Examining the Effects of the Nitrogen Environment on Growth and N2-Fixation of Endophytic Herbaspirillum seropedicae in Maize Seedlings by Applying 11C Radiotracing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth and Root Inoculation with H. seropedicae Bacteria
2.2. Production and Administration of Radioactive 11CO2
2.3. Measuring Metabolic Partitioning of Plant Fixed 11C
2.4. Autoradiography
2.5. Fluorescence Imaging
2.6. Root-Associated Bacteria Growth Performance—Drop Plate Assay
2.7. Liquid Culture Bacteria Growth Performance—Spectrophotometric Assay
2.8. Microbial Nitrogenase Activity—Acetylene Reduction Assay
2.9. Statistical Analysis
3. Results and Discussion
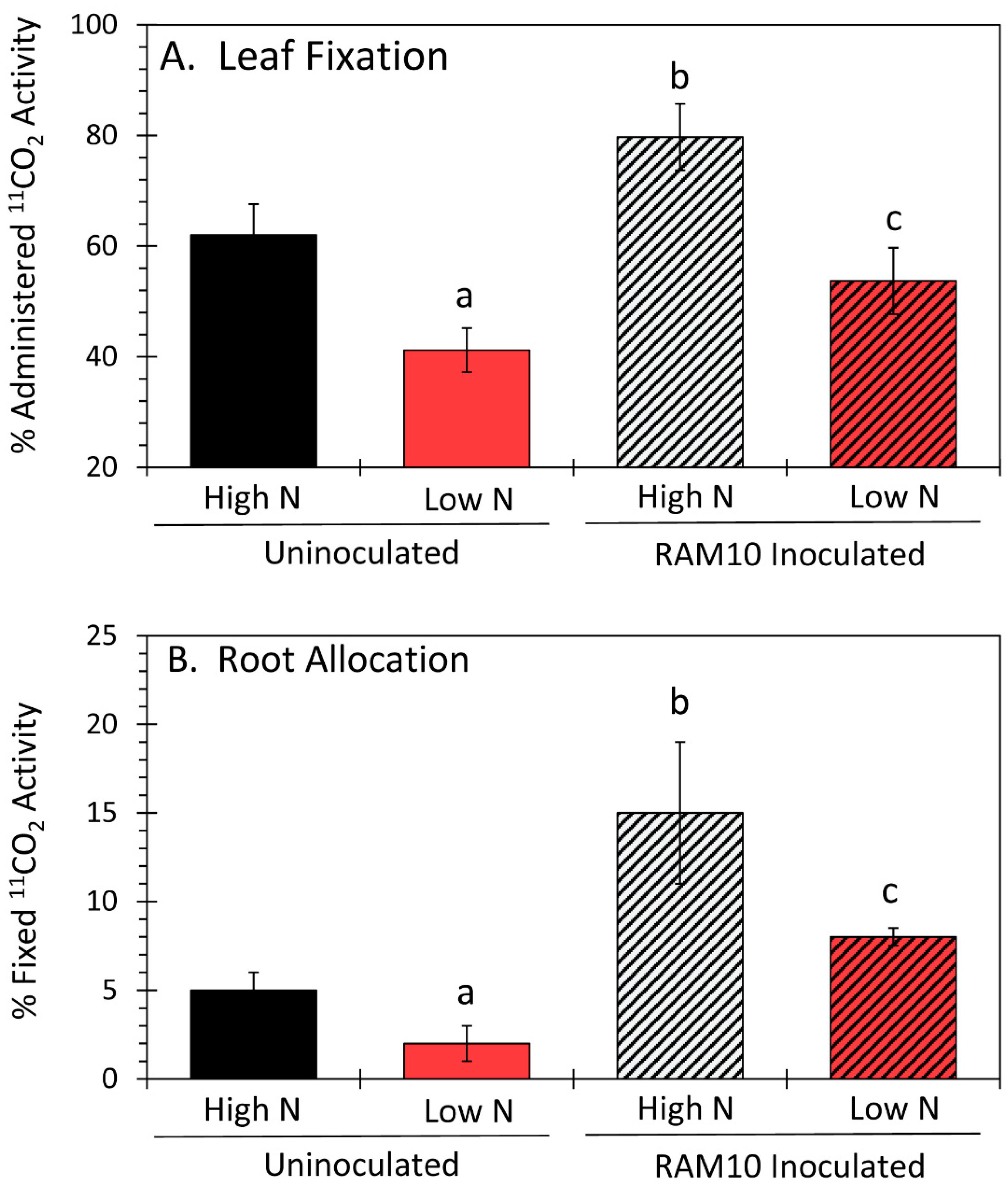
3.1. Microorganisms Can Influence Their Host’s Ability to Assimilate Carbon and Allocate It Belowground
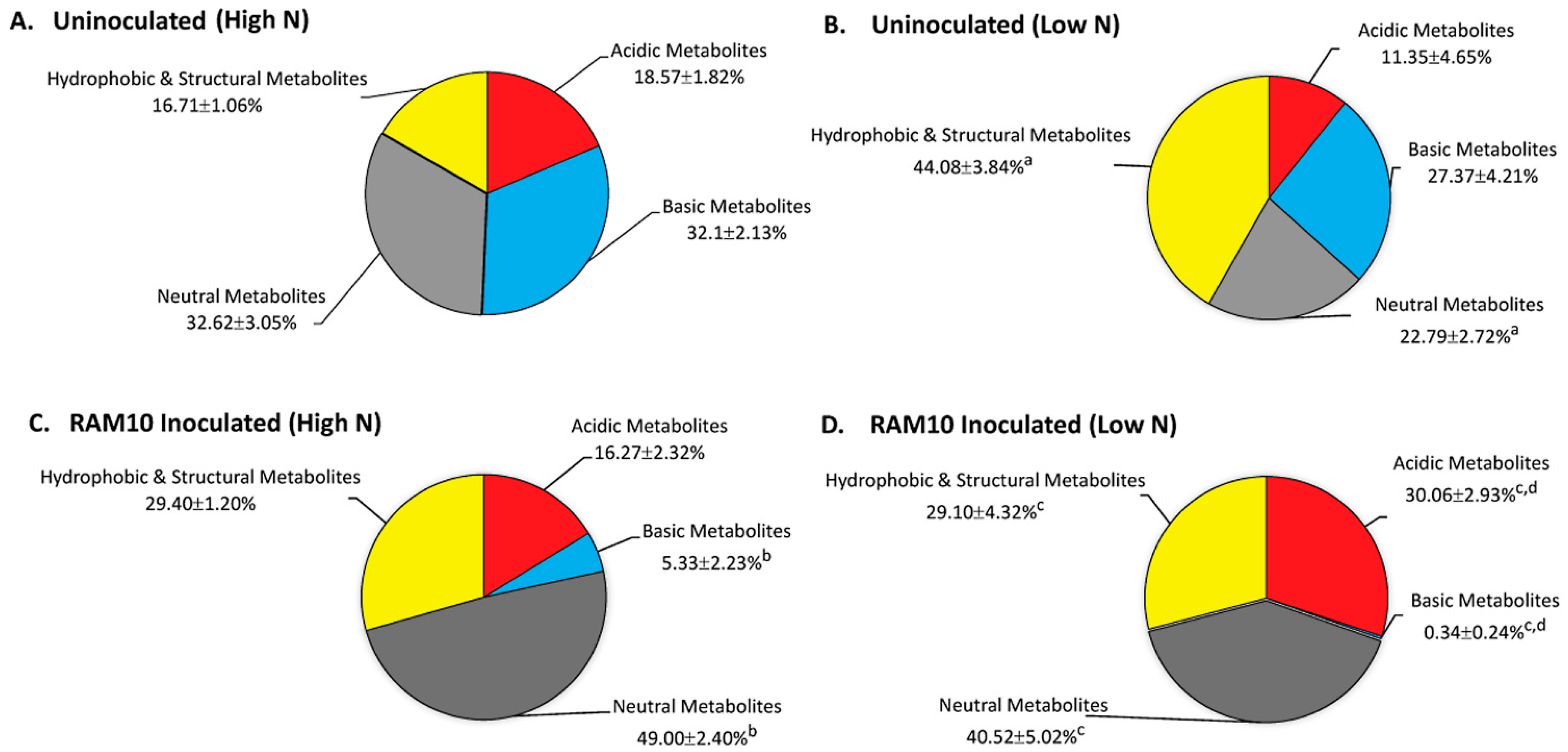
3.2. Microorganisms Can Influence Their Host’s Carbon Metabolism
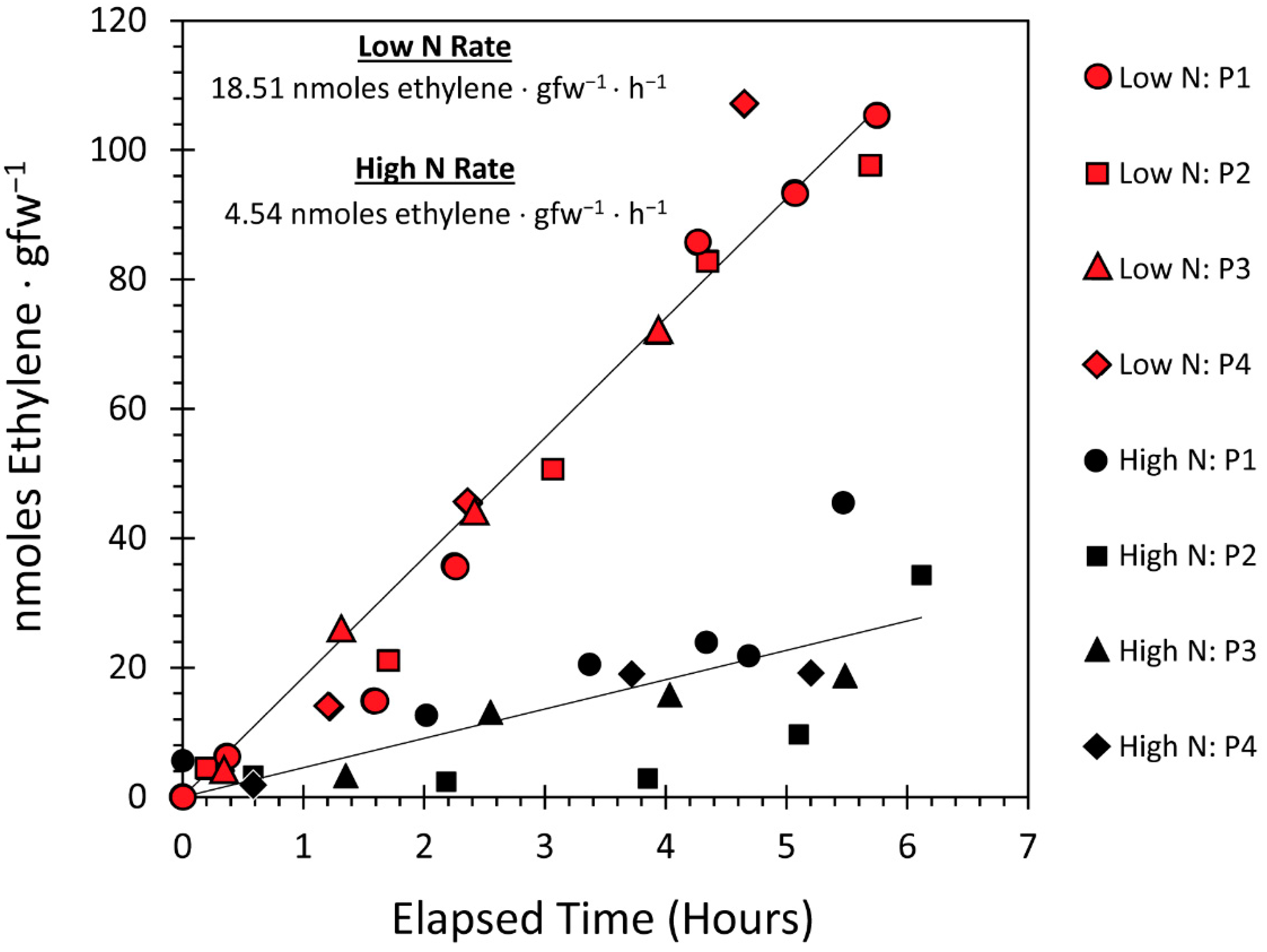
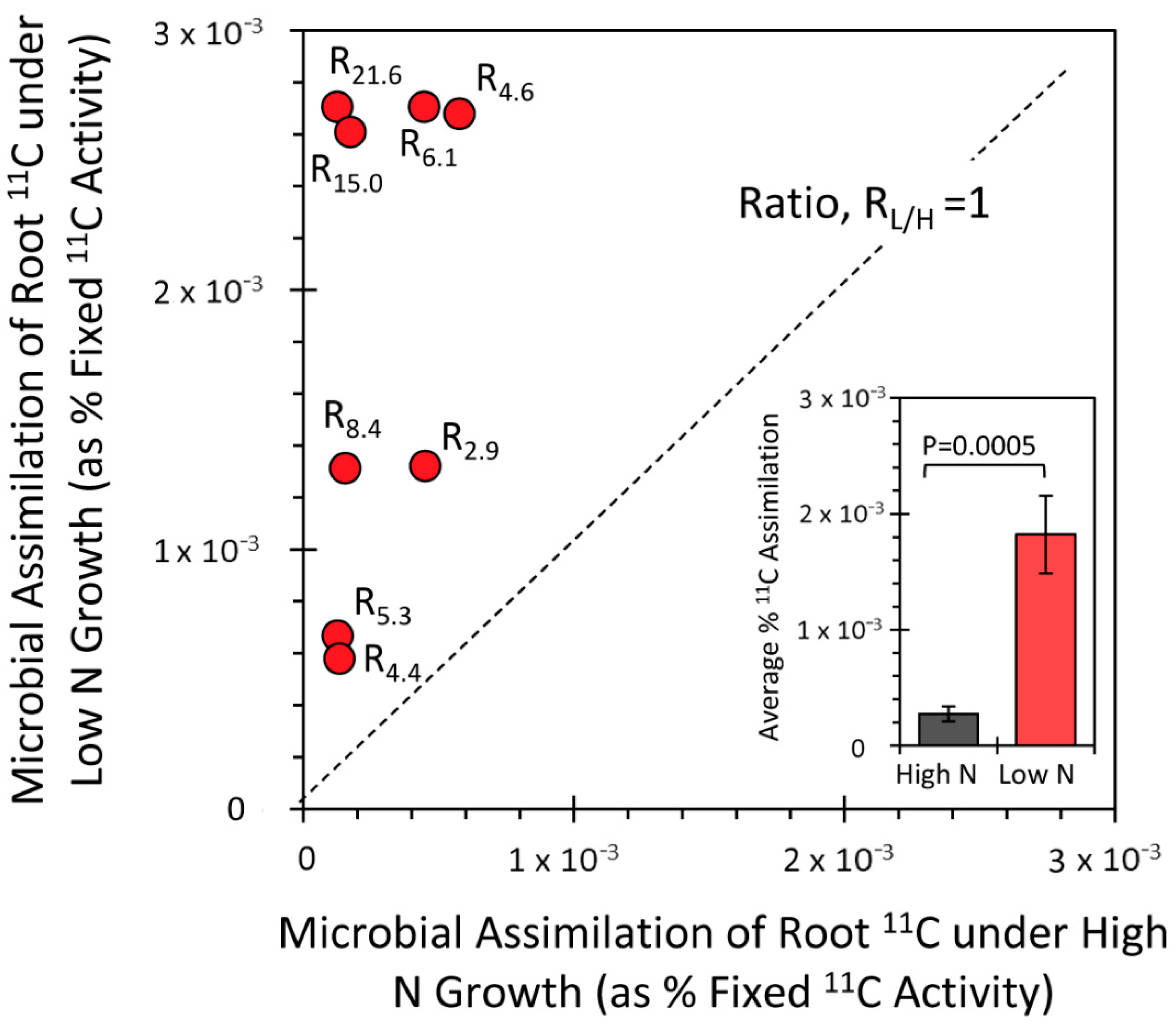
3.3. Effect of the N Environment on BNF and Its Influence on Microbial Demand for Plant-Borne Carbon
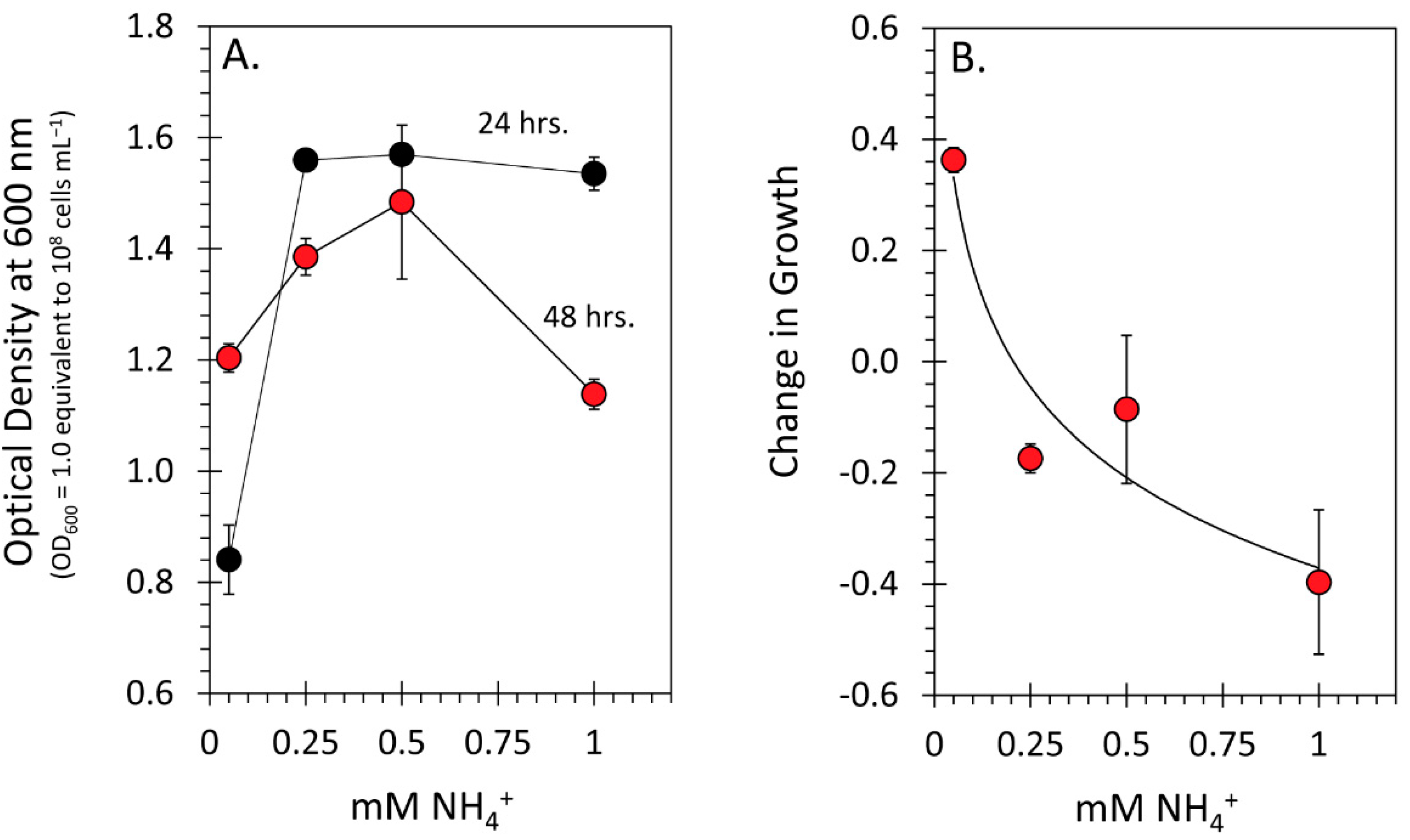
3.4. Effect of the N Environment on Microbial Growth Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, G.; Panwar, J.; Sayeed, A.M.; Jha, P.N. Endophytic nitrogen-fixing bacteria as biofertilizer. In Sustainable Agriculture Reviews; Lichthouse, E., Ed.; Springer Science+Business Media Dordrect: Berlin, Germany, 2012; pp. 183–221. [Google Scholar]
- Tkacz, A.; Poole, P. Role of root microbiota in plant productivity. J. Exp. Bot. 2015, 66, 2167–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [PubMed]
- do Amaral, F.P.; Agtuca, B.J.; Stacey, G. Setaria Root–Microbe Interactions. In Genetics and Genomics of Setaria; Springer: Berlin, Germany, 2017; pp. 239–250. [Google Scholar]
- Reinhold-Hurek, B.; Hurek, T. Life in grasses: Diazotrophic endophytes. Trends Microbiol. 1998, 6, 139–144. [Google Scholar] [CrossRef]
- Reinhold-Hurek, B.; Hurek, T. Living inside plants: Bacterial endophytes. Curr. Opin. Plant Biol. 2011, 14, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Pankievicz, V.C.S.; Amaral, F.P.; Santos, K.F.D.; Agtuca, B.; Xu, Y.; Schueller, M.J.; Arisi, A.C.M.; Steffens, M.B.R.; de Souza, E.M.; Pedrosa, F.O.; et al. Robust biological nitrogen fixation in a model grass-bacterial association. Plant J. 2015, 81, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludden, P.W.; Burris, R.H. Nitrogenase: Properties and regulation. In Biochemical Basis of Plant Breeding; Neyra, C.A., Ed.; CRC Press, Inc.: Boca Raton, FL, USA, 1986. [Google Scholar]
- Hartmann, A.; Fu, H.; Burris, R.H. Regulation of nitrogenase activity by ammonium chloride in Azospirillum Spp. J. Bacteriol. 1986, 165, 864–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, H.; Burris, R.H. Ammonium inhibition of nitrogenase activity in Herbaspirillum seropedicae. J. Bacteriol. 1989, 171, 3168–3175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cejudo, F.J.; Paneque, A. Short-term nitrate inhibition of nitrogen fixation in Azotobacter chroococcum. J. Bacteriol. 1986, 165, 240–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burris, R.H.; Hartmann, A.; Zhang, Y.; Fu, H. Control of nitrogenase in Azospirillum sp. Plant Soil 1991, 137, 127–134. [Google Scholar] [CrossRef]
- Nordlund, S.; Högbom, M. ADP-ribosylation, a mechanism regulating nitrogenase activity. FEBS J. 2013, 280, 3484–3490. [Google Scholar] [CrossRef] [PubMed]
- Ludden, P.W.; Burris, R.H. Activating factor for the iron protein of nitrogenase from Rhodospirillum rubrum. Science 1976, 194, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Ljungström, E.; Yates, M.G.; Nordlund, S. Purification of the activating enzyme of the Fe-protein of nitrogenase from Azospirillum brasilense. Biochim. Biophys. Acta 1989, 994, 210–214. [Google Scholar] [CrossRef]
- Baldani, J.I.; Baldani, V.L.D.; Seldin, L.; Doebereiner, J. Characterization of Herbaspirillum seropedicae gen. nov, sp. nov., a root-associated nitrogen-fixing bacterium. Int. J. Syst. Bacteriol. 1986, 36, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Waller, S.; Wilder, S.L.; Schueller, M.J.; Housh, A.B.; Ferrieri, R.A. Quantifying plant-borne carbon assimilation by root-associating bacteria. Microorganisms 2020, 8, 700. [Google Scholar] [CrossRef] [PubMed]
- Ferrieri, R.A.; Wolf, A.P. The chemistry of positron-emitting nucleogenic (hot) atoms with regard to preparation of labeled compounds of practical utility. Radiochim. Acta 1983, 34, 69–83. [Google Scholar] [CrossRef]
- Ferrieri, R.A. Production and application of synthetic precursors labeled with carbon-11 and fluorine-18. In Handbook of Radiopharmaceuticals: Radiochemistry and Applications; Welch, M.J., Redvanly, C.S., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2003. [Google Scholar]
- Ferrieri, R.A.; Gray, D.W.; Babst, B.A.; Schueller, M.J.; Schlyer, D.J.; Thorpe, M.R.; Orians, C.M.; Lerdau, M. Use of carbon-11 in Populus shows that exogenous jasmonic acid increases biosynthesis of isoprene from recently fixed carbon. Plant Cell Environ. 2005, 25, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Ferrieri, R.A.; Herman, E.; Babst, B.; Schueller, M.J. Managing the soil nitrogen cycle in agroecosystems. In Soil Nitrogen Uses and Environmental Impacts 2018 Mar 15; CRC Press: Boca Raton, FL, USA, 2018; pp. 343–360. [Google Scholar]
- Housh, A.; Powell, G.; Scott, S.; Anstaett, A.; Gerheart, A.; Benoit, M.; Guthrie, J.M.; Higgins, B.; Wilder, S.L.; Schueller, M.J.; et al. Mutant Azospirillum brasilense bacterium elicit beneficial physiological and metabolic responses in Zea mays that contribute to increased host iron assimilation. ISME J. 2021, 15, 1505–1522. [Google Scholar] [CrossRef] [PubMed]
- Agtuca, B.; Rieger, E.; Hilger, K.; Song, L.; Robert, C.A.M.; Erb, M.; Karve, A.; Ferrieri, R.A. Carbon-11 reveals opposing roles of auxin and salicylate in regulating leaf physiology, leaf metabolism and resource allocation patterns that impact root growth in Zea mays. J. Plant Growth Regul. 2014, 33, 328–339. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waller, S.; Wilder, S.L.; Schueller, M.J.; Housh, A.B.; Scott, S.; Benoit, M.; Powell, A.; Powell, G.; Ferrieri, R.A. Examining the Effects of the Nitrogen Environment on Growth and N2-Fixation of Endophytic Herbaspirillum seropedicae in Maize Seedlings by Applying 11C Radiotracing. Microorganisms 2021, 9, 1582. https://doi.org/10.3390/microorganisms9081582
Waller S, Wilder SL, Schueller MJ, Housh AB, Scott S, Benoit M, Powell A, Powell G, Ferrieri RA. Examining the Effects of the Nitrogen Environment on Growth and N2-Fixation of Endophytic Herbaspirillum seropedicae in Maize Seedlings by Applying 11C Radiotracing. Microorganisms. 2021; 9(8):1582. https://doi.org/10.3390/microorganisms9081582
Chicago/Turabian StyleWaller, Spenser, Stacy L. Wilder, Michael J. Schueller, Alexandra B. Housh, Stephanie Scott, Mary Benoit, Avery Powell, Garren Powell, and Richard A. Ferrieri. 2021. "Examining the Effects of the Nitrogen Environment on Growth and N2-Fixation of Endophytic Herbaspirillum seropedicae in Maize Seedlings by Applying 11C Radiotracing" Microorganisms 9, no. 8: 1582. https://doi.org/10.3390/microorganisms9081582
APA StyleWaller, S., Wilder, S. L., Schueller, M. J., Housh, A. B., Scott, S., Benoit, M., Powell, A., Powell, G., & Ferrieri, R. A. (2021). Examining the Effects of the Nitrogen Environment on Growth and N2-Fixation of Endophytic Herbaspirillum seropedicae in Maize Seedlings by Applying 11C Radiotracing. Microorganisms, 9(8), 1582. https://doi.org/10.3390/microorganisms9081582





