito Holobiont: Fresh Insight into Mosquito-Microbiota Interactions. Microbiome 2018, 6, 49. [Google Scholar] [CrossRef]
- Capinera, J.L. (Ed.) Encyclopedia of Entomology; Springer: Dordrecht, The Netherlands, 2005; ISBN 978-0-306-48380-6. [Google Scholar]
- Clements, A.N. The Biology of Mosquitoes: Development, Nutrition and Reproduction; Chapman & Hall: London, UK, 1992; ISBN 978-0-412-40180-0. [Google Scholar]
- Touré, D.S.; Ouattara, A.F.; Kra, K.D.; Kwadjo, K.E.; Koné, M.; Doumbia, M.; Doannio, J.M.C. Impact of egg laying delay on reproduction, gorging habit and mortality in gravid females Anopheles gambiae (Diptera Culicidae). Bull. Soc. Pathol. Exot. 2017, 110, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Clements, A.N. The Biology of Mosquitoes: Sensory Reception and Behaviour; CABI: Wallingford, UK, 1999; ISBN 978-0-85199-313-3. [Google Scholar]
- Day, J.F. Mosquito Oviposition Behavior and Vector Control. Insects 2016, 7, 65. [Google Scholar] [CrossRef]
- Bentley, M.D.; Day, J.F. Chemical Ecology and Behavioral Aspects of Mosquito Oviposition. Annu. Rev. Entomol. 1989, 34, 401–421. [Google Scholar] [CrossRef]
- McMeniman, C.J. Chapter 11—Disruption of Mosquito Olfaction. In Genetic Control of Malaria and Dengue; Adelman, Z.N., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 227–252. ISBN 978-0-12-800246-9. [Google Scholar]
- Baik, L.S.; Carlson, J.R. The Mosquito Taste System and Disease Control. Proc. Natl. Acad. Sci. USA 2020, 117, 32848–32856. [Google Scholar] [CrossRef]
- Dethier, V.G.; Browne, B.L.; Smith, C.N. The Designation of Chemicals in Terms of the Responses They Elicit from Insects. J. Econ. Entomol. 1960, 53, 134–136. [Google Scholar] [CrossRef]
- Ranasinghe, H.A.K.; Amarasinghe, L.D. Naturally Occurring Microbiota Associated with Mosquito Breeding Habitats and Their Effects on Mosquito Larvae. Available online: https://www.hindawi.com/journals/bmri/2020/4065315/ (accessed on 4 March 2021).
- Coon, K.L.; Vogel, K.J.; Brown, M.R.; Strand, M.R. Mosquitoes Rely on Their Gut Microbiota for Development. Mol. Ecol. 2014, 23, 2727–2739. [Google Scholar] [CrossRef] [PubMed]
- Dada, N.; Jumas-Bilak, E.; Manguin, S.; Seidu, R.; Stenström, T.-A.; Overgaard, H.J. Comparative Assessment of the Bacterial Communities Associated with Aedes Aegypti Larvae and Water from Domestic Water Storage Containers. Parasites Vectors 2014, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Dickson, L.B.; Jiolle, D.; Minard, G.; Moltini-Conclois, I.; Volant, S.; Ghozlane, A.; Bouchier, C.; Ayala, D.; Paupy, C.; Moro, C.V.; et al. Carryover Effects of Larval Exposure to Different Environmental Bacteria Drive Adult Trait Variation in a Mosquito Vector. Sci. Adv. 2017, 3, e1700585. [Google Scholar] [CrossRef]
- Minard, G.; Tran, F.-H.; Van, V.T.; Fournier, C.; Potier, P.; Roiz, D.; Mavingui, P.; Moro, C.V. Shared Larval Rearing Environment, Sex, Female Size and Genetic Diversity Shape Ae. Albopictus Bacterial Microbiota. PLoS ONE 2018, 13, e0194521. [Google Scholar] [CrossRef] [PubMed]
- Alfano, N.; Tagliapietra, V.; Rosso, F.; Manica, M.; Arnoldi, D.; Pindo, M.; Rizzoli, A. Changes in Microbiota Across Developmental Stages of Aedes Koreicus, an Invasive Mosquito Vector in Europe: Indications for Microbiota-Based Control Strategies. Front. Microbiol. 2019, 10, 2832. [Google Scholar] [CrossRef]
- Nilsson, L.K.J.; de Oliveira, M.R.; Marinotti, O.; Rocha, E.M.; Håkansson, S.; Tadei, W.P.; de Souza, A.Q.L.; Terenius, O. Characterization of Bacterial Communities in Breeding Waters of Anopheles Darlingi in Manaus in the Amazon Basin Malaria-Endemic Area. Microb. Ecol. 2019, 78, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Nyasembe, V.O.; Torto, B. Volatile Phytochemicals as Mosquito Semiochemicals. Phytochem. Lett. 2014, 8, 196–201. [Google Scholar] [CrossRef]
- Arbaoui, A.A.; Chua, T.H. Bacteria as a Source of Oviposition Attractant for Aedes Aegypti Mosquitoes. Trop. Biomed. 2014, 31, 134–142. [Google Scholar]
- Ponnusamy, L.; Xu, N.; Nojima, S.; Wesson, D.M.; Schal, C.; Apperson, C.S. Identification of Bacteria and Bacteria-Associated Chemical Cues That Mediate Oviposition Site Preferences by Aedes Aegypti. Proc. Natl. Acad. Sci. USA 2008, 105, 9262–9267. [Google Scholar] [CrossRef] [PubMed]
- Sumba, L.A.; Guda, T.O.; Deng, A.L.; Hassanali, A.; Beier, J.C.; Knols, B.G.J. Mediation of Oviposition Site Selection in the African Malaria Mosquito Anopheles Gambiae (Diptera: Culicidae) by Semiochemicals of Microbial Origin. Int. J. Trop. Insect Sci. 2004, 24, 260–265. [Google Scholar] [CrossRef]
- Lindh, J.M.; Kännaste, A.; Knols, B.G.J.; Faye, I.; Borg-Karlson, A.-K. Oviposition Responses of Anopheles Gambiae s.s. (Diptera: Culicidae) and Identification of Volatiles from Bacteria-Containing Solutions. J. Med. Entomol. 2008, 45, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Varela, M.; Lindh, J.; Lindsay, S.W.; Fillinger, U. Habitat Discrimination by Gravid Anopheles Gambiae Sensu Lato—A Push-Pull System. Malar. J. 2014, 13, 1–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lindh, J.M.; Okal, M.N.; Herrera-Varela, M.; Borg-Karlson, A.-K.; Torto, B.; Lindsay, S.W.; Fillinger, U. Discovery of an Oviposition Attractant for Gravid Malaria Vectors of the Anopheles Gambiae Species Complex. Malar. J. 2015, 14, 119. [Google Scholar] [CrossRef]
- Eneh, L.K.; Saijo, H.; Borg-Karlson, A.-K.; Lindh, J.M.; Rajarao, G.K. Cedrol, a Malaria Mosquito Oviposition Attractant Is Produced by Fungi Isolated from Rhizomes of the Grass Cyperus Rotundus. Malar. J. 2016, 15, 478. [Google Scholar] [CrossRef]
- Fillinger, U.; Sombroek, H.; Majambere, S.; van Loon, E.; Takken, W.; Lindsay, S.W. Identifying the Most Productive Breeding Sites for Malaria Mosquitoes in The Gambia. Malar. J. 2009, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Ndenga, B.A.; Simbauni, J.A.; Mbugi, J.P.; Githeko, A.K.; Fillinger, U. Productivity of Malaria Vectors from Different Habitat Types in the Western Kenya Highlands. PLoS ONE 2011, 6, e19473. [Google Scholar] [CrossRef] [PubMed]
- Gouagna, L.C.; Rakotondranary, M.; Boyer, S.; Lempérière, G.; Dehecq, J.-S.; Fontenille, D. Abiotic and Biotic Factors Associated with the Presence of Anopheles Arabiensis Immatures and Their Abundance in Naturally Occurring and Man-Made Aquatic Habitats. Parasites Vectors 2012, 5, 96. [Google Scholar] [CrossRef]
- Lantova, L.; Volf, P. Mosquito and Sand Fly Gregarines of the Genus Ascogregarina and Psychodiella (Apicomplexa: Eugregarinorida, Aseptatorina)—Overview of Their Taxonomy, Life Cycle, Host Specificity and Pathogenicity. Infect. Genet. Evol. 2014, 28, 616–627. [Google Scholar] [CrossRef]
- Reeves, W.K. Oviposition by Aedes Aegypti (Diptera: Culicidae) in Relation to Conspecific Larvae Infected with Internal Symbiotes. J. Vector Ecol. 2004, 29, 159–163. [Google Scholar]
- Ponnusamy, L.; Schal, C.; Wesson, D.M.; Arellano, C.; Apperson, C.S. Oviposition Responses of Aedes Mosquitoes to Bacterial Isolates from Attractive Bamboo Infusions. Parasites Vectors 2015, 8, 486. [Google Scholar] [CrossRef] [PubMed]
- Zahiri, N.S.; Mulla, M.S. Ovipositional and Ovicidal Effects of the Microbial Agent Bacillus thuringiensis israelensis on Culex quinquefasciatus Say (Diptera: Culicidae). J. Vector Ecol. 2006, 31, 29–34. [Google Scholar] [CrossRef]
- Futami, K.; Kongere, J.O.; Mwania, M.S.; Lutiali, P.A.; Njenga, S.M.; Minakawa, N. Effects of Bacillus thuringiensis Israelensis on Anopheles arabiensis. J. Am. Mosq. Control Assoc. 2011, 27, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Stoops, C.A. Influence of Bacillus Thuringiensis Var. Israelensis on Oviposition of Aedes Albopictus (Skuse). J. Vector Ecol. 2005, 30, 41–44. [Google Scholar] [PubMed]
- Wasi Ahmad, N.; Lee, H.; Wan, R.; Lian, A.; Chee Dhang, C.; Azahari, A.; Sadiyah, I. Oviposition Behaviour of Aedes Albopictus in Temephos and Bacillus Thuringiensis Israelensis-Treated Ovitraps. Dengue Bull. 2009, 33, 209–217. [Google Scholar]
- Zettel Nalen, C.M.; Allan, S.A.; Becnel, J.J.; Kaufman, P.E. Oviposition Substrate Selection by Florida Mosquitoes in Response to Pathogen-Infected Conspecific Larvae. J. Vector Ecol. 2013, 38, 182–187. [Google Scholar] [CrossRef]
- Lowenberger, C.A.; Rau, M.E. Selective Oviposition by Aedes Aegypti (Diptera: Culicidae) in Response to a Larval Parasite, Plagiorchis Elegans (Trematoda: Plagiorchiidae). Environ. Entomol. 1994, 23, 1269–1276. [Google Scholar] [CrossRef]
- Zahiri, N.; Rau, M.E. Oviposition Attraction and Repellency of Aedes Aegypti (Diptera: Culicidae) to Waters from Conspecific Larvae Subjected to Crowding, Confinement, Starvation, or Infection. J. Med Entomol. 1998, 35, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Becnel, J.J.; Johnson, M.A. Mosquito Host Range and Specificity of Edhazardia Aedis (Microspora: Culicosporidae). J. Am. Mosq. Control Assoc. 1993, 9, 269–274. [Google Scholar]
- Becnel, J.J.; Garcia, J.J.; Johnson, M.A. Edhazardia Aedis (Microspora: Culicosporidae) Effects on the Reproductive Capacity of Aedes Aegypti (Diptera: Culicidae). J. Med. Entomol. 1995, 32, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Agnew, P.; Koella, J. Life History Interactions with Environmental Conditions in a Host–Parasite Relationship and the Parasite’s Mode of Transmission. Evol. Ecol. 1999, 13, 67–91. [Google Scholar] [CrossRef]
- Becnel, J.J.; Johnson, M.A. Impact of Edhazardia Aedis (Microsporidia: Culicosporidae) on a Seminatural Population of Aedes Aegypti (Diptera: Culicidae). Biol. Control 2000, 18, 39–48. [Google Scholar] [CrossRef]
- Grigsby, A.; Kelly, B.J.; Sanscrainte, N.D.; Becnel, J.J.; Short, S.M. Propagation of the Microsporidian Parasite Edhazardia Aedis in Aedes Aegypti Mosquitoes. J. Vis. Exp. 2020, e61574. [Google Scholar] [CrossRef]
- Schwab, A.E.; Lewis, D.J.; Rau, M.E. The Impact of Selective Oviposition and Infection with Plagiorchis Elegans on Aedes Aegypti Pre-Imago Population Dynamics at Optimal Food Availability. J. Med. Entomol. 2003, 40, 830–840. [Google Scholar] [CrossRef]
- Ben-Dov, E. Bacillus Thuringiensis Subsp. Israelensis and Its Dipteran-Specific Toxins. Toxins 2014, 6, 1222–1243. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.L.; Jordan, H.R.; Serewis-Pond, C.; Zheng, L.; Benbow, M.E.; Small, P.L.; Tomberlin, J.K. Mycobacterium Ulcerans Toxin, Mycolactone May Enhance Host-Seeking and Oviposition Behaviour by Aedes Aegypti (L.) (Diptera: Culicidae). Environ. Microbiol. 2017, 19, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A Meta-Analysis of Preference–Performance Relationships in Phytophagous Insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar] [CrossRef]
- Rejmánková, E.; Roberts, D.R.; Manguin, S.; Pope, K.O.; Komárek, J.; Post, R.A. Anopheles Albimanus (Diptera: Culicidae) and Cyanobacteria: An Example of Larval Habitat Selection. Environ. Entomol. 1996, 25, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Martínez, M.G.; Rodríguez, M.H.; Arredondo-Jiménez, J.I.; Méndez-Sánchez, J.D. Phormidium Animalis (Cyanobacteria: Oscillatoriaceae) Supports Larval Development of Anopheles Albimanus. J. Am. Mosq. Control Assoc. 2003, 19, 155–158. [Google Scholar] [PubMed]
- Beehler, J.W.; Millar, J.G.; Mulla, M.S. Protein Hydrolysates and Associated Bacterial Contaminants as Oviposition Attractants for the Mosquito Culex Quinquefasciatus. Med. Vet. Entomol. 1994, 8, 381–385. [Google Scholar] [CrossRef]
- Díaz-Nieto, L.M.; D Alessio, C.; Perotti, M.A.; Berón, C.M. Culex Pipiens Development Is Greatly Influenced by Native Bacteria and Exogenous Yeast. PLoS ONE 2016, 11, e0153133. [Google Scholar] [CrossRef]
- Souza, R.S.; Virginio, F.; Riback, T.I.S.; Suesdek, L.; Barufi, J.B.; Genta, F.A. Microorganism-Based Larval Diets Affect Mosquito Development, Size and Nutritional Reserves in the Yellow Fever Mosquito Aedes Aegypti (Diptera: Culicidae). Front. Physiol. 2019, 10, 152. [Google Scholar] [CrossRef]
- Roberts, D.M. Egg Hatching of Mosquitoes Aedes Caspius and Ae. Vittatus Stimulated by Water Vibrations. Med Vet. Entomol. 2001, 15, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, B.; Foster, W. Water Agitation: A Stimulus for Egg Hatching of Anopheles Gambiae. In Proceedings of the Entomological Society of America Annual Meeting 2008, Reno, NV, USA, 16–19 November 2008. [Google Scholar]
- Impoinvil, D.E.; Cardenas, G.A.; Gihture, J.I.; Mbogo, C.M.; Beier, J.C. Constant Temperature and Time Period Effects on Anopheles Gambiae Egg Hatching. J. Am. Mosq. Control Assoc. 2007, 23, 124–130. [Google Scholar] [CrossRef]
- Walker, E.D.; Merritt, R.W. The Significance of Leaf Detritus to Mosquito (Diptera: Culicidae) Productivity from Treeholes. Environ. Entomol. 1988, 17, 199–206. [Google Scholar] [CrossRef]
- Walker, E.D.; Lawson, D.L.; Merritt, R.W.; Morgan, W.T.; Klug, M.J. Nutrient Dynamics, Bacterial Populations, and Mosquito Productivity in Tree Hole Ecosystems and Microcosms. Ecology 1991, 72, 1529–1546. [Google Scholar] [CrossRef]
- Rozeboom, L.E. The Effect of Bacteria on the Hatching of Mosquito Eggs. Am. J. Hyg. 1934, 20, 496–501. [Google Scholar] [CrossRef]
- Ponnusamy, L.; Böröczky, K.; Wesson, D.M.; Schal, C.; Apperson, C.S. Bacteria Stimulate Hatching of Yellow Fever Mosquito Eggs. PLoS ONE 2011, 6, e24409. [Google Scholar] [CrossRef]
- Gjullin, C.M.; Hegarty, C.P.; Bollen, W.B. The Necessity of a Low Oxygen Concentration for the Hatching of Aedes Mosquito Eggs. J. Cell. Comp. Physiol. 1941, 17, 193–202. [Google Scholar] [CrossRef]
- Fallis, S.P.; Snow, K.R. The Hatching Stimulus for Eggs of Aedes Punctor (Diptera: Culicidae). Ecol. Entomol. 1983, 8, 23–28. [Google Scholar] [CrossRef]
- Judson, C.L.; Hokama, Y.; Haydock, I. The Physiology of Hatching of Aedine Mosquito Eggs: Some Larval Responses to the Hatching Stimulus. J. Insect Physiol. 1965, 11, 1169–1177. [Google Scholar] [CrossRef]
- Flor-Weiler, L.B.; Rooney, A.P.; Behle, R.W.; Muturi, E.J. Characterization of Tolypocladium Cylindrosporum (Hypocreales: Ophiocordycipitaceae) and Its Impact Against Aedes Aegypti and Aedes Albopictus Eggs at Low Temperature. J. Am. Mosq. Control Assoc. 2017, 33, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Montalva, C.; Silva, J.J.; Rocha, L.F.N.; Luz, C.; Humber, R.A. Characterization of Tolypocladium Cylindrosporum (Hypocreales, Ophiocordycipitaceae) Isolates from Brazil and Their Efficacy against Aedes Aegypti (Diptera, Culicidae). J. Appl. Microbiol. 2019, 126, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Coon, K.L.; Valzania, L.; Brown, M.R.; Strand, M.R. Predaceous Toxorhynchites Mosquitoes Require a Living Gut Microbiota to Develop. Proc. R. Soc. B Biol. Sci. 2020, 287, 20192705. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.A.; Matusovsky, B.; Brackney, D.E.; Steven, B. Generation of Axenic Aedes Aegypti Demonstrate Live Bacteria Are Not Required for Mosquito Development. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Coon, K.L.; Valzania, L.; McKinney, D.A.; Vogel, K.J.; Brown, M.R.; Strand, M.R. Bacteria-Mediated Hypoxia Functions as a Signal for Mosquito Development. Proc. Natl. Acad. Sci. USA 2017, 114, E5362–E5369. [Google Scholar] [CrossRef]
- Valzania, L.; Coon, K.L.; Vogel, K.J.; Brown, M.R.; Strand, M.R. Hypoxia-Induced Transcription Factor Signaling Is Essential for Larval Growth of the Mosquito Aedes Aegypti. Proc. Natl. Acad. Sci. USA 2018, 115, 457–465. [Google Scholar] [CrossRef]
- Wang, Y.; Eum, J.H.; Harrison, R.E.; Valzania, L.; Yang, X.; Johnson, J.A.; Huck, D.T.; Brown, M.R.; Strand, M.R. Riboflavin Instability Is a Key Factor Underlying the Requirement of a Gut Microbiota for Mosquito Development. Proc. Natl. Acad. Sci. USA 2021, 118, e2101080118. [Google Scholar] [CrossRef]
- Valzania, L.; Martinson, V.G.; Harrison, R.E.; Boyd, B.M.; Coon, K.L.; Brown, M.R.; Strand, M.R. Both Living Bacteria and Eukaryotes in the Mosquito Gut Promote Growth of Larvae. PLoS Negl. Trop. Dis. 2018, 12, e0006638. [Google Scholar] [CrossRef]
- Steyn, A.; Roets, F.; Botha, A. Yeasts Associated with Culex Pipiens and Culex Theileri Mosquito Larvae and the Effect of Selected Yeast Strains on the Ontogeny of Culex Pipiens. Microb. Ecol. 2016, 71, 747–760. [Google Scholar] [CrossRef]
- Bukhari, T.; Middelman, A.; Koenraadt, C.J.M.; Takken, W.; Knols, B.G.J. Factors Affecting Fungus-Induced Larval Mortality in Anopheles Gambiae and Anopheles Stephensi. Malar. J. 2010, 9, 22. [Google Scholar] [CrossRef]
- Scholte, E.-J.; Knols, B.G.J.; Samson, R.A.; Takken, W. Entomopathogenic Fungi for Mosquito Control: A Review. J. Insect Sci. 2004, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Butt, T.M.; Greenfield, B.P.J.; Greig, C.; Maffeis, T.G.G.; Taylor, J.W.D.; Piasecka, J.; Dudley, E.; Abdulla, A.; Dubovskiy, I.M.; Garrido-Jurado, I.; et al. Metarhizium Anisopliae Pathogenesis of Mosquito Larvae: A Verdict of Accidental Death. PLoS ONE 2013, 8, e81686. [Google Scholar] [CrossRef]
- Lovett, B.; Bilgo, E.; Millogo, S.A.; Ouattarra, A.K.; Sare, I.; Gnambani, E.J.; Dabire, R.K.; Diabate, A.; Leger, R.J.S. Transgenic Metarhizium Rapidly Kills Mosquitoes in a Malaria-Endemic Region of Burkina Faso. Science 2019, 364, 894–897. [Google Scholar] [CrossRef]
- Federici, B.A. Virus Pathogens of Culicidae (Mosquitos). Bull. World Health Organ. 1977, 55, 25–36. [Google Scholar]
- Johnson, R.M.; Rasgon, J.L. Densonucleosis Viruses (‘Densoviruses’) for Mosquito and Pathogen Control. Curr. Opin. Insect Sci. 2018, 28, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A. Bacillus Thuringiensis Serovariety Israelensis and Bacillus Sphaericus for Mosquito Control. J. Am. Mosq. Control Assoc. 2007, 23, 133–163. [Google Scholar] [CrossRef]
- Brühl, C.A.; Després, L.; Frör, O.; Patil, C.D.; Poulin, B.; Tetreau, G.; Allgeier, S. Environmental and Socioeconomic Effects of Mosquito Control in Europe Using the Biocide Bacillus Thuringiensis Subsp. Israelensis (Bti). Sci. Total Environ. 2020, 724, 137800. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Aimanova, K.G.; Gill, S.S. Aedes Cadherin Receptor That Mediates Bacillus Thuringiensis Cry11A Toxicity Is Essential for Mosquito Development. PLoS Negl. Trop. Dis. 2020, 14, e0007948. [Google Scholar] [CrossRef]
- Bhattarai, U.R.; Doherty, J.-F.; Dowle, E.; Gemmell, N.J. The Adaptiveness of Host Behavioural Manipulation Assessed Using Tinbergen’s Four Questions. Trends Parasitol. 2021, 37, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, T.; Fukatsu, T. Relevance of Microbial Symbiosis to Insect Behavior. Curr. Opin. Insect Sci. 2020, 39, 91–100. [Google Scholar] [CrossRef] [PubMed]
| Microorganisms | Species | Condition/Concentration | Mosquito Species | Semiochemicals | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Aedes aegypti | Aedes albopictus | Anopheles gambiae | An. arabiensis | Culex quinquefasciatus | |||||
| Bacteria | Bacillus thuringiensis | 106 CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | |
| 107 CFU/mL | attractivity/stimulation | attractivity/stimulation | − | − | − | ||||
| 108 CFU/mL | no response | repellency/deterrence | − | − | − | ||||
| Bacillus thuringiensis var. israelensis | 0.5–2 mg/L (for Cx. quinquefasciatus), 8 mg/L (for Ae. albopictus), 2–6 mg/L (for An. arabiensis) | − | no response or attractivity/stimulation | − | no response | repellency/deterrence | [50,51,52,53] | ||
| Brevundimonas vesicularis | 106 CFU/mL | attractivity/stimulation | attractivity/stimulation | − | − | − | [49] | ||
| 107 CFU/mL | attractivity/stimulation | no response | − | − | − | ||||
| 108 CFU/mL | no response | repellency/deterrence | − | − | − | ||||
| Citrobacter freundii | 106 CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | ||
| 107 CFU/mL | attractivity/stimulation | attractivity/stimulation | − | − | − | ||||
| Comamonas spp | [4.2 × 107; 8.1 × 107] CFU/mL | − | − | attractivity/stimulation | − | − | 2-Methyl-3-decanol, methyl-1-butanol, 2-phenylethanol, phenylmethanol, alkyl-pyrazines, 3-methylbutanoic acid | [40] | |
| Enterobacter asburiae | [106;107] CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | ||
| Enterobacter cancerogenus | [106;107] CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | ||
| Enterobacter gergoviae | 106 CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | ||
| 108 CFU/mL | no response | repellency/deterrence | − | − | − | ||||
| Enterobacter ludwigii | 106 CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | ||
| 107 CFU/mL | no response | attractivity/stimulation | − | − | − | ||||
| Exiguobacterium spp | [5.2 × 107; 5.3 × 107] CFU/mL | − | − | attractivity/stimulation | - | - | 2-Methyl-3-decanol, methyl-1-butanol, 2-phenylethanol, phenylmethanol, alkyl-pyrazines, 3-methylbutanoic acid | [40] | |
| Lactococcus lactis | 106 CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | ||
| 107 CFU/mL | attractivity/stimulation | attractivity/stimulation | − | − | − | ||||
| Micrococcus. spp | [7.7 × 106; 1.8 × 107] CFU/mL | − | − | attractivity/stimulation | − | − | 2-Methyl-3-decanol, methyl-1-butanol, 2-phenylethanol, phenylmethanol, alkyl-pyrazines, 3-methylbutanoic acid | [40] | |
| Proteus spp | [6.9 × 107; 3.2 × 108] CFU/mL | − | − | attractivity/stimulation | − | − | 2-Methyl-3-decanol, methyl-1-butanol, 2-phenylethanol, phenylmethanol, alkyl-pyrazines, 3-methylbutanoic acid | [40] | |
| Pseudomonas fulva | 107 CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | ||
| Pseudomonas plecoglossicida | 106 CFU/mL | no response | repellency/deterrence | − | − | − | [49] | ||
| 107 CFU/mL | no response | attractivity/stimulation | − | − | − | ||||
| Rhizobium huautlense | 108 CFU/mL | repellency/deterrence | no response | − | − | − | [49] | ||
| Shigella dysenteriae | [106;107] CFU/mL | attractivity/stimulation | no response | − | − | − | [49] | ||
| Vibrio metschnikovii | [2 × 108; 4 × 108] CFU/mL | − | − | attractivity/stimulation | − | − | 2-Methyl-3-decanol, methyl-1-butanol, 2-phenylethanol, phenylmethanol, alkyl-pyrazines, 3-methylbutanoic acid | [40] | |
| Fungi | Fusarium fujikuroi complex | − | − | attractivity/stimulation | − | − | Cedrol | [43] | |
| Fusarium falciforme | − | − | attractivity/stimulation | − | − | ||||
| Smittium morbosum | infected larvae | repellency/deterrence | − | − | − | − | [48] | ||
| Candidatus near pseudoglaebosa | infected larvae | attractivity/stimulation | − | − | − | − | |||
| Edhazardia aedis | repellency/deterrence | − | − | − | − | [54] | |||
| Protist | Ascogregarina taiwanensis | infected larvae (12–97 trophozoites) | attractivity/stimulation | − | − | − | − | [48] | |
| Trematode | Plagiorchis elegans | infected larvae | repellency/deterrence | − | − | − | − | [55,56] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girard, M.; Martin, E.; Vallon, L.; Raquin, V.; Bellet, C.; Rozier, Y.; Desouhant, E.; Hay, A.-E.; Luis, P.; Valiente Moro, C.; et al. Microorganisms Associated with Mosquito Oviposition Sites: Implications for Habitat Selection and Insect Life Histories. Microorganisms 2021, 9, 1589. https://doi.org/10.3390/microorganisms9081589
Girard M, Martin E, Vallon L, Raquin V, Bellet C, Rozier Y, Desouhant E, Hay A-E, Luis P, Valiente Moro C, et al. Microorganisms Associated with Mosquito Oviposition Sites: Implications for Habitat Selection and Insect Life Histories. Microorganisms. 2021; 9(8):1589. https://doi.org/10.3390/microorganisms9081589
Chicago/Turabian StyleGirard, Maxime, Edwige Martin, Laurent Vallon, Vincent Raquin, Christophe Bellet, Yves Rozier, Emmanuel Desouhant, Anne-Emmanuelle Hay, Patricia Luis, Claire Valiente Moro, and et al. 2021. "Microorganisms Associated with Mosquito Oviposition Sites: Implications for Habitat Selection and Insect Life Histories" Microorganisms 9, no. 8: 1589. https://doi.org/10.3390/microorganisms9081589
APA StyleGirard, M., Martin, E., Vallon, L., Raquin, V., Bellet, C., Rozier, Y., Desouhant, E., Hay, A.-E., Luis, P., Valiente Moro, C., & Minard, G. (2021). Microorganisms Associated with Mosquito Oviposition Sites: Implications for Habitat Selection and Insect Life Histories. Microorganisms, 9(8), 1589. https://doi.org/10.3390/microorganisms9081589








