How to Handle CT-Guided Abscess Drainages in Microbiological Analyses? Sterile Vials vs. Blood Culture Bottles for Transport and Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Descriptive Parameters
2.4. CT Imaging Protocol
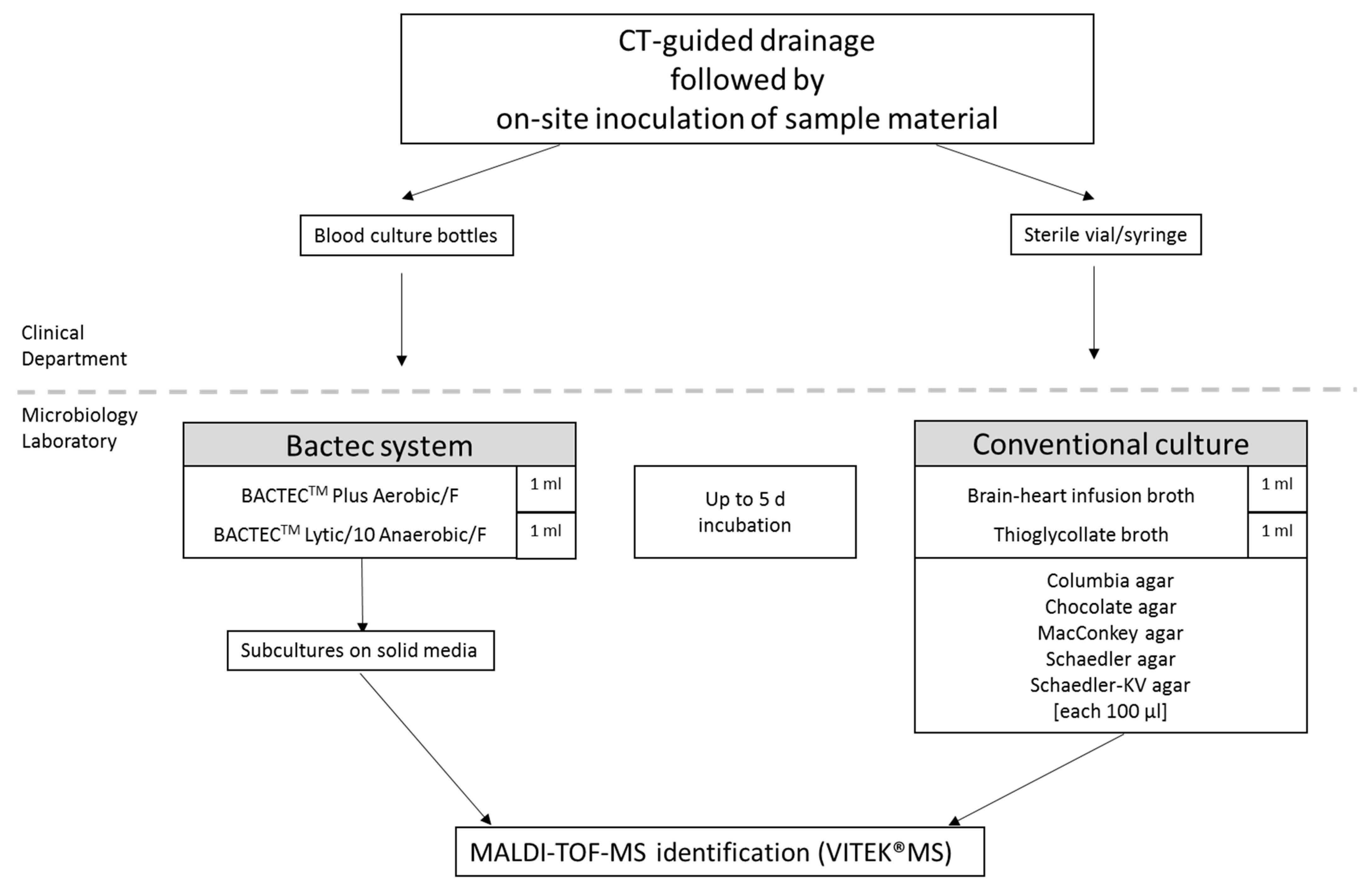
2.5. Collection and Specimens Processing
2.6. Outcome Parameters
2.7. Statistical Assessment
2.8. Ethical Clearance
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duszak, R.L.; Levy, J.M.; Akins, E.W.; Bakal, C.W.; Denny, D.D.; Martin, L.G.; van Moore, A.; Pentecost, M.J.; Roberts, A.C.; Vogelzang, R.L.; et al. Percutaneous catheter drainage of infected intra-abdominal fluid collections. American College of Radiology. ACR Appropriateness Criteria. Radiology 2000, 215, 1067–1075. [Google Scholar] [PubMed]
- Johnson, W.C.; Gerzof, S.G.; Robbins, A.H.; Nabseth, D.C. Treatment of Abdominal Abscesses. Ann. Surg. 1981, 194, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.S.; Wilson, S.E. Intraabdominal Abscesses: Image-Guided Diagnosis and Therapy. Clin. Infect. Dis. 1996, 23, 28–36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sartelli, M.; Chichom-Mefire, A.; Labricciosa, F.M.; Hardcastle, T.; Abu-Zidan, F.M.; Adesunkanmi, A.K.; Ansaloni, L.; Bala, M.; Balogh, Z.J.; Beltrán, M.A.; et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J. Emerg. Surg. 2017, 12, 1–34. [Google Scholar] [CrossRef]
- McGillen, K.; Boos, J.; Nathavitharana, R.; Brook, A.; Sun, M.R.; Siewert, B.; Raptopoulos, V.; Kane, R.; Sheiman, R.; Brook, O.R. Diagnostic yield and clinical impact of microbiologic diagnosis from CT-guided drainage in patients previously treated with empiric antibiotics. Abdom. Radiol. 2017, 42, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Rankine, J.J.; Barron, D.A.; Robinson, P.; Millner, P.A.; Dickson, R.A. Therapeutic impact of percutaneous spinal biopsy in spinal infection. Postgrad. Med. J. 2004, 80, 607–609. [Google Scholar] [CrossRef]
- Enoch, D.A.; Cargill, J.S.; Laing, R.; Herbert, S.; Corrah, T.W.; Brown, N.M. Value of CT-guided biopsy in the diagnosis of septic discitis. J. Clin. Pathol. 2007, 61, 750–753. [Google Scholar] [CrossRef]
- Renaud, L.; Kelly, J.S. Proceedings: Response of identified ventromedial hypothalamic nucleus neurons to putative neurotransmitters applied by microiontophoresis. Br. J. Pharmacol. 1975, 55, 277–278. [Google Scholar]
- Asai, N.; Ohkuni, Y.; Yamazaki, I.; Kaneko, N.; Aoshima, M.; Kawamura, Y. Therapeutic impact of CT-guided percutaneous catheter drainage in treatment of deep tissue abscesses. Braz. J. Infect. Dis. 2013, 17, 483–486. [Google Scholar] [CrossRef]
- Gnannt, R.; Fischer, M.A.; Baechler, T.; Clavien, P.-A.; Karlo, C.; Seifert, B.; Lesurtel, M.; Alkadhi, H. Distinguishing Infected from Noninfected Abdominal Fluid Collections after Surgery. Investig. Radiol. 2015, 50, 17–23. [Google Scholar] [CrossRef]
- Vansonnenberg, E.; Mueller, P.R.; Ferrucci, J.T. Percutaneous drainage of 250 abdominal abscesses and fluid collections. Part I: Results, failures, and complications. Radiology 1984, 151, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Chew, F.S.; Kline, M.J. Diagnostic Yield of CT-guided Percutaneous Aspiration Procedures in Suspected Spontaneous Infectious Diskitis. Radiology 2001, 218, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Spira, D.; Germann, T.; Lehner, B.; Hemmer, S.; Akbar, M.; Jesser, J.; Weber, M.-A.; Rehnitz, C. CT-Guided Biopsy in Suspected Spondylodiscitis—The Association of Paravertebral Inflammation with Microbial Pathogen Detection. PLoS ONE 2016, 11, e0146399. [Google Scholar] [CrossRef] [PubMed]
- Bijllaardt, W.V.D.; Van Der Jagt, O.P.; Peijs, M.; Janssens, M.; Buiting, A.G.; Reuwer, A.Q. Culturing periprosthetic tissue in blood culture bottles results in isolation of additional microorganisms. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 245–252. [Google Scholar] [CrossRef]
- Peel, T.; Dylla, B.L.; Hughes, J.G.; Lynch, D.T.; Greenwood-Quaintance, K.E.; Cheng, A.C.; Mandrekar, J.N.; Patel, R. Improved Diagnosis of Prosthetic Joint Infection by Culturing Periprosthetic Tissue Specimens in Blood Culture Bottles. mBio 2016, 7, e01776-15. [Google Scholar] [CrossRef]
- Duployez, C.; Wallet, F.; Migaud, H.; Senneville, E.; Loiez, C. Culturing Periprosthetic Tissues in BacT/Alert® Virtuo Blood Culture Bottles for a Short Duration of Post-operative Empirical Antibiotic Therapy. J. Bone Jt. Infect. 2020, 5, 145–150. [Google Scholar] [CrossRef]
- Sanabria, A.; Røkeberg, M.E.O.; Johannessen, M.; Sollid, J.E.; Simonsen, G.S.; Hanssen, A.-M. Culturing periprosthetic tissue in BacT/Alert® Virtuo blood culture system leads to improved and faster detection of prosthetic joint infections. BMC Infect. Dis. 2019, 19. [Google Scholar] [CrossRef]
- Li, C.; Ojeda-Thies, C.; Trampuz, A. Culture of periprosthetic tissue in blood culture bottles for diagnosing periprosthetic joint infection. BMC Musculoskelet. Disord. 2019, 20, 299. [Google Scholar] [CrossRef]
- Peel, T.N.; Sedarski, J.A.; Dylla, B.L.; Shannon, S.K.; Amirahmadi, F.; Hughes, J.G.; Cheng, A.C.; Patel, R. Laboratory Workflow Analysis of Culture of Periprosthetic Tissues in Blood Culture Bottles. J. Clin. Microbiol. 2017, 55, 2817–2826. [Google Scholar] [CrossRef]
- Çetin, E.S.; Kaya, S.; Demirci, M.; Aridogan, B.C. Comparison of the BACTEC blood culture system versus conventional methods for culture of normally sterile body fluids. Adv. Ther. 2007, 24, 1271–1277. [Google Scholar] [CrossRef]
- Sorlin, P.; Mansoor, I.; Dagyaran, C.; Struelens, M.J. Comparison of resin-containing BACTECTM Plus Aerobic/F* medium with conventional methods for culture of normally sterile body fluids. J. Med. Microbiol. 2000, 49, 787–791. [Google Scholar] [CrossRef][Green Version]
- Calderaro, A.; Martinelli, M.; Montecchini, S.; Motta, F.; Covan, S.; Larini, S.; Medici, M.C.; Arcangeletti, M.C.; Chezzi, C.; De Conto, F.; et al. Higher recovery rate of microorganisms from cerebrospinal fluid samples by the BACTEC culture system in comparison with agar culture. Diagn. Microbiol. Infect. Dis. 2016, 84, 281–286. [Google Scholar] [CrossRef]
- Sajjad, M.; Khan, Z.A.; Khan, M.S. Ascitic Fluid Culture in Cirrhotic Patients with Spontaneous Bacterial Peritonitis. J. Coll. Physicians Surg. Pak. 2016, 26, 658–661. [Google Scholar]
- Hughes, J.G.; Vetter, E.A.; Patel, R.; Schleck, C.D.; Harmsen, S.; Turgeant, L.T.; Cockerill, F.R. Culture with BACTEC Peds Plus/F Bottle Compared with Conventional Methods for Detection of Bacteria in Synovial Fluid. J. Clin. Microbiol. 2001, 39, 4468–4471. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Tang, J.; Wang, Q.; Jiang, Y.; Zhang, X. Sonication of Explanted Prosthesis Combined with Incubation in BD Bactec Bottles for Pathogen-Based Diagnosis of Prosthetic Joint Infection. J. Clin. Microbiol. 2014, 53, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Alm, E.W.; Stahl, D.A.; Raskin, L. Characterization of universal small-subunit rRNA hybridization probes for quantitative molecular microbial ecology studies. Appl. Environ. Microbiol. 1996, 62, 4504–4513. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Kappe, R.; Okeke, C.N.; Fauser, C.; Maiwald, M.; Sonntag, H.-G. Molecular probes for the detection of pathogenic fungi in the presence of human tissue. J. Med. Microbiol. 1998, 47, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Kappe, R.; Fauser, C.; Okeke, C.N.; Maiwald, M. Universal fungus-specific primer systems and group-specific hybridization oligonucleotides for 18S rDNA. Mycoses 1996, 39, 25–30. [Google Scholar] [CrossRef]
- Einsele, H.; Hebart, H.; Roller, G.; Löffler, J.; Rothenhofer, I.; Müller, C.A.; Bowden, R.A.; van Burik, J.; Engelhard, D.; Kanz, L.; et al. Detection and identification of fungal pathogens in blood by using molecular probes. J. Clin. Microbiol. 1997, 35, 1353–1360. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Akcam, F.Z.; Yayli, G.; Uskun, E.; Kaya, O.; Demir, C. Evaluation of the Bactec microbial detection system for culturing miscellaneous sterile body fluids. Res. Microbiol. 2006, 157, 433–436. [Google Scholar] [CrossRef]
- Allen, B.; Barnhart, H.; Bashir, M.; Nieman, C.; Breault, S.; Jaffe, T.A. Diagnostic Accuracy of Intra-abdominal Fluid Collection Characterization in the Era of Multidetector Computed Tomography. Am. Surg. 2012, 78, 185–189. [Google Scholar] [CrossRef]
- Radosa, C.G.; Radosa, J.C.; Laniado, M.; Brandt, J.; Streitzig, J.; Seppelt, D.; Volk, A.; Plodeck, V.; Kühn, J.P.; Hoffmann, R.-T. Infected versus sterile abdominal fluid collections in postoperative CT: A scoring system based on clinical and imaging findings. Abdom. Radiol. 2020, 45, 2871–2878. [Google Scholar] [CrossRef]
- Prasad, K.N.; Mishra, A.M.; Gupta, D.; Husain, N.; Husain, M.; Gupta, R.K. Analysis of microbial etiology and mortality in patients with brain abscess. J. Infect. 2006, 53, 221–227. [Google Scholar] [CrossRef]
- Hanson, K.E.; Slechta, E.S.; Killpack, J.A.; Heyrend, C.; Lunt, T.; Daly, J.A.; Hemmert, A.C.; Blaschke, A.J. Preclinical Assessment of a Fully Automated Multiplex PCR Panel for Detection of Central Nervous System Pathogens. J. Clin. Microbiol. 2016, 54, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Maneg, D.; Sponsel, J.; Müller, I.; Lohr, B.; Penders, J.; Madlener, K.; Hunfeld, K.-P. Advantages and Limitations of Direct PCR Amplification of Bacterial 16S-rDNA from Resected Heart Tissue or Swabs Followed by Direct Sequencing for Diagnosing Infective Endocarditis: A Retrospective Analysis in the Routine Clinical Setting. BioMed Res. Int. 2016, 2016, 7923874. [Google Scholar] [CrossRef] [PubMed]
- Bast, A.; Dohmen, P.M.; Podbielski, A.; Warnke, P. Rapid Microbiological Diagnostics from Explanted Heart Valves by a Multiplex PCR Assay. J. Clin. Microbiol. 2019, 57, e01575-18. [Google Scholar] [CrossRef] [PubMed]
- Echavarría, M.; Marcone, D.; Querci, M.; Seoane, A.; Ypas, M.; Videla, C.; O’Farrell, C.; Vidaurreta, S.; Ekstrom, J.; Carballal, G. Clinical impact of rapid molecular detection of respiratory pathogens in patients with acute respiratory infection. J. Clin. Virol. 2018, 108, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Kehrmann, J.; Chapot, V.; Buer, J.; Rating, P.; Bornfeld, N.; Steinmann, J. Diagnostic performance of blood culture bottles for vitreous culture compared to conventional microbiological cultures in patients with suspected endophthalmitis. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 889–895. [Google Scholar] [CrossRef]
- Portillo, M.E.; Salvadó, M.; Trampuz, A.; Siverio, A.; Alier, A.; Sorlí, L.; Martínez, S.; Pérez-Prieto, D.; Horcajada, J.P.; Puig-Verdie, L. Improved Diagnosis of Orthopedic Implant-Associated Infection by Inoculation of Sonication Fluid into Blood Culture Bottles. J. Clin. Microbiol. 2015, 53, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Simor, A.E.; Scythes, K.; Meaney, H.; Louie, M. Evaluation of the BacT/Alert® microbial detection system with FAN aerobic and FAN anaerobic bottles for culturing normally sterile body fluids other than blood. Diagn. Microbiol. Infect. Dis. 2000, 37, 5–9. [Google Scholar] [CrossRef]
- Kuhajda, I.; Zarogoulidis, K.; Tsirgogianni, K.; Tsavlis, D.; Kioumis, I.; Kosmidis, C.; Tsakiridis, K.; Mpakas, A.; Zarogoulidis, P.; Zissimopoulos, A.; et al. Lung abscess-etiology, diagnostic and treatment options. Ann. Transl. Med. 2015, 3, 183. [Google Scholar] [CrossRef]
- Xiong, Y.-M.; Rao, X. Clinical and Microbiological Characteristics of Patients with Complicated Intra-abdominal Infections in Intensive Care Unit. Curr. Med. Sci. 2020, 40, 104–109. [Google Scholar] [CrossRef]
- Cottle, L.; Riordan, T. Infectious spondylodiscitis. J. Infect. 2008, 56, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Yazbeck, M.F.; Dahdel, M.; Kalra, A.; Browne, A.S.; Pratter, M.R. Lung abscess: Update on microbiology and management. Am. J. Ther. 2014, 21, 217–221. [Google Scholar] [CrossRef] [PubMed]
- De Waele, J.; Lipman, J.; Sakr, Y.; Marshall, J.C.; Vanhems, P.; Barrera Groba, C.; Leone, M.; Vincent, J.-L. Abdominal infections in the intensive care unit: Characteristics, treatment and determinants of outcome. BMC Infect. Dis. 2014, 14, 420. [Google Scholar] [CrossRef]
- Gouliouris, T.; Aliyu, S.H.; Brown, N.M. Spondylodiscitis: Update on diagnosis and management. J. Antimicrob. Chemother. 2010, 65 (Suppl. 3), iii11–iii24. [Google Scholar] [CrossRef]
- Jenkins, S.G. Infections due to anaerobic bacteria and the role of antimicrobial susceptibility testing of anaerobes. Rev. Med Microbiol. 2001, 12, 1–12. [Google Scholar] [CrossRef]
- Gajdács, M.; Spengler, G.; Urbán, E. Identification and Antimicrobial Susceptibility Testing of Anaerobic Bacteria: Rubik’s Cube of Clinical Microbiology? Antibiotics 2017, 6, 25. [Google Scholar] [CrossRef]
- Band, V.I.; Weiss, D.S. Heteroresistance: A cause of unexplained antibiotic treatment failure? PLoS Pathog. 2019, 15, e1007726. [Google Scholar] [CrossRef] [PubMed]
- Brukner, I.; Oughton, M. A Fundamental Change in Antibiotic Susceptibility Testing Would Better Prevent Therapeutic Failure: From Individual to Population-Based Analysis. Front. Microbiol. 2020, 11, 1820. [Google Scholar] [CrossRef] [PubMed]
| Parameter | |
|---|---|
| years (±SD) | |
| Age | 63.18 (±15.78) |
| in % | |
| Sex | |
| Male | 69 |
| Female | 31 |
| Sample acquisition site | |
| Thoracic | 21 |
| Abdominal | 69 |
| Musculoskeletal/soft tissue | 10 |
| Referring ward | |
| General and Visceral Surgery | 43 |
| Intensive Care Unit | 23 |
| Internal Medicine | 20 |
| Urology | 5 |
| Other surgical departments | 8 |
| Other departments | 1 |
| Previous surgery | 67 |
| Previous puncture sample acquisition | 13 |
| Blood Culture Method | Conventional Method | p-Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Total (n = 100) | 1.29 | 1.21 | 1.41 | 1.62 | 1 |
| Sample Acquisition Site | |||||
| thoracic (n = 21) | 0.28 | 0.71 | 0.19 | 0.51 | 0.6250 |
| abdominal (n = 69) | 1.56 | 1.14 | 1.75 | 1.61 | 0.7011 |
| musculoskeletal/soft tissue (n = 10) | 1.50 | 1.50 | 1.60 | 2.11 | 1 |
| Microorganism | Blood Culture Method | Conventional Method |
|---|---|---|
| Aerobes | ||
| Gram-negative rods | 42 | 41 |
| Gram-positive cocci | 65 | 52 |
| Gram-positive bacilli | 5 | 4 |
| Anaerobes | 10 | 33 |
| Fungi | 7 | 11 |
| total | 129 | 141 |
| Samples Detected Positive by | ||||||
|---|---|---|---|---|---|---|
| Blood Culture Method | Conventional Culture Method | |||||
| n | % | n | % | p-Value | Fleiss’ Kappa (0.95 Confidence Interval) | |
| Total | 71 | 100 | 64 | 100 | 0.2906 | 0.751 (0.614, 0.888) |
| Detection of | ||||||
| >2 isolates | 33 | 46 | 34 | 53 | 0.4406 | 0.741 (0.574, 0.908) |
| anaerobic bacteria | 9 | 13 | 23 | 36 | 0.0015 | 0.447 (0.235, 0.659) |
| aerobic gram-negative rods | 34 | 48 | 33 | 52 | 0.6698 | 0.968 (0.905, 1) |
| aerobic gram-positive cocci | 51 | 72 | 41 | 64 | 0.3334 | 0.550 (0.330, 0.769) |
| gram-positive bacilli | 5 | 7 | 4 | 6 | 0.8538 | 0.466 (0.021, 0.910) |
| fungi | 7 | 10 | 11 | 17 | 0.2110 | 0.664 (0.398, 0.930) |
| n = 61 | Blood Culture Method | Conventional Method | p-Value | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| 43.55 h | 23.18; 47.37 | 28.62 h | 21.67; 47.00 | 0.0722 | |
| Sample Acquisition Site | Anaerobic Isolates Detected by Conventional Method | p-Value | |
|---|---|---|---|
| Yes | No | ||
| thoracic (n = 21) | 0 | 21 | p < 0.0001 |
| abdominal (n = 69) | 30 | 39 | |
| musculoskeletal/soft tissue (n = 10) | 3 | 7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skusa, R.; Skusa, C.; Wohlfarth, M.; Hahn, A.; Frickmann, H.; Weber, M.-A.; Podbielski, A.; Warnke, P. How to Handle CT-Guided Abscess Drainages in Microbiological Analyses? Sterile Vials vs. Blood Culture Bottles for Transport and Processing. Microorganisms 2021, 9, 1510. https://doi.org/10.3390/microorganisms9071510
Skusa R, Skusa C, Wohlfarth M, Hahn A, Frickmann H, Weber M-A, Podbielski A, Warnke P. How to Handle CT-Guided Abscess Drainages in Microbiological Analyses? Sterile Vials vs. Blood Culture Bottles for Transport and Processing. Microorganisms. 2021; 9(7):1510. https://doi.org/10.3390/microorganisms9071510
Chicago/Turabian StyleSkusa, Romy, Christopher Skusa, Moritz Wohlfarth, Andreas Hahn, Hagen Frickmann, Marc-André Weber, Andreas Podbielski, and Philipp Warnke. 2021. "How to Handle CT-Guided Abscess Drainages in Microbiological Analyses? Sterile Vials vs. Blood Culture Bottles for Transport and Processing" Microorganisms 9, no. 7: 1510. https://doi.org/10.3390/microorganisms9071510
APA StyleSkusa, R., Skusa, C., Wohlfarth, M., Hahn, A., Frickmann, H., Weber, M.-A., Podbielski, A., & Warnke, P. (2021). How to Handle CT-Guided Abscess Drainages in Microbiological Analyses? Sterile Vials vs. Blood Culture Bottles for Transport and Processing. Microorganisms, 9(7), 1510. https://doi.org/10.3390/microorganisms9071510






