Experimental Selection of Paromomycin Resistance in Leishmania donovani Amastigotes Induces Variable Genomic Polymorphisms
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Amastigote Susceptibilty to Paromomycin and Amphotericin B
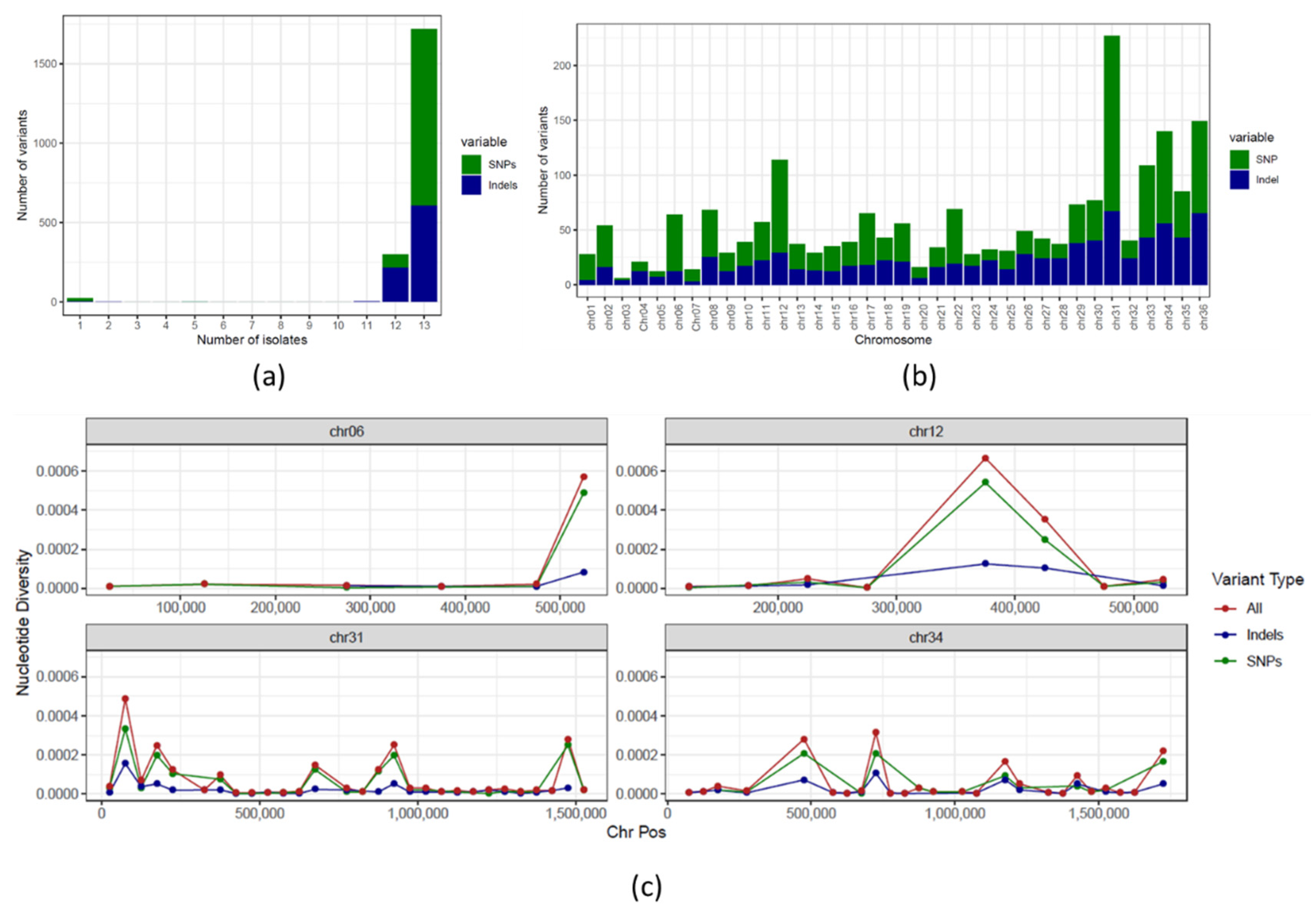
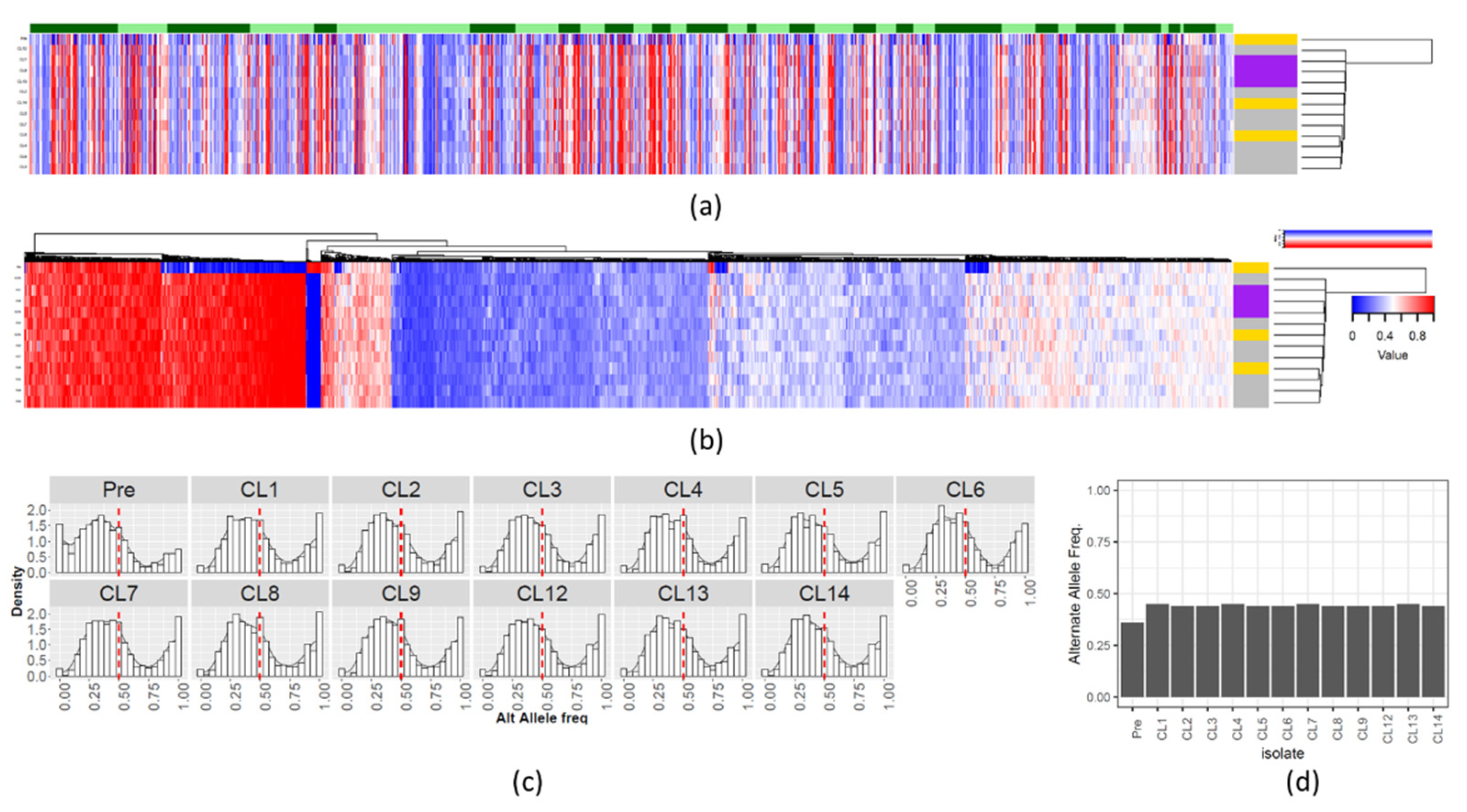
3.2. SNPs and Indels
3.3. Chromosomal Copy Number Variation (CCNV)
3.4. Gene Copy Number Variations (CNV)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maslov, D.A.; Opperdoes, F.R.; Kostygov, A.Y.; Hashimi, H.; Lukeš, J.; Yurchenko, V. Recent advances in trypanosomatid research: Genome organization, expression, metabolism, taxonomy and evolution. Parasitology 2018, 146, 1–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musa, A.M.; Younis, B.; Fadlalla, A.; Royce, C.; Balasegaram, M.; Wasunna, M.; Hailu, A.; Edwards, T.; Omollo, R.; Mudawi, M.; et al. Paromomycin for the treatment of visceral leishmaniasis in Sudan: A randomized, open-label, dose-finding study. PLoS Negl. Trop. Dis. 2010, 4, e855. [Google Scholar] [CrossRef] [PubMed]
- Hailu, A.; Musa, A.; Wasunna, M.; Balasegaram, M.; Yifru, S.; Mengistu, G.; Hurissa, Z.; Hailu, W.; Weldegebreal, T.; Tesfaye, S.; et al. Geographical variation in the response of visceral leishmaniasis to paromomycin in East Africa: A multicentre, open-label, randomized trial. PLoS Negl. Trop. Dis. 2010, 4, e709. [Google Scholar] [CrossRef] [Green Version]
- van Griensven, J.; Balasegaram, M.; Meheus, F.; Alvar, J.; Lynen, L.; Boelaert, M. Combination therapy for visceral leishmaniasis. Lancet Infect. Dis. 2010, 10, 184–194. [Google Scholar] [CrossRef]
- el-On, J.; Bazarsky, E.; Sneir, R. Leishmania major: In vitro and in vivo anti-leishmanial activity of paromomycin ointment (Leshcutan) combined with the immunomodulator Imiquimod. Exp. Parasitol. 2007, 116, 156–162. [Google Scholar] [CrossRef]
- Scott, J.A.; Davidson, R.N.; Moody, A.H.; Grant, H.R.; Felmingham, D.; Scott, G.M.; Olliaro, P.; Bryceson, A.D. Aminosidine (paromomycin) in the treatment of leishmaniasis imported into the United Kingdom. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 617–619. [Google Scholar] [CrossRef]
- Wiwanitkit, V. Interest in paromomycin for the treatment of visceral leishmaniasis (kala-azar). Clin. Risk Manag. 2012, 8, 323–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrickx, S.; Boulet, G.; Mondelaers, A.; Dujardin, J.C.; Rijal, S.; Lachaud, L.; Cos, P.; Delputte, P.; Maes, L. Experimental selection of paromomycin and miltefosine resistance in intracellular amastigotes of Leishmania donovani and L. infantum. Parasitol. Res. 2014, 113, 1875–1881. [Google Scholar] [CrossRef]
- Hendrickx, S.; Inocencio da Luz, R.A.; Bhandari, V.; Kuypers, K.; Shaw, C.D.; Lonchamp, J.; Salotra, P.; Carter, K.; Sundar, S.; Rijal, S.; et al. Experimental induction of paromomycin resistance in antimony-resistant strains of L. donovani: Outcome dependent on in vitro selection protocol. PLoS Negl. Trop. Dis. 2012, 6, e1664. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, S.; Mondelaers, A.; Eberhardt, E.; Delputte, P.; Cos, P.; Maes, L. In Vivo Selection of Paromomycin and Miltefosine Resistance in Leishmania donovani and L. infantum in a Syrian Hamster Model. Antimicrob. Agents Chemother. 2015, 59, 4714–4718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhingran, A.; Chawla, B.; Saxena, S.; Barrett, M.P.; Madhubala, R. Paromomycin: Uptake and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 2009, 164, 111–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrickx, S.; Beyers, J.; Mondelaers, A.; Eberhardt, E.; Lachaud, L.; Delputte, P.; Cos, P.; Maes, L. Evidence of a drug-specific impact of experimentally selected paromomycin and miltefosine resistance on parasite fitness in Leishmania infantum. J. Antimicrob. Chemother. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslett, M.; Aurrecoechea, C.; Berriman, M.; Brestelli, J.; Brunk, B.P.; Carrington, M.; Depledge, D.P.; Fischer, S.; Gajria, B.; Gao, X.; et al. TriTrypDB: A functional genomic resource for the Trypanosomatidae. Nucleic Acids Res. 2009, 38, D457–D462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [Green Version]
- Garrison, E.; Marth, G. Haplotype-Based Variant Detection from Short-Read Sequencing. Available online: https://arxiv.org/pdf/1207.3907.pdf (accessed on 19 July 2021).
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef] [Green Version]
- Reis-Cunha, J.L.; Baptista, R.P.; Rodrigues-Luiz, G.F.; Coqueiro-dos-Santos, A.; Valdivia, H.O.; de Almeida, L.V.; Cardoso, M.S.; D’Ávila, D.A.; Dias, F.H.C.; Fujiwara, R.T.; et al. Whole genome sequencing of Trypanosoma cruzi field isolates reveals extensive genomic variability and complex aneuploidy patterns within TcII DTU. BMC Genom. 2018, 19, 816. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Subgroup, G.P.D.P. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/.2019 (accessed on 19 July 2021).
- Wickham, H. Ggplot2, 2nd ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Dumetz, F.; Imamura, H.; Sanders, M.; Seblova, V.; Myskova, J.; Pescher, P.; Vanaerschot, M.; Meehan, C.J.; Cuypers, B.; De Muylder, G.; et al. Modulation of Aneuploidy in Leishmania donovaniduring Adaptation to Different In Vitro and In Vivo Environments and Its Impact on Gene Expression. mBio 2017, 8, e00599-17. [Google Scholar] [CrossRef] [Green Version]
- Prieto Barja, P.; Pescher, P.; Bussotti, G.; Dumetz, F.; Imamura, H.; Kedra, D.; Domagalska, M.; Chaumeau, V.; Himmelbauer, H.; Pages, M.; et al. Haplotype selection as an adaptive mechanism in the protozoan pathogen Leishmania donovani. Nat. Ecol. Evol. 2017, 1, 1961–1969. [Google Scholar] [CrossRef]
- Subramanian, A.; Sarkar, R.R. Comparison of codon usage bias across Leishmania and Trypanosomatids to understand mRNA secondary structure, relative protein abundance and pathway functions. Genomics 2015, 106, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Das, S.; Das, S.; Shadab, M.; Chowdhury, R.; Tripathy, S.; Ali, N. Genome Plasticity in Cultured Leishmania donovani: Comparison of Early and Late Passages. Front. Microbiol. 2018, 9, 1279. [Google Scholar] [CrossRef] [PubMed]
- Camacho, E.; González-de la Fuente, S.; Rastrojo, A.; Peiró-Pastor, R.; Solana, J.C.; Tabera, L.; Gamarro, F.; Carrasco-Ramiro, F.; Requena, J.M.; Aguado, B. Complete assembly of the Leishmania donovani (HU3 strain) genome and transcriptome annotation. Sci. Rep. 2019, 9, 6127. [Google Scholar] [CrossRef]
- Davis, B.D. Mechanism of bactericidal action of aminoglycosides. Microbiol. Rev. 1987, 51, 341–350. [Google Scholar] [CrossRef]
- Davis, B.D.; Chen, L.L.; Tai, P.C. Misread protein creates membrane channels: An essential step in the bactericidal action of aminoglycosides. Proc. Natl. Acad. Sci. USA 1986, 83, 6164–6168. [Google Scholar] [CrossRef] [Green Version]
- Fong, D.; Chan, M.M.; Rodriguez, R.; Gately, L.J.; Berman, J.D.; Grogl, M. Paromomycin resistance in Leishmania tropica: Lack of correlation with mutation in the small subunit ribosomal RNA gene. Am. J. Trop. Med. Hyg. 1994, 51, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Maarouf, M.; Adeline, M.T.; Solignac, M.; Vautrin, D.; Robert-Gero, M. Development and characterization of paromomycin-resistant Leishmania donovani promastigotes. Parasite 1998, 5, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Maarouf, M.; de Kouchkovsky, Y.; Brown, S.; Petit, P.X.; Robert-Gero, M. In vivo interference of paromomycin with mitochondrial activity of Leishmania. Exp. Cell Res. 1997, 232, 339–348. [Google Scholar] [CrossRef]
- Hobbie, S.N.; Kaiser, M.; Schmidt, S.; Shcherbakov, D.; Janusic, T.; Brun, R.; Bottger, E.C. Genetic Reconstruction of Protozoan rRNA Decoding Sites Provides a Rationale for Paromomycin Activity against Leishmania and Trypanosoma. PLoS Negl. Trop. Dis. 2011, 5, e1161. [Google Scholar] [CrossRef]
- Horvath, A.; Nebohacova, M.; Lukes, J.; Maslov, D.A. Unusual polypeptide synthesis in the kinetoplast-mitochondria from Leishmania tarentolae. Identification of individual de novo translation products. J. Biol. Chem. 2002, 277, 7222–7230. [Google Scholar] [CrossRef] [Green Version]
- Chawla, B.; Jhingran, A.; Panigrahi, A.; Stuart, K.D.; Madhubala, R. Paromomycin affects translation and vesicle-mediated trafficking as revealed by proteomics of paromomycin -susceptible -resistant Leishmania donovani. PLoS ONE 2011, 6, e26660. [Google Scholar] [CrossRef]
- Sundar, S.; Jha, T.K.; Thakur, C.P.; Sinha, P.K.; Bhattacharya, S.K. Injectable paromomycin for Visceral leishmaniasis in India. N. Engl. J. Med. 2007, 356, 2571–2581. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Banerjee, A.; Kamran, M.; Ejazi, S.A.; Asad, M.; Ali, N.; Chakrabarti, S. A chemical inhibitor of heat shock protein 78 (HSP78) from Leishmania donovani represents a potential antileishmanial drug candidate. J. Biol. Chem. 2020, 295, 9934–9947. [Google Scholar] [CrossRef]
- Mukherjee, S.; Xu, W.; Hsu, F.F.; Patel, J.; Huang, J.; Zhang, K. Sterol methyltransferase is required for optimal mitochondrial function and virulence in Leishmania major. Mol. Microbiol. 2019, 111, 65–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pountain, A.W.; Weidt, S.K.; Regnault, C.; Bates, P.A.; Donachie, A.M.; Dickens, N.J.; Barrett, M.P. Genomic instability at the locus of sterol C24-methyltransferase promotes amphotericin B resistance in Leishmania parasites. PLoS Negl. Trop. Dis. 2019, 13, e0007052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastrojo, A.; García-Hernández, R.; Vargas, P.; Camacho, E.; Corvo, L.; Imamura, H.; Dujardin, J.C.; Castanys, S.; Aguado, B.; Gamarro, F.; et al. Genomic and transcriptomic alterations in Leishmania donovani lines experimentally resistant to antileishmanial drugs. Int. J. Parasitology. Drugs Drug Resist. 2018, 8, 246–264. [Google Scholar] [CrossRef]
- Hendrickx, S.; Leemans, A.; Mondelaers, A.; Rijal, S.; Khanal, B.; Dujardin, J.C.; Delputte, P.; Cos, P.; Maes, L. Comparative Fitness of a Parent Leishmania donovani Clinical Isolate and Its Experimentally Derived Paromomycin-Resistant Strain. PLoS ONE 2015, 10, e0140139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrickx, S.; Van Bockstal, L.; Aslan, H.; Sadlova, J.; Maes, L.; Volf, P.; Caljon, G. Transmission potential of paromomycin-resistant Leishmania infantum and Leishmania donovani. J. Antimicrob. Chemother. 2019. [Google Scholar] [CrossRef] [PubMed]
- Silva-Jardim, I.; Horta, M.F.; Ramalho-Pinto, F.J. The Leishmania chagasi proteasome: Role in promastigotes growth and amastigotes survival within murine macrophages. Acta Trop. 2004, 91, 121–130. [Google Scholar] [CrossRef]
- Shaw, C.D.; Imamura, H.; Downing, T.; Blackburn, G.; Westrop, G.D.; Cotton, J.A.; Berriman, M.; Sanders, M.; Rijal, S.; Coombs, G.H.; et al. Genomic and Metabolomic Polymorphism among Experimentally Selected Paromomycin-Resistant Leishmania donovani Strains. Antimicrob. Agents Chemother. 2019, 64. [Google Scholar] [CrossRef] [Green Version]
- Maarouf, M.; Lawrence, F.; Brown, S.; Robert-Gero, M. Biochemical alterations in paromomycin-treated Leishmania donovani promastigotes. Parasitol. Res. 1997, 83, 198–202. [Google Scholar] [CrossRef]
- Gazanion, É.; Fernández-Prada, C.; Papadopoulou, B.; Leprohon, P.; Ouellette, M. Cos-Seq for high-throughput identification of drug target and resistance mechanisms in the protozoan parasite Leishmania. Proc. Natl. Acad. Sci. USA 2016, 113, E3012–E3021. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Nowicki, C.; Blankenfeldt, W. Structural basis for the relaxed substrate selectivity of Leishmania mexicana broad specificity aminotransferase. Mol. Biochem. Parasitol. 2015, 202, 34–37. [Google Scholar] [CrossRef]
- Palmieri, F. The mitochondrial transporter family SLC25: Identification, properties and physiopathology. Mol. Asp. Med. 2013, 34, 465–484. [Google Scholar] [CrossRef]
- Bahl, S.; Parashar, S.; Malhotra, H.; Raje, M.; Mukhopadhyay, A. Functional Characterization of Monomeric GTPase Rab1 in the Secretory Pathway of Leishmania. J. Biol. Chem. 2015, 290, 29993–30005. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, C.; San Francisco, J.; Gutiérrez, B.; González, J. Role of the Ubiquitin-Proteasome Systems in the Biology and Virulence of Protozoan Parasites. Biomed. Res. Int. 2015, 2015, 141526. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, V.; Sundar, S.; Dujardin, J.C.; Salotra, P. Elucidation of cellular mechanisms involved in experimental paromomycin resistance in Leishmania donovani. Antimicrob. Agents Chemother. 2014, 58, 2580–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivier, M.; Atayde, V.D.; Isnard, A.; Hassani, K.; Shio, M.T. Leishmania virulence factors: Focus on the metalloprotease GP63. Microbes. Infect 2012, 14, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Kühberger, R.; Piepersberg, W.; Petzet, A.; Buckel, P.; Böck, A. Alteration of ribosomal protein L6 in gentamicin-resistant strains of Escherichia coli. Effects on fidelity of protein synthesis. Biochemistry 1979, 18, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Shah, P.; Baharia, R.K.; Tandon, R.; Khare, P.; Sundar, S.; Sahasrabuddhe, A.A.; Siddiqi, M.I.; Dube, A. Over-expression of 60s ribosomal L23a is associated with cellular proliferation in SAG resistant clinical isolates of Leishmania donovani. PLoS Negl. Trop. Dis. 2013, 7, e2527. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.; Gonçalves, L.O.; Liarte, D.B.; Lima, D.A.; Guimarães, F.G.; de Melo Resende, D.; Santi, A.M.M.; de Oliveira, L.M.; Velloso, J.P.L.; Delfino, R.G.; et al. Comparative transcriptomic analysis of antimony resistant and susceptible Leishmania infantum lines. Parasites Vectors 2020, 13, 600. [Google Scholar] [CrossRef] [PubMed]
- De Pablos, L.M.; Ferreira, T.R.; Dowle, A.A.; Forrester, S.; Parry, E.; Newling, K.; Walrad, P.B. The mRNA-bound Proteome of Leishmania mexicana: Novel Genetic Insight into an Ancient Parasite. Mol. Cell. Proteom. Mcp 2019, 18, 1271–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppock, D.L.; Kopman, C.; Scandalis, S.; Gilleran, S. Preferential gene expression in quiescent human lung fibroblasts. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 1993, 4, 483–493. [Google Scholar]
- Grossman, I.; Alon, A.; Ilani, T.; Fass, D. An inhibitory antibody blocks the first step in the dithiol/disulfide relay mechanism of the enzyme QSOX1. J. Mol. Biol. 2013, 425, 4366–4378. [Google Scholar] [CrossRef]
- Verma, A.; Bhandari, V.; Deep, D.K.; Sundar, S.; Dujardin, J.C.; Singh, R.; Salotra, P. Transcriptome profiling identifies genes/pathways associated with experimental resistance to paromomycin in Leishmania donovani. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 370–377. [Google Scholar] [CrossRef]


| Strain | PMM IC50 (µM) 1 | AmB IC50 (nM) |
|---|---|---|
| Wild-type (preselection) | 45.0 ± 5.6 | 9.7 ± 0.2 |
| Clone 1 | 417.4 ± 15.1 | 20.9 ± 6.6 |
| Clone 2 | 196.8 ±11.2 | 15.4 ± 2.0 |
| Clone 3 | 213.0 ± 7.3 | 19.6 ± 4.5 |
| Clone 4 | 157.0 ± 9.6 | 22.4 ± 6.1 |
| Clone 5 | 129.7 ± 13.1 | 10.5 ± 1.0 |
| Clone 6 | 57.1 ± 6.8 | 11.8 ± 2.1 |
| Clone 7 | 154.0 ± 14.9 | 22.0 ± 5.1 |
| Clone 8 | 313.1 ± 14.3 | 15.3 ± 0.9 |
| Clone 9 | 132.5 ± 9.0 | 13.8 ± 0.8 |
| Clone 12 | 171.3 ± 5.7 | 13.7 ± 1.7 |
| Clone 13 | 310.2 ± 11.8 | 17.0 ± 3.5 |
| Clone 14 | 71.2 ± 2.8 | ND |
| Gene ID | Chr | Pos1 | Pos2 | Gene Annotation | Correlation | p | Max | Min | Max − Min |
|---|---|---|---|---|---|---|---|---|---|
| LdBPK_020370.1 | chr02 | 171561 | 171713 | hypothetical protein unknown function | 0.73 | 4.96 × 10−3 | 2.09 | 0.88 | 1.21 |
| LdBPK_070160.1 | chr07 | 67067 | 67567 | hypothetical protein conserved | 0.73 | 4.54 × 10−3 | 1.21 | 0.73 | 0.48 |
| LdBPK_070980.1 | chr07 | 404916 | 406232 | hypothetical protein conserved | 0.74 | 3.96 × 10−3 | 1.13 | 0.85 | 0.28 |
| LdBPK_141050.1 | chr14 | 424294 | 425424 | ADP/ATP mitochondrial carrier-like protein | 0.76 | 2.45 × 10−3 | 1.52 | 1.18 | 0.34 |
| LdBPK_180600.1 | chr18 | 242208 | 242483 | hypothetical protein | 0.76 | 2.47 × 10−3 | 1.15 | 0.77 | 0.38 |
| LdBPK_230470.1 | chr23 | 162446 | 163672 | aldose 1-epimerase-like protein | 0.71 | 6.97 × 10−3 | 1.54 | 1.31 | 0.23 |
| LdBPK_250660.1 | chr25 | 217458 | 219455 | predicted zinc finger protein | 0.76 | 2.84 × 10−3 | 1.17 | 0.96 | 0.21 |
| LdBPK_250800.1 | chr25 | 276286 | 277551 | hypothetical protein unknown function | 0.77 | 2.10 × 10−3 | 1.09 | 0.89 | 0.2 |
| LdBPK_270620.1 | chr27 | 239023 | 239625 | ras-related protein RAB1A putative | 0.76 | 2.58 × 10−3 | 1.3 | 0.78 | 0.52 |
| LdBPK_271080.1 | chr27 | 441116 | 442363 | RING-H2 zinc finger putative | 0.76 | 2.47 × 10−3 | 1.13 | 0.88 | 0.25 |
| LdBPK_271370.1 | chr27 | 539921 | 540925 | proteasome regulatory non-ATP-ase subunit 3 putative | 0.70 | 7.57 × 10−3 | 1.26 | 0.84 | 0.42 |
| LdBPK_271520.1 | chr27 | 591692 | 592333 | Eukaryotic translation initiation factor 4E−1 | 0.71 | 6.85 × 10−3 | 1.15 | 0.89 | 0.26 |
| LdBPK_271540.1 | chr27 | 607146 | 609893 | BRO1-like domain/ALIX V-shaped domain binding to HIV putative | 0.75 | 3.40 × 10−3 | 1.19 | 0.96 | 0.23 |
| LdBPK_280530.1 | chr28 | 180433 | 182571 | TPR repeat putative | 0.72 | 5.12 × 10−3 | 1.06 | 0.87 | 0.19 |
| LdBPK_280600.1 | chr28 | 209904 | 211604 | major surface protease gp63 putative | 0.72 | 5.67 × 10−3 | 1.02 | 0.86 | 0.16 |
| LdBPK_280630.1 | chr28 | 222510 | 224015 | hypothetical protein unknown function | 0.75 | 3.31 × 10−3 | 1.06 | 0.9 | 0.16 |
| LdBPK_301200.1 | chr30 | 387893 | 388444 | hypothetical protein conserved | 0.76 | 2.82 × 10−3 | 1.04 | 0.82 | 0.22 |
| LdBPK_302290.1 | chr30 | 855859 | 857973 | tubulin-tyrosine ligase-like protein | 0.81 | 8.65 × 10−4 | 1.08 | 0.88 | 0.2 |
| LdBPK_302310.1 | chr30 | 864017 | 867931 | hypothetical protein conserved | 0.74 | 3.75 × 10−3 | 1.02 | 0.9 | 0.12 |
| LdBPK_302740.1 | chr30 | 1031040 | 1032266 | TPR domain protein conserved | 0.73 | 4.95 × 10−3 | 1 | 0.84 | 0.16 |
| LdBPK_321520.1 | chr32 | 572415 | 582050 | phosphatidylinositol 3-related kinase putative | 0.88 | 7.93 × 10−5 | 1.02 | 0.93 | 0.09 |
| LdBPK_330670.1 | chr33 | 209670 | 210923 | intraflagellar transport protein 57/55 putative | 0.71 | 6.32 × 10−3 | 1.44 | 1.2 | 0.24 |
| LdBPK_330890.1 | chr33 | 292053 | 294803 | hypothetical protein unknown function | 0.76 | 2.47 × 10−3 | 1.45 | 1.25 | 0.2 |
| LdBPK_151060.1 | chr33 | 429431 | 429940 | 60S ribosomal protein L6 putative | 0.71 | 6.78 × 10−3 | 1.36 | 0.98 | 0.38 |
| LdBPK_331560.1 | chr33 | 594808 | 595194 | RNA-binding protein putative | 0.78 | 1.70 × 10−3 | 1.58 | 1.21 | 0.37 |
| LdBPK_332810.1 | chr33 | 1086018 | 1089704 | hypothetical protein conserved | 0.71 | 7.02 × 10−3 | 1.43 | 1.25 | 0.18 |
| LdBPK_332950.1 | chr33 | 1165769 | 1167190 | transcription elongation factor-like protein | 0.71 | 6.23 × 10−3 | 1.49 | 1.24 | 0.25 |
| LdBPK_341840.1 | chr34 | 802817 | 803974 | protein kinase putative | 0.72 | 5.11 × 10−3 | 1.04 | 0.8 | 0.24 |
| LdBPK_343220.1 | chr34 | 1362570 | 1364477 | DNA topoisomerase IB large subunit | 0.78 | 1.48 × 10−3 | 1.02 | 0.84 | 0.18 |
| LdBPK_343390.1 | chr34 | 1417106 | 1417627 | Complex 1 protein (LYR family) putative | 0.74 | 4.04 × 10−3 | 1.09 | 0.81 | 0.28 |
| LdBPK_361330.1 | chr36 | 475562 | 475984 | hypothetical protein (fragment) | 0.86 | 1.79 × 10−4 | 0.52 | 0.27 | 0.25 |
| LdBPK_361780.1 | chr36 | 673858 | 675498 | hypothetical protein conserved | 0.88 | 8.22 × 10−5 | 1.12 | 0.89 | 0.23 |
| LdBPK_362310.1 | chr36 | 878592 | 880163 | protein-tyrosine phosphatase 1-like protein | 0.73 | 4.69 × 10−3 | 1.02 | 0.9 | 0.12 |
| LdBPK_241390.1 | chr24 | 496214 | 498880 | hypothetical protein conserved | −0.80 | 1.13 × 10−3 | 1.27 | 0.94 | 0.33 |
| LdBPK_261490.1 | chr26 | 541997 | 546778 | hypothetical protein unknown function | −0.72 | 5.61 × 10−3 | 1.65 | 1.37 | 0.28 |
| LdBPK_261660.1 | chr26 | 599794 | 600579 | hypothetical protein conserved | −0.79 | 1.19 × 10−3 | 1.61 | 1.25 | 0.36 |
| LdBPK_291180.1 | chr29 | 429699 | 429965 | ribosomal protein L1aputative (fragment) | −0.81 | 7.78 × 10−4 | 1.25 | 0.89 | 0.36 |
| LdBPK_300440.1 | chr30 | 142693 | 144375 | quiescin sulfhydryl oxidase putative | −0.72 | 5.96 × 10−3 | 1 | 0.81 | 0.19 |
| LdBPK_363720.1 | chr36 | 1394227 | 1395405 | phytoene synthase putative | −0.79 | 1.33 × 10−3 | 1.06 | 0.79 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendrickx, S.; Reis-Cunha, J.L.; Forrester, S.; Jeffares, D.C.; Caljon, G. Experimental Selection of Paromomycin Resistance in Leishmania donovani Amastigotes Induces Variable Genomic Polymorphisms. Microorganisms 2021, 9, 1546. https://doi.org/10.3390/microorganisms9081546
Hendrickx S, Reis-Cunha JL, Forrester S, Jeffares DC, Caljon G. Experimental Selection of Paromomycin Resistance in Leishmania donovani Amastigotes Induces Variable Genomic Polymorphisms. Microorganisms. 2021; 9(8):1546. https://doi.org/10.3390/microorganisms9081546
Chicago/Turabian StyleHendrickx, Sarah, João Luís Reis-Cunha, Sarah Forrester, Daniel C. Jeffares, and Guy Caljon. 2021. "Experimental Selection of Paromomycin Resistance in Leishmania donovani Amastigotes Induces Variable Genomic Polymorphisms" Microorganisms 9, no. 8: 1546. https://doi.org/10.3390/microorganisms9081546
APA StyleHendrickx, S., Reis-Cunha, J. L., Forrester, S., Jeffares, D. C., & Caljon, G. (2021). Experimental Selection of Paromomycin Resistance in Leishmania donovani Amastigotes Induces Variable Genomic Polymorphisms. Microorganisms, 9(8), 1546. https://doi.org/10.3390/microorganisms9081546







